Abstract
Legionella pneumophila has been increasingly recognized as a cause of community-acquired pneumonia (CAP) and an important public health problem worldwide. We conducted the present study to assess trends in epidemiology, diagnosis, clinical features, treatment, and outcomes of sporadic community-acquired L. pneumophila pneumonia requiring hospitalization at a university hospital over a 15-year period (1995–2010). Among 3934 nonimmunosuppressed hospitalized patients with CAP, 214 (5.4%) had L. pneumophila pneumonia (16 cases were categorized as travel-associated pneumonia, and 21 were part of small clusters). Since the introduction of the urinary antigen test, the diagnosis of L. pneumophila using this method remained stable over the years (p = 0.42); however, diagnosis by means of seroconversion and culture decreased (p < 0.001 and p = 0.001, respectively).
The median age of patients with L. pneumophila pneumonia was 58.2 years (SD 13.8), and 76.4% were male. At least 1 comorbid condition was present in 119 (55.6%) patients with L. pneumophila pneumonia, mainly chronic heart disease, diabetes mellitus, and chronic pulmonary disease. The frequency of older patients (aged >65 yr) and comorbidities among patients with L. pneumophila pneumonia increased over the years (p = 0.06 and p = 0.02, respectively). In addition, 100 (46.9%) patients were classified into high-risk classes according to the Pneumonia Severity Index (groups IV–V). Twenty-four (11.2%) patients with L. pneumophila pneumonia received inappropriate empirical antibiotic therapy at hospital admission. Compared with patients who received appropriate empirical antibiotic, patients who received inappropriate therapy more frequently had acute onset of illness (p = 0.004), pleuritic chest pain (p = 0.03), and pleural effusion (p = 0.05). The number of patients who received macrolides decreased over the study period (p < 0.001), whereas the number of patients who received levofloxacin increased (p < 0.001). No significant difference was found in the outcomes between patients who received erythromycin and clarithromycin. However, compared with macrolide use during hospital admission, levofloxacin therapy was associated with a trend toward a shorter time to reach clinical stability (median, 3 vs. 5 d; p = 0.09) and a shorter length of hospital stay (median, 7 vs. 10 d; p < 0.001).
Regarding outcomes, 38 (17.8%) patients required intensive care unit (ICU) admission, and the inhospital case-fatality rate was 6.1% (13 of 214 patients). The frequency of ICU admission (p = 0.34) and the need for mechanical ventilation (p = 0.57) remained stable over the study period, but the inhospital case-fatality rate decreased (p = 0.04). In the logistic regression analysis, independent factors associated with severe disease (ICU admission and death) were current/former smoker (odds ratio [OR], 2.96; 95% confidence interval [CI], 1.01–8.62), macrolide use (OR, 2.40; 95% CI, 1.03–5.56), initial inappropriate therapy (OR, 2.97; 95% CI, 1.01–8.74), and high-risk Pneumonia Severity Index classes (OR, 9.1; 95% CI, 3.52–23.4).
In conclusion, L. pneumophila is a relatively frequent causative pathogen among hospitalized patients with CAP and is associated with high morbidity. The annual number of L. pneumophila cases remained stable over the study period. In recent years, there have been significant changes in diagnosis and treatment, and the inhospital case-fatality rate of L. pneumophila pneumonia has decreased.
INTRODUCTION
Legionella species cause 2 clinical syndromes, known as Legionnaires disease and Pontiac fever. Legionnaires disease is an acute, serious, and sometimes lethal pneumonia, whereas Pontiac fever is generally a self-limited, nonpneumonic, influenza-like condition. Since the original description of Legionnaires disease in 1977, Legionella pneumophila has been increasingly recognized as a cause of sporadic and epidemic community-acquired pneumonia (CAP) in all age groups and in both healthy and immunosuppressed hosts.4,6,16,22–24 L. pneumophila is particularly frequent among patients with CAP who require admission to an intensive care unit (ICU).31,37 Therefore, L. pneumophila continues to be an important public health problem worldwide.
Prospective studies have reported major differences in the frequencies of L. pneumophila causing CAP. These differences may be due to variances in the locations studied, the specific patient populations included, and the extent and nature of the microbiologic techniques used. Similarly, seasonal variations in the incidence of Legionnaires disease have been described.1,23,25,36 In addition, in recent years, new diagnostic tests (urinary antigen test and polymerase chain reaction) and antibiotic therapies (third-generation fluoroquinolones and newer macrolides) for Legionella pneumonia have become available. Although their use may have had an impact on identifying cases and on case-fatality rates, comprehensive clinical studies analyzing the issue are scarce. Most data regarding trends in Legionnaires disease are from passive surveillance systems.1,23 Therefore, new information is required for a better understanding of the disease burden.
We conducted the present study to assess trends in epidemiology, diagnosis, clinical features, treatment, and outcomes of sporadic community-acquired L. pneumophila pneumonia cases in a large prospective cohort of nonimmunosuppressed patients requiring hospitalization at a university hospital over a 15-year period (1995–2010).
PATIENTS AND METHODS
Setting, Patients, and Study Design
This observational study was conducted at an 800-bed tertiary teaching hospital for adults in Barcelona, Spain. The hospital serves an urban area of 900,000 inhabitants. Nonimmunosuppressed patients admitted to the hospital with CAP from February 13, 1995, through December 31, 2010, were prospectively recruited and followed. Patients with CAP were identified at the emergency department by the attending physicians and/or the study investigators. Clinical and laboratory data on all patients were prospectively recorded using a computer-assisted protocol. Patients with neutropenia, solid organ transplantation, chemotherapy, acquired immunodeficiency syndrome (AIDS) or current corticosteroid therapy (≥20 mg prednisone/d or equivalent) at admission were excluded. This observational study was approved by the institutional review board, and all patients included gave informed consent.
For the purposes of the study, we analyzed data from confirmed cases of sporadic community-acquired L. pneumophila pneumonia, diagnosed with 1 or more of the following methods: urine antigen test, isolation of Legionella in sputum, transthoracic needle aspiration specimen, or pleural fluid, and/or a fourfold increase in the antibody titer in serologic methods. Cases of community-acquired L. pneumophila pneumonia were defined as travel associated if the patient had stayed at or visited an accommodation site during the disease incubation period (15 d before symptom onset), in accordance with the criteria of the European Legionnaires Disease Surveillance Network.
Clinical Assessment, Antibiotic Therapy, and Follow-Up
Patients were seen daily during their hospital stay by 1 or more of the investigators. Data were collected on epidemiology, demographic characteristics, comorbidities, causative organisms, antibiotic susceptibilities, biochemical analysis, empirical antibiotic therapy, and outcomes, including mortality. A long-term follow-up visit took place 1 month after discharge. To stratify patients according to risk, we used the Pneumonia Severity Index (PSI).11 Clinical stability was considered as described elsewhere.15
Antibiotic therapy was initiated in the emergency department in accordance with the hospital guidelines, which recommended the administration of a β-lactam (either ceftriaxone sodium 1 g IV once/d or amoxicillin/clavulanate potassium 1 g IV 3 times/d) with or without a macrolide; from 1998 onward, levofloxacin (500 mg IV once/d) was also allowed. Combination therapy was recommended for patients with clinical suspicion of a Legionella species or an atypical pathogen, or in the absence of a demonstrative finding on sputum Gram stain results. Patients with a urine antigen test result positive for Legionella at admission were treated with macrolide (with or without rifampin, at the discretion of the physician) or levofloxacin (500 mg IV once/d). Patients initially treated with other antibiotics were switched to appropriate therapy. Combined amoxicillin/clavulanate was recommended for patients with clinical suspicion of aspiration pneumonia in order to provide adequate antianaerobic coverage, as described elsewhere.20
Definitions
Pneumonia was defined as an acute illness associated with 1 or more of the following signs and symptoms: new cough with or without sputum production, pleuritic chest pain, dyspnea, fever or hypothermia, altered breath sounds on auscultation, leukocytosis, plus the presence of a new infiltrate on a chest radiograph. Pneumococcal pneumonia was diagnosed in patients with 1 or more cultures positive for Streptococcus pneumoniae obtained from blood, normally sterile fluids, or sputum, and/or positive urinary antigen test detection. Only good quality samples of sputum (<10 squamous epithelial cells and >25 leukocytes per field) were accepted for processing.
