Abstract
Rationale:
Although immunomodulatory therapy has been clearly stated as an important landmark in treatment of ulcerative colitis, significantly improving the quality of life for patients with inflammatory bowel disease, there are several aspects to be considered regarding the possible side-effects of anti-TNF alpha agents. In spite of a good safety profile, biologic TNF antagonists may induce paradoxical inflammation, which can manifest as sarcoid-like granulomatosis, consisting of noncaseating granulomas in the affected organs.
Patient concerns:
We report the case of a 30-year-old male patient, with no personal or familial history of lung disease, with a personal history of ulcerative colitis (UC), under clinical remission following infliximab therapy in maintenance dose, who was admitted for treatment administration, but also for dyspnea, nocturnal sweating, and nonproductive cough.
Diagnoses:
Based on clinical manifestations, biological landmarks excluding various infections, CT scan, fibrobronchoscopy with bronchoalveolar lavage for culture and immunohistochemical examination, followed by mediastinoscopy with sampling of paratracheal lymph node, which underwent histopathological examination, the patient was diagnosed with drug- induced stage II pulmonary sarcoidosis.
Interventions:
Since the patient had developed severe allergic reaction after being administered Infliximab at admission, the biological treatment was immediately discontinued. Following the diagnosis of pulmonary sarcoidosis, corticotherapy was initiated.
Patient outcomes:
After corticotherapy was initiated, the patient had a favorable outcome at 3 months reevaluation, both regarding the course of ulcerative colitis and sarcoidosis.
Lessons:
Patients under biological therapy using anti-TNF alpha agents must be carefully monitored, in order to early identify potential paradoxical inflammation (such as sarcoidosis) as a side-effect. The drug-related pulmonary disease tends to improve upon withdrawal of the drug, with occasional requirement of steroid treatment. However, a thorough strategy should be assembled in the case of UC relapse in this patient category, with switching to adalimumab or surgical approach as main possibilities.
Keywords: infliximab, sarcoidosis, ulcerative colitis
1. Introduction
Anti-TNF therapy has significantly improved the quality of life for patients with inflammatory bowel disease (IBD), changing the algorithm for treating this pathology; however, certain aspects must be taken into consideration regarding this class of therapeutic agents. There are several side-effects to be considered when monitoring patients with IBD under biological therapy, including various infections and inflammatory responses, such as sarcoidosis, with several cases reported to have developed sarcoidosis during biological anti-TNF therapy. Sarcoidosis represents a systemic chronic granulomatous disease of unknown etiology, predominantly involving the lungs, but also being able to target other organs or tissues in the body, such as the lymphatic system, liver, and skin. The characteristic histopathological findings are noncaseating granulomas in the affected organs. The management of sarcoidosis in this context is currently under debate, although it is clearly stated that anti-TNF agents represent essential tools in treating refractory sarcoidosis.
2. Case report
A 30-year-old man with a 5-year history of ulcerative colitis (UC) with pancolic extension (Fig. 1) in clinical remission was admitted for treatment administration (infliximab- maintenance dose 5 mg/kgc (500 mg) at 8 weeks) (IFX), but also evaluation for dyspnea, nocturnal sweating, and nonproductive cough.
Figure 1.
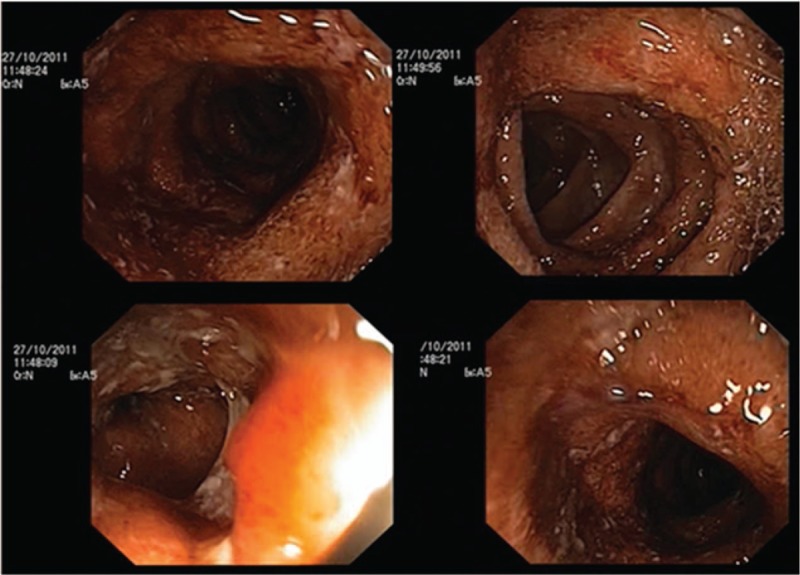
Colonoscopic aspects of the colon at UC diagnosis. UC = ulcerative colitis.
At admission, the patient was having 2 to 3 bowel movements per day, without rectal bleeding or other digestive-related symptoms. The family history for inflammatory bowel diseases was negative, and the patient was a nonsmoker, without personal history of respiratory disease. Medication history for UC included 5-ASA derivatives, azathioprine, corticotherapy, all without inducing clinical remission, consequently requiring biological therapy; IFX was introduced 3 years and 8 months prior to the current admission, being well-tolerated and inducing clinical remission.
The physical examination revealed no changes at cardiac and pulmonary auscultation, absence of fever, a mildly tender abdomen to palpation in the left lower quadrant and hepatomegaly grade 1. Biological findings included: normal hematological panel, presence