Tobacco smoking was recorded when a patient had smoked more than 10 cigarettes per day for at least 1 year. Alcohol abuse was considered if alcohol intake was more than 3 standard drinks per day. Vaccinated patients included all individuals who had received pneumococcal vaccine in the previous 5 years or influenza vaccine in the previous year. The diagnosis of septic shock was based on a systolic blood pressure of less than 90 mm Hg and peripheral hypoperfusion with the need for vasopressors.3 Empirical antibiotic therapy was defined as antibiotics received on the first day of therapy for pneumonia. Initial inappropriate therapy was defined as the absence of antimicrobial agents directed at a specific type of organism or administration of an antibiotic to which the organism was resistant, according to susceptibility test criteria for lower respiratory tract pathogens. Initial inappropriate therapy was considered in patients with Legionella pneumonia who did not receive macrolides, levofloxacin, or tetracyclines at hospital admission. Patients with aspiration pneumonia who had not received anaerobic coverage (that is, amoxicillin-clavulanate) were considered to have received inappropriate empirical antibiotic therapy.
Complications were defined as any untoward circumstances occurring during hospitalization. The composite outcome of ICU admission or death was used to evaluate severe disease. Inhospital case-fatality rate was defined as death from any cause during hospitalization.
Microbiologic Studies and Etiologic Diagnosis
Pathogens in blood, normally sterile fluids, sputum, and other samples were investigated using standard microbiologic procedures. Isolation of Legionella species was attempted in sputum samples and other samples by the selective medium buffered charcoal yeast extract-α. L. pneumophila serogroup 1 antigen in urine was detected by an immunochromatographic method (NOW Legionella Urinary Antigen Test; Binax Inc., Portland, ME) or enzyme-linked immunosorbent assay (ELISA-Bartels, Bartels, Trinity Biotech, Wicklow, Ireland). The S. pneumoniae antigen in urine was detected by a rapid immunochromatographic assay (NOW Assay, Binax Inc.). Standard serologic methods were used to determine antibodies against atypical agents. Enzyme immunoassay was used to detect antibodies against L. pneumophila serogroups 1–6. Microbiologic studies were performed at the discretion of the physicians. Antimicrobial susceptibility was tested by the microdilution method, following methods and criteria of the Clinical and Laboratory Standards Institute.
Statistical Analysis
We used the chi-square test for trend analysis to account for multiple comparisons to determine whether the slope of the trend line differed from 0. To detect significant differences between Legionella and pneumococcal pneumonia, we used the chi-square test or Fisher exact test for categorical variables and the t test or Mann-Whitney U test for continuous variables, depending on the results of the Kolmogorov-Smirnov normality test. A logistic regression analysis was carried out to evaluate associations between independent variables and severe disease. Significant (p < 0.10) and clinically important variables (age >70 yr, male sex, comorbidities, tobacco smoking, alcohol abuse, and high-risk PSI classes) were included in the multivariate analysis. High-risk PSI classes were chosen as a marker of severity to avoid collinearity with other variables already included in this score and due to the low number of patients who had severe disease. The relative risks were expressed as odds ratios (OR) and 95% confidence intervals (CI). The goodness-of-fit of the model was evaluated by the Hosmer-Lemeshow test. In addition, a receiver operating characteristic (ROC) curve and an area under curve (AUC) were used to evaluate the discriminatory power and predictive value of the PSI for identifying severe L. pneumophila pneumonia. P values ≤0.05 were considered statistically significant. All reported p values are 2-tailed. Data were analyzed using SPSS statistical software (v. 15.0, SPSS Inc., Chicago, IL).
RESULTS
General Features of the Study Population
During the 15-year prospective study period, 3934 nonimmunosuppressed patients with CAP required hospitalization. The median age was 70 years (interquartile range [IQR], 50–79); 2691 (68.4%) patients were male, and 2989 (76%) had at least 1 comorbidity, mainly chronic pulmonary disease (28%), chronic heart disease (23.5%), and diabetes mellitus (20.9%). Septic shock and altered mental state at hospital admission were diagnosed in 288 (7.3%) and 574 (14.6%) patients, respectively. Chest X-ray with multilobar pneumonia was evidenced in 1289 (32.8%) patients. A total of 2312 (58.8%) patients were classified into high-risk classes according to the PSI (groups IV–V). Overall, S. pneumoniae (1346 cases) was the most frequent causative pathogen, followed by aspiration pneumonia (287 cases), Legionella species (215 cases), and Haemophilus influenzae (205 cases). Three hundred fifty-nine (9.1%) patients required ICU admission, and 305 (7.8%) patients died within 30 days of hospitalization.
Diagnosis and Epidemiology of Community-Acquired L. pneumophila Pneumonia
Among the 215 patients with community-acquired Legionella species pneumonia, 1 patient had L. longbeachae pneumonia and 214 patients had L. pneumophila pneumonia. All patients had L. pneumophila serogroup 1. Sixteen (7.4%) cases were categorized as travel-associated L. pneumophila pneumonia, and 21 (9.8%) were part of small clusters (15 patients in 2002 and 6 patients in 2004). No epidemics occurred in our hospital area during the study period.
The diagnosis of the 214 L. pneumophila pneumonia cases was established using 1 or more of the following methods: urinary antigen test (n = 194), seroconversion (n = 95), sputum culture (n = 37), transthoracic needle aspiration specimen culture (n = 9), and pleural fluid culture (n = 3). As shown in Figure 1, the diagnosis of L. pneumophila with the urinary antigen test remained stable over the years; however, the diagnosis using seroconversion and cultures decreased (p = 0.42, p < 0.001, and p = 0.001, respectively).
FIGURE 1.
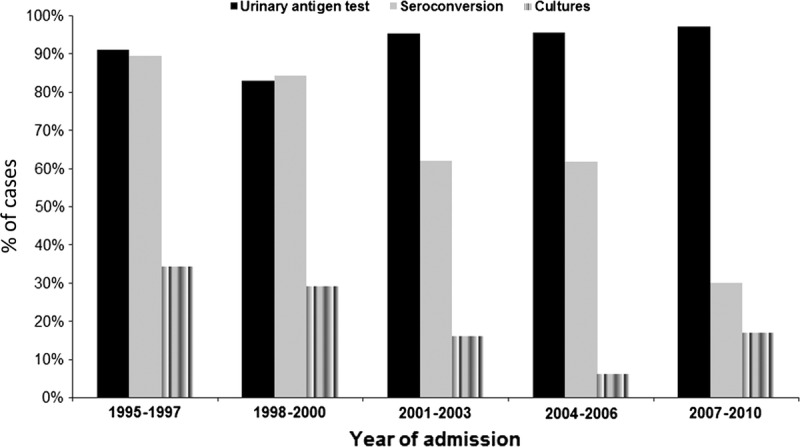
Tests used to diagnose community-acquired L. pneumophila pneumonia over the study period.
The median annual number of L. pneumophila pneumonia cases was 14 (range, 3–20). As shown in Figure 2A, although the number of annual L. pneumophila pneumonia cases remained stable over the years, the percentage of annual L. pneumophila pneumonia cases in relation to the number of hospitalized patients with CAP decreased in recent years (from 9.2% in 2005 to 1.5% in 2010; p < 0.001). Moreover, 171 (79.9%) cases occurred during summer and fall (from June to December) (Figure 2B).
FIGURE 2.
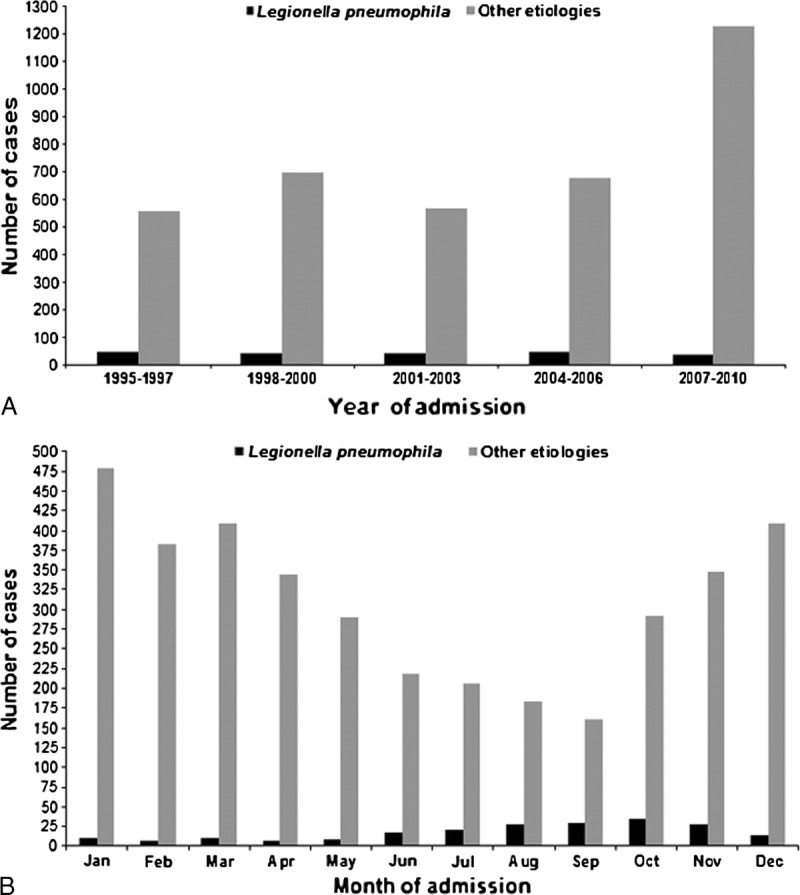
Number of community-acquired L. pneumophila pneumonia cases by year (A) and by month (B) over the study period, in relation to number of CAP cases from other etiologies.
Demographic and Clinical Features of Patients With Community-Acquired L. pneumophila Pneumonia
The mean age of patients with L. pneumophila pneumonia was 58.2 years (SD 13.8). L. pneumophila pneumonia occurred more commonly in patients aged 50–69 years, and in male patients (76.4%). Patients aged <70 years comprised 78.2% of the total L. pneumophila pneumonia cases. At least 1 comorbid condition was present in 119 (55.6%) patients with L. pneumophila pneumonia, mainly chronic heart disease, diabetes mellitus, and chronic pulmonary disease. The frequency of male sex remained stable over the study period (p = 0.11). However, the frequency of older patients (aged >65 yr) and comorbidities increased (p = 0.06 and p = 0.02, respectively) (Figure 3). Most patients with L. pneumophila pneumonia were current/former smokers (72.8%).
FIGURE 3.
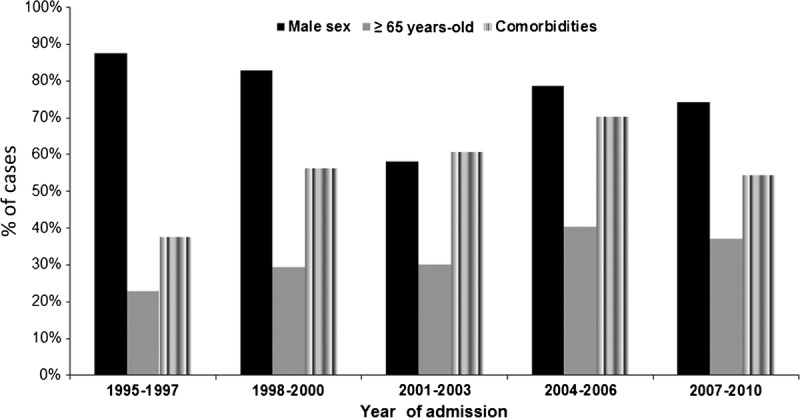
Demographic data for patients with community-acquired L. pneumophila pneumonia.
In addition, 100 (46.9%) patients were classified into high-risk classes according to the PSI (groups IV–V), identifying 78% of patients with severe disease (ICU admission or death). Area under ROC curve to predict severe disease for PSI was 0.76 (95% CI, 0.68–0.85).
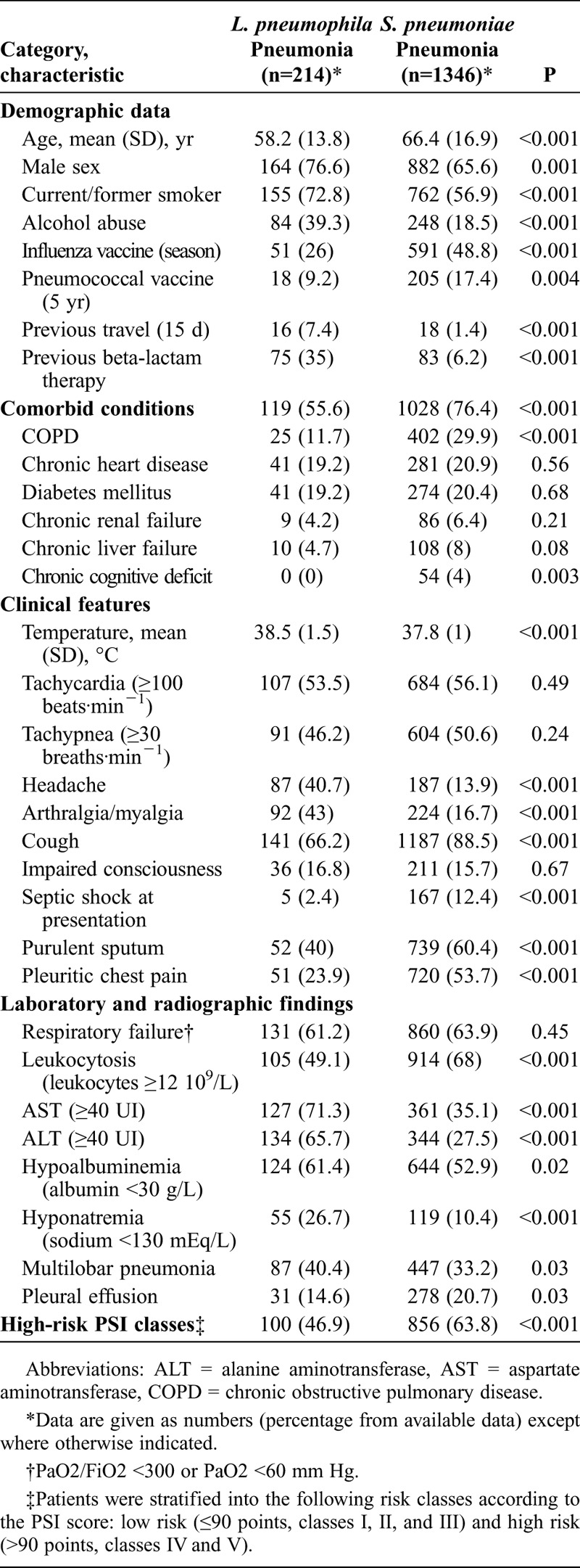
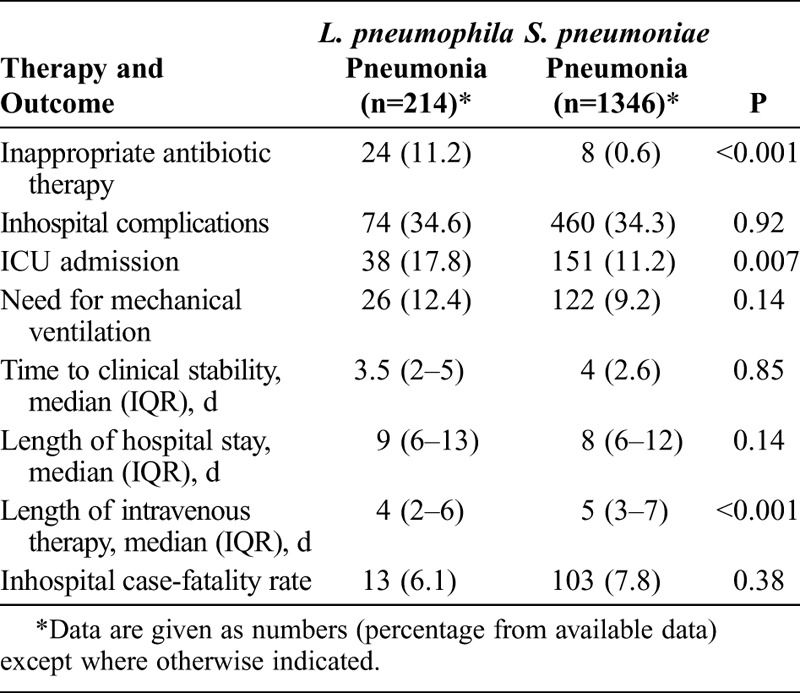
Demographic and clinical features were compared in patients with L. pneumophila pneumonia and patients with pneumococcal pneumonia (Table 1). Patients with L. pneumophila pneumonia had higher axillary temperature at hospital admission (95% CI of mean difference, 0.5–0.9 °C) and were younger (95% CI of mean difference, 6.1–10.2 yr). By contrast, patients with pneumococcal pneumonia were more likely to have comorbidities, mainly chronic pulmonary disease, chronic liver disease, and chronic cognitive deficit. Patients with pneumococcal pneumonia had more frequently received prior seasonal influenza and pneumococcal vaccine. Conversely, alcohol abuse and current/former smoking were more common in patients with L. pneumophila pneumonia. Significantly, patients with L. pneumophila pneumonia more frequently had received prior outpatient β-lactam treatment (medication was administered from symptoms onset to hospitalization) and had a history of previous travel. Regarding clinical features, patients with L. pneumophila pneumonia were less likely to have cough, purulent sputum, pleuritic chest pain, pleural effusion, and septic shock at hospital admission. Conversely, they more commonly had headache, arthralgia/myalgia, hyponatremia, and multilobar pneumonia, and had higher transaminase values.
TABLE 1.
Characteristics of Patients With L. pneumophila and S. pneumoniae Pneumonia
Antimicrobial Treatment and Outcome of Community-Acquired L. pneumophila Pneumonia
Twenty-four (11.2%) patients with L. pneumophila pneumonia received inappropriate empirical antibiotic therapy at hospital admission. The frequency of patients who received inappropriate empirical antibiotic therapy at hospital admission was stable over the years (p = 0.67). Compared with patients who received appropriate empirical antibiotic, patients who received inappropriate therapy more frequently had acute onset of illness (p = 0.004), pleuritic chest pain (p = 0.03), and pleural effusion (p = 0.05).
Among 190 patients who received appropriate empirical antibiotic, 111 received levofloxacin; 74, macrolides (48 erythromycin 500 mg IV every 6 h, 24 clarithromycin 500 mg IV once/d, 1 azithromycin, and 1 roxithromycin); 3 received combination therapy with levofloxacin and macrolides; 1, doxycycline; and 1, clindamycin. Combination therapy with rifampicin was administered to 50 patients (in 2 patients with levofloxacin). The median duration of intravenous macrolide and levofloxacin therapy was 5 days (IQR, 3–7) and 4 days (IQR, 3–7), respectively. The total duration of macrolide therapy was 25 days (IQR, 21–28) and of levofloxacin therapy was 14 days (IQR, 11–19) (p < 0.001). The number of patients who received macrolides decreased over the years (p < 0.001), whereas the number of patients who received levofloxacin increased (p < 0.001) (Figure 4). Levofloxacin was administered more frequently to patients whose diagnosis was made with the urinary antigen test than in patients whose diagnosis was made with culture or serology (61.3% vs. 30%; p = 0.007). Compared with macrolide use during hospital admission, levofloxacin use was associated with a trend toward a shorter time to reach clinical stability (median, 3 vs. 5 d; p = 0.09) and shorter length of hospital stay (median, 7 vs. 10 d; p < 0.001).
FIGURE 4.
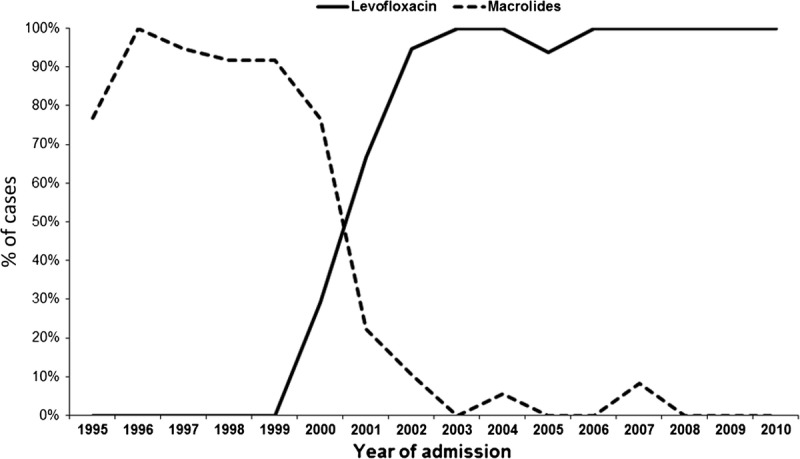
Antibiotic therapy in patients with community-acquired L. pneumophila pneumonia.
Regarding outcomes, 38 (17.8%) patients required ICU admission, and 13 (6.1%) patients died during hospitalization. Among the 13 patients with L. pneumophila pneumonia who died, causes of death were respiratory failure (7 patients), multiorgan failure (3 patients), acute cardiac event (1 patient), and renal failure (1 patient). No cause of death was established for 1 patient. The frequency of ICU admission (p = 0.34) and the need for mechanical ventilation (p = 0.57) remained stable over the years. By contrast, the inhospital case-fatality rate decreased (p = 0.04) (Figure 5).
FIGURE 5.
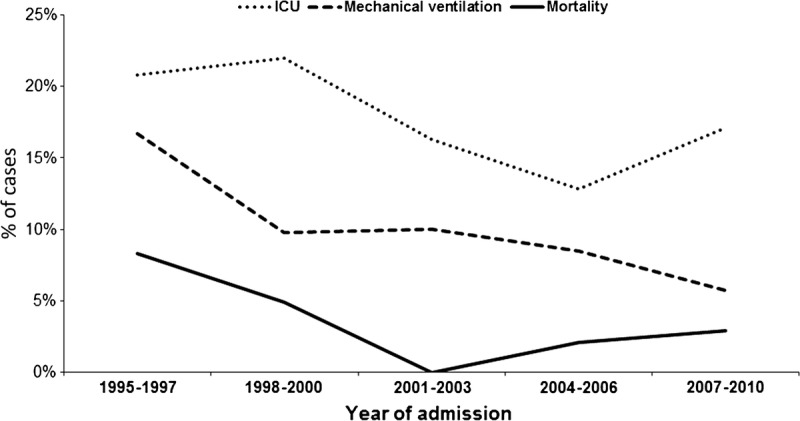
Outcomes of patients with community-acquired L. pneumophila pneumonia.
Compared with patients with pneumococcal pneumonia, patients with L. pneumophila pneumonia more frequently received inappropriate empirical antibiotic therapy and were more likely to require ICU admission. No significant differences were found in time to reach clinical stability and length of hospital stay. However, inhospital case-fatality rate was lower in patients with L. pneumophila pneumonia (Table 2).
TABLE 2.
Therapy and Outcomes of Patients With L. pneumophila and S. pneumoniae Pneumonia
Factors Associated With Severe Community-Acquired L. pneumophila Pneumonia
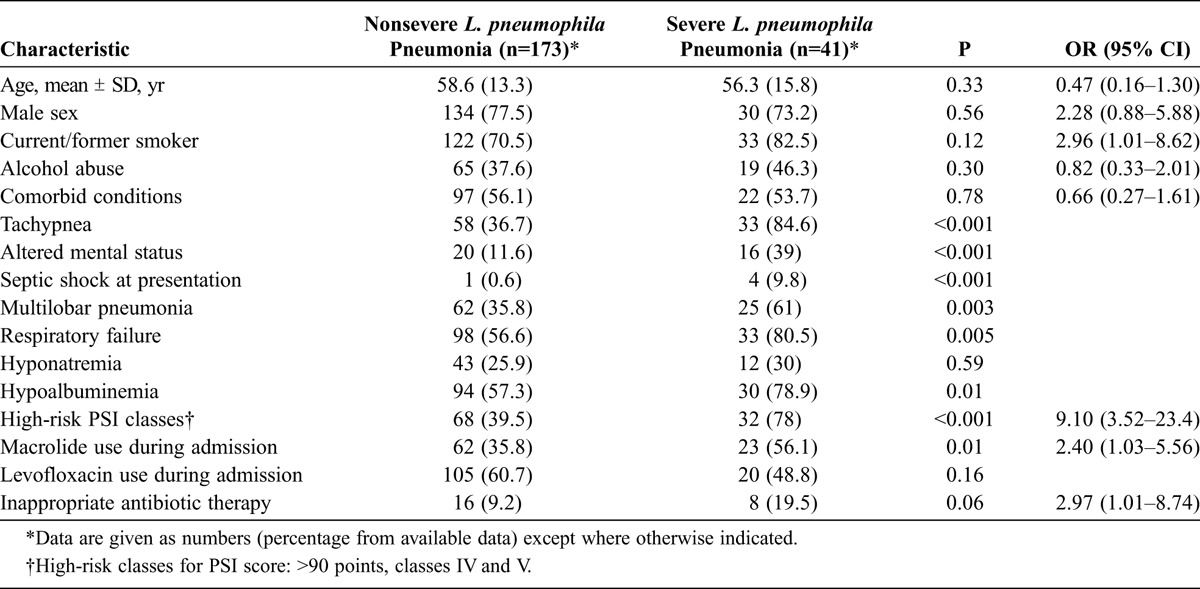
Forty-one (19.1%) patients with L. pneumophila pneumonia developed severe disease (ICU admission or death). Risk factors related with severe disease in this group of patients are detailed in Table 3. No significant differences were found regarding age, sex, and comorbidities. By contrast, altered mental state, septic shock, tachypnea, respiratory failure, high-risk PSI classes, hypoalbuminemia, multilobar pneumonia in chest X-rays, inappropriate empirical antibiotic therapy, and the use of macrolides during hospital admission were more frequent in patients with severe L. pneumophila pneumonia. In the logistic regression analysis, independent factors associated with severe disease were current/former smoker (OR, 2.96; 95% CI, 1.01–8.62), macrolide use (OR, 2.40; 95% CI, 1.03–5.56), initial inappropriate therapy (OR, 2.97; 95% CI, 1.01–8.74), and high-risk PSI classes (OR, 9.1; 95% CI, 3.52–23.4). The goodness-of-fit of the model was 0.20.
TABLE 3.
Factors Associated With Severe Disease (ICU Admission and Death) in Patients With L. pneumophila Pneumonia: Multivariate Analysis
DISCUSSION
In the current 15-year prospective study of a large cohort of nonimmunosuppressed patients with CAP requiring hospitalization, we document the following findings: 1) L. pneumophila is a frequent causative pathogen; 2) the annual number of sporadic L. pneumophila pneumonia cases remained stable over the years of the study; 3) L. pneumophila pneumonia is associated with high morbidity, as evidenced by the high proportion of patients requiring ICU admission; 4) changes have occurred in the diagnosis (the use of the urinary antigen test remained stable, but the use of serology and culture decreased), treatment (levofloxacin has progressively replaced macrolides), and prognosis (the inhospital case-fatality rate decreased) of L. pneumophila pneumonia in recent years, 5) levofloxacin use was associated with a shorter time to reach clinical stability and shorter length of hospital stay in patients with L. pneumophila pneumonia, and 6) independent factors associated with poor prognosis (ICU admission or death) among patients with Legionella pneumonia were current/former smoker, macrolide use, initial inappropriate therapy, and high-risk PSI classes.
Legionella species have been increasingly recognized as a cause of both sporadic and epidemic CAP requiring hospitalization. In Europe and the United States, L. pneumophila is responsible for 95% of cases of Legionnaires disease.40 In a 2008 report39 applying sophisticated diagnostic tools in patients with CAP, L. pneumophila was diagnosed in 3.8% of hospitalized patients. In the present study carried out in Barcelona, Spain, L. pneumophila accounted for 5.4% of all cases of CAP (the third most frequent causative pathogen). However, studies testing for Legionella urinary antigen test, specialized cultures for Legionella, and the Legionella serologic test reported an incidence of Legionnaires disease between 12.5% and 14%.36,41 Table 4 shows the frequency of Legionella pneumonia in patients with CAP and the diagnostic tests used.
TABLE 4.
Frequency of Legionella Pneumonia in Hospitalized Adult Patients With CAP
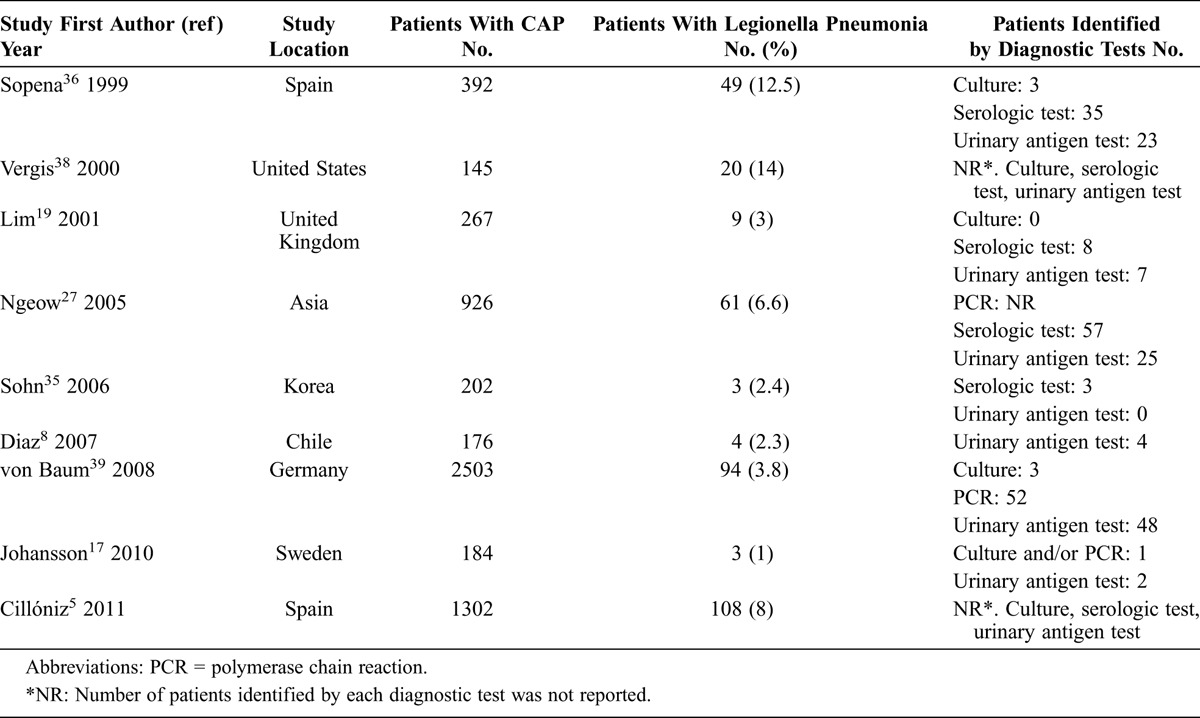
In the present study, 16 (7.4%) patients were categorized as having travel-associated L. pneumophila pneumonia. An analysis of Legionnaires disease cases between 1980 and 1998 in the United States showed that an average of 20% of legionellosis cases were travel associated.1 The European Working Group for Legionella Infections (EWGLI) and the United States Centers for Disease Control and Prevention have identified numerous cases of travel-associated Legionnaires disease; the most commonly identified source of infection has been contaminated water in hotels.14 Surveillance programs are critical for the detection of travel-associated Legionnaires disease to implement timely preventive actions.
Although the frequency of the L. pneumophila urinary antigen test among patients with pneumonia increased over the study period (from 20.4% in 1995–1997 to 42.9% in 2006–2010; p < 0.001), the annual number of L. pneumophila cases remained stable during the study, with a predominance of cases in the summer and fall. Correspondingly, the EWGLI reported that the number of annual cases during 2007–2008 was similar to the number during 2005–2006 for 36 European countries.18 By contrast, Neil et al23 found an increase in reported legionellosis cases in the United States, mainly during 2003–2005. Factors that might have contributed to the increase in reported legionellosis cases include an increasing population of persons at risk for infection, improved diagnosis and reporting, and increased use of urine antigen testing. Ng et al26 documented a recent decrease in legionellosis incidence in Ontario, Canada. Notably, however, investigators found no evidence that changes in diagnostic testing were responsible for the increases and decreases in cases, respectively.23,26 These data highlight the differences in the frequencies of L. pneumophila causing CAP due to the variances in the locations studied and the specific patient populations included in the reports.
In other geographic areas (Australia, New Zealand, and Japan), L. longbeachae infection occurs as often as L. pneumophila infection.40 In addition, some studies have documented that L. longbeachae–derived Legionnaires disease has increased worldwide. In the Netherlands between 2000 and 2004, the first 5 cases of L. longbeachae pneumonia were reported.7,40 In the current study, in 2009, 1 patient had L. longbeachae pneumonia. Another report found that 10% of Legionella infections were caused by species other than L. pneumophila.39 Risk factors for and clinical presentation of L. longbeachae pneumonia are similar to those of other legionelloses. However, gardening activities and use of potting mixes are risk factors that are so far unique to L. longbeachae infection.40 Significantly, although the urinary antigen test can be used to diagnose infections with L. pneumophila serotype 1, it is not sensitive for diagnosis of infections caused by other Legionella species. The widespread application of the urinary antigen test may lead to underrecognition of other Legionella species.41 Interestingly, we found that the diagnosis of Legionella using cultures has decreased over the years. In this regard, it should be noted that although patients with Legionnaires disease frequently have nonpurulent or insufficient sputum, these samples can often yield the microorganism in culture.41
Regarding the demographic features of patients with L. pneumophila pneumonia, we found that the highest number of cases occurred in persons aged 40–69 years, and that males accounted for more than 70% of cases. However, we noted a trend toward a higher number of cases in older ages (aged >65 yr) over the 15-year study (from 22.9% in 1995–1997 to 37.1% in 2007–2010). We also found that the prevalence of comorbid conditions among patients with L. pneumophila pneumonia increased (from 37.5% in 1995–1997 to 54.3% in 2007–2010). Additionally, a large proportion of patients with L. pneumophila pneumonia were current/former smokers and heavy drinkers. Significantly, previous studies have demonstrated a strong association between Legionella infection and these demographic features.6
Although there is no single clinical manifestation that distinguishes Legionnaires disease from other types of pneumonia, it has been suggested that there is a clinical profile that increases the likelihood of the diagnosis. Compared with patients with pneumococcal pneumonia, we found that patients with L. pneumophila pneumonia more frequently received prior outpatient β-lactam treatment and were less likely to have cough, purulent sputum, pleuritic chest pain, pleural effusion, and septic shock at hospital admission. By contrast, they more frequently had a history of previous travel and were more likely to have headache, arthralgia/myalgia, hyponatremia, and multilobar pneumonia. Similarly, they had higher transaminase values. Thus, certain clinical features may allow recognition of Legionella pneumonia, and physicians should consider them when evaluating patients with CAP in the emergency department. However, it appears that it is difficult to express a reliable scoring system, and there are no convincing data in favor of a syndromic approach to the management of this infection.10,13,21
In the present study, the number of patients who received macrolides decreased over the years, while the number of patients who received levofloxacin increased. Compared with macrolide use during hospital admission, levofloxacin use was associated with a trend toward a shorter time to reach clinical stability and shorter length of hospital stay. By contrast, the use of macrolides during hospital admission was independently associated with poor prognosis. It is important to note that no significant difference on outcomes was found between patients who received erythromycin and clarithromycin. Observational studies2,22,33 comparing levofloxacin with older macrolides in the treatment of Legionnaires disease reported that levofloxacin was associated with faster resolution of pneumonia symptoms, more rapid achievement of clinical stability, and shorter length of hospital stay compared with older macrolides. In addition, treatment failures with erythromycin have been reported.29 Nevertheless, it should be emphasized that none of these studies was a randomized trial. Levofloxacin has been shown to be superior to erythromycin in inhibiting the intracellular growth of L. pneumophila in both in vitro and animal models.12,29 Moreover, observational studies9,28,30 have demonstrated the safety and efficacy of azithromycin for the treatment of hospitalized patients with Legionnaires disease. In a prospective, open-label, noncomparative study,30 azithromycin was well tolerated and efficacious in the treatment of 25 hospitalized patients with community-acquired Legionella pneumonia. The overall cure rate among clinically evaluable patients was 95% at 10–14 days after therapy and 96% at 4–6 weeks after therapy. Although in vitro and in vivo studies have demonstrated that the efficacy of azithromycin is comparable to that of quinolones, to our knowledge no comparative clinical studies have been performed.
Twenty-four (11.2%) patients with L. pneumophila pneumonia received inappropriate empirical antibiotic therapy at hospital admission. Patients who received inappropriate therapy more frequently had acute onset of illness, pleuritic chest pain, and pleural effusion. The frequency of patients who received inappropriate therapy at hospital admission remained stable over the years. However, inappropriate empirical antibiotic therapy was independently associated with poor prognosis. In 2008, a large study39 in Germany also found a high rate of discordant initial antimicrobial treatment (30%) in patients with Legionella pneumonia. In that study, most patients receiving a discordant initial treatment were given aminopenicillins plus β-lactamase inhibitors or cephalosporins. Significantly, inappropriate empirical antibiotic therapy has been associated with early failure and higher mortality in patients with CAP. In this regard, L. pneumophila was the pathogen most frequently associated with discordant therapy in a report by our group.32 Since Legionella pneumonia is difficult to diagnose clinically, and universal broad-spectrum antibiotic therapy may not be the answer, some authors have recommended the use of the urinary antigen test for all patients with CAP who require hospitalization.41 A sequential approach is currently performed at our institution: Legionella urinary antigen test and specific Legionella cultures are reserved for patients with high-risk pneumonia for whom demonstrative results of sputum Gram staining are not available (poor-quality samples and/or samples in which predominant morphotypes were not detected), and who have negative pneumococcal urinary antigen test.
To our knowledge, no prior study has evaluated the usefulness of CAP-specific scores on patients with L. pneumophila pneumonia. We found that high-risk PSI classes identify nearly 80% of patients with severe disease (ICU admission or death), and the accuracy of this score is good as demonstrated by the statistical analysis (AUC, 0.76). AUC obtained from PSI score in our study was close to those obtained in other studies aimed at predicting mortality or severe disease in patients with CAP.34
Factors associated with poor prognosis in hospitalized patients with Legionella pneumonia are not well defined. In the current study, independent factors associated with severe disease were current/former smoker, macrolide use, initial inappropriate therapy, and high-risk PSI classes. The frequency of ICU admission and the need for mechanical ventilation remained stable over the years. By contrast, there was a decrease in the inhospital case-fatality rate. A previous study1 also reported a substantial fall in the rate of mortality due to Legionella pneumonia. Investigators considered that because the urine antigen test is more sensitive than culture or serologic testing, it is possible that its use led to the detection of disease in patients with milder forms of legionellosis, in whom case-fatality rates are lower. In addition, it is probable that these patients were administered treatment more quickly. Interestingly, the frequency of patients with L. pneumophila pneumonia and high-risk PSI score at hospital admission remained stable over the 15-year study period (from 56.3% in 1995–1997 to 48.6% in 2007–2010; p = 0.34). Similarly, in the present study no significant difference in time from hospital admission to antibiotic administration was found between patients who had the urinary antigen test and those who had culture performed (data not shown). However, another reason for the decrease in mortality may be the changes in empirical antibiotic treatment of hospitalized patients with CAP.
The strengths of the current study include its prospective nature, the large cohort of consecutive hospitalized patients with community-acquired L. pneumophila pneumonia, and the comprehensive clinical data collection. In addition, the L. pneumophila pneumonia cases were sporadic, and no bias occurred due to epidemics during the study period. Nevertheless, several limitations should be acknowledged. The study was performed at a single institution, and so variances in the locations and specific patient populations should be taken into account. In addition, microbiologic tests for Legionella species were not performed in all hospitalized patients. Similarly, other tests used to identify L. pneumophila in patients with pneumonia, such as polymerase chain reaction, were not performed. Finally, the low number of patients with Legionella pneumonia who died in the present study limits our conclusions regarding this topic.
In conclusion, L. pneumophila is a relatively frequent causative pathogen among hospitalized patients with CAP and is associated with high morbidity. The annual number of L. pneumophila cases remained stable over the 15 years of the study. During the last years, significant changes have occurred in diagnosis and treatment, and the inhospital case-fatality rate of L. pneumophila pneumonia has decreased.
Footnotes
Abbreviations: AUC = area under curve, CAP = community-acquired pneumonia, CI = confidence interval, EWGLI = European Working Group for Legionella Infections, ICU = intensive care unit, IQR = interquartile range, IV = intravenous, OR = odds ratio, PSI = Pneumonia Severity Index, PCR = polymerase chain reaction, ROC = receiver operating characteristic, SD = standard deviation
Financial support: This study was supported by the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III and co-financed by the European Regional Development Fund “A way to achieve Europe” ERDF, Spanish Network for Research in Infectious Diseases (REIPI RD06/0008). Dr. Viasus is the recipient of a research grant from the Institut d’Investigació Biomèdica de Bellvitge (IDIBELL). Dr Garcia-Vidal is the recipient of a Juan de la Cierva research grant from the Instituto de Salud Carlos III, Madrid, Spain.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Benin AL, Benson RF, Besser RE. Trends in Legionnaires disease, 1980–1998: declining mortality and new patterns of diagnosis. Clin Infect Dis. 2002; 35: 1039–1046. [DOI] [PubMed] [Google Scholar]
- 2.Blazquez Garrido RM, Espinosa Parra FJ, Alemany Frances L, Ramos Guevara RM, Sanchez-Nieto JM, Segovia Hernandez M, Serrano Martinez JA, Huerta FH. Antimicrobial chemotherapy for Legionnaires disease: levofloxacin versus macrolides. Clin Infect Dis. 2005; 40: 800–806. [DOI] [PubMed] [Google Scholar]
- 3.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992; 101: 1644–1655. [DOI] [PubMed] [Google Scholar]
- 4.Carratala J, Garcia-Vidal C. An update on Legionella. Curr Opin Infect Dis. 2010; 23: 152–157. [DOI] [PubMed] [Google Scholar]
- 5.CillÆniz C, Ewig S, Polverino E, Marcos MA, Esquinas C, Gabarrus A, Mensa J, Torres A. Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax. 2011; 66: 340–346. [DOI] [PubMed] [Google Scholar]
- 6.Darby J, Buising K. Could it be Legionella? Aust Fam Physician. 2008; 37: 812–815. [PubMed] [Google Scholar]
- 7.den Boer JW, Yzerman EP, Jansen R, Bruin JP, Verhoef LP, Neve G, van der Zwaluw K. Legionnaires’ disease and gardening. Clin Microbiol Infect. 2007; 13: 88–91. [DOI] [PubMed] [Google Scholar]
- 8.Diaz A, Barria P, Niederman M, Restrepo MI, Dreyse J, Fuentes G, Couble B, Saldias F. Etiology of community-acquired pneumonia in hospitalized patients in Chile: the increasing prevalence of respiratory viruses among classic pathogens. Chest. 2007; 131: 779–787. [DOI] [PubMed] [Google Scholar]
- 9.Falco V, Molina I, Juste C, Crespo M, Almirante B, Pigrau C, Ferrer A, Bravo C, Palomar M, Pahissa A. [Treatment for Legionnaires’ disease. Macrolides or quinolones?] Enferm Infecc Microbiol Clin. 2006; 24: 360–364. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Sabe N, Roson B, Carratala J, Dorca J, Manresa F, Gudiol F. Clinical diagnosis of Legionella pneumonia revisited: evaluation of the Community-Based Pneumonia Incidence Study Group scoring system. Clin Infect Dis. 2003; 37: 483–489. [DOI] [PubMed] [Google Scholar]
- 11.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997; 336: 243–250. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Vidal C, Carratala J. Current clinical management of Legionnaires’ disease. Expert Rev Anti Infect Ther. 2006; 4: 995–1004. [DOI] [PubMed] [Google Scholar]
- 13.Gupta SK, Imperiale TF, Sarosi GA. Evaluation of the Winthrop-University Hospital criteria to identify Legionella pneumonia. Chest. 2001; 120: 1064–1071. [DOI] [PubMed] [Google Scholar]
- 14.Guyard C, Low DE. Legionella infections and travel associated legionellosis. Travel Med Infect Dis. 2011; 9: 176–186. [DOI] [PubMed] [Google Scholar]
- 15.Halm EA, Fine MJ, Marrie TJ, Coley CM, Kapoor WN, Obrosky DS, Singer DE. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998; 279: 1452–1457. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson KL, Miceli MH, Tarrand JJ, Kontoyiannis DP. Legionella pneumonia in cancer patients. Medicine (Baltimore). 2008; 87: 152–159. [DOI] [PubMed] [Google Scholar]
- 17.Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010; 50: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph CA, Ricketts KD; European Working Group for Legionella Infections. Legionnaires disease in Europe 2007–2008. Euro Surveill. 2010; 15: 19493.20197022 [Google Scholar]
- 19.Lim WS, Macfarlane JT, Boswell TC, Harrison TG, Rose D, Leinonen M, Saikku P. Study of Community Acquired Pneumonia Aetiology (SCAPA) in adults admitted to hospital: implications for management guidelines. Thorax. 2001; 56: 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG. Infectious Disease Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007; 44 (Suppl 2): S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulazimoglu L, Yu VL. Can Legionnaires disease be diagnosed by clinical criteria? A critical review. Chest. 2001; 120: 1049–1053. [DOI] [PubMed] [Google Scholar]
- 22.Mykietiuk A, Carratala J, Fernandez-Sabe N, Dorca J, Verdaguer R, Manresa F, Gudiol F. Clinical outcomes for hospitalized patients with Legionella pneumonia in the antigenuria era: the influence of levofloxacin therapy. Clin Infect Dis. 2005; 40: 794–799. [DOI] [PubMed] [Google Scholar]
- 23.Neil K, Berkelman R. Increasing incidence of legionellosis in the United States, 1990-2005: changing epidemiologic trends. Clin Infect Dis. 2008; 47: 591–599. [DOI] [PubMed] [Google Scholar]
- 24.Newton HJ, Ang DK, van Driel IR, Hartland EL. Molecular pathogenesis of infections caused by Legionella pneumophila. Clin Microbiol Rev. 2010; 23: 274–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng V, Tang P, Fisman DN. Our evolving understanding of legionellosis epidemiology: learning to count. Clin Infect Dis. 2008; 47: 600–602. [DOI] [PubMed] [Google Scholar]
- 26.Ng V, Tang P, Jamieson F, Guyard C, Low DE, Fisman DN. Laboratory-based evaluation of legionellosis epidemiology in Ontario, Canada, 1978 to 2006. BMC Infect Dis. 2009; 9: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngeow YF, Suwanjutha S, Chantarojanasriri T, Wang F, Saniel M, Alejandria M, Hsueh PR, Ping-Ing L, Park SC, Sohn JW, Aziah AM, Liu Y, Seto WH, Ngan CC, Hadiarto M, Hood A, Cheong YM. An Asian study on the prevalence of atypical respiratory pathogens in community-acquired pneumonia. Int J Infect Dis. 2005; 9: 144–153. [DOI] [PubMed] [Google Scholar]
- 28.Pedro-Botet ML, Garcia-Cruz A, Tural C, Mateu L, Sopena N, Roure S, Rey-Joly C, Sabria M. Severe Legionnaires’ disease successfully treated with levofloxacin and azithromycin. J Chemother. 2006; 18: 559–561. [DOI] [PubMed] [Google Scholar]
- 29.Pedro-Botet L, Yu VL. Legionella: macrolides or quinolones? Clin Microbiol Infect. 2006; 12 (Suppl 3): 25–30. [DOI] [PubMed] [Google Scholar]
- 30.Plouffe JF, Breiman RF, Fields BS, Herbert M, Inverso J, Knirsch C, Kolokathis A, Marrie TJ, Nicolle L, Schwartz DB. Azithromycin in the treatment of Legionella pneumonia requiring hospitalization. Clin Infect Dis. 2003; 37: 1475–1480. [DOI] [PubMed] [Google Scholar]
- 31.Rello J, Bodi M, Mariscal D, Navarro M, Diaz E, Gallego M, Valles J. Microbiological testing and outcome of patients with severe community-acquired pneumonia. Chest. 2003; 123: 174–180. [DOI] [PubMed] [Google Scholar]
- 32.Roson B, Carratala J, Fernandez-Sabe N, Tubau F, Manresa F, Gudiol F. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med. 2004; 164: 502–508. [DOI] [PubMed] [Google Scholar]
- 33.Sabria M, Pedro-Botet ML, Gomez J, Roig J, Vilaseca B, Sopena N, Banos V; Legionnaires Disease Therapy Group. Fluoroquinolones vs macrolides in the treatment of Legionnaires disease. Chest. 2005; 128: 1401–1405. [DOI] [PubMed] [Google Scholar]
- 34.Singanayagam A, Chalmers JD, Hill AT. Severity assessment in community-acquired pneumonia: a review. QJM. 2009; 102: 379–388. [DOI] [PubMed] [Google Scholar]
- 35.Sohn JW, Park SC, Choi YH, Woo HJ, Cho YK, Lee JS, Sim HS, Kim MJ. Atypical pathogens as etiologic agents in hospitalized patients with community-acquired pneumonia in Korea: a prospective multi-center study. J Korean Med Sci. 2006; 21: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sopena N, Sabria M, Pedro-Botet ML, Manterola JM, Matas L, Dominguez J, Modol JM, Tudela P, Ausina V, Foz M. Prospective study of community-acquired pneumonia of bacterial etiology in adults. Eur J Clin Microbiol Infect Dis. 1999; 18: 852–858. [DOI] [PubMed] [Google Scholar]
- 37.Vergis EN, Akbas E, Yu VL. Legionella as a cause of severe pneumonia. Semin Respir Crit Care Med. 2000; 21: 295–304. [DOI] [PubMed] [Google Scholar]
- 38.Vergis EN, Indorf A, File TM, Jr, Phillips J, Bates J, Tan J, Sarosi GA, Grayston JT, Summersgill J, Yu VL. Azithromycin vs cefuroxime plus erythromycin for empirical treatment of community-acquired pneumonia in hospitalized patients: a prospective, randomized, multicenter trial. Arch Intern Med. 2000; 160: 1294–1300. [DOI] [PubMed] [Google Scholar]
- 39.von Baum H, Ewig S, Marre R, Suttorp N, Gonschior S, Welte T, Luck C; Competence Network for Community Acquired Pneumonia Study Group. Community-acquired Legionella pneumonia: new insights from the German Competence Network for Community-Acquired Pneumonia. Clin Infect Dis. 2008; 46: 1356–1364. [DOI] [PubMed] [Google Scholar]
- 40.Whiley H, Bentham R. Legionella longbeachae and legionellosis. Emerg Infect Dis. 2011; 17: 579–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu VL, Stout JE. Community-acquired legionnaires disease: implications for underdiagnosis and laboratory testing. Clin Infect Dis. 2008; 46: 1365–1367. [DOI] [PubMed] [Google Scholar]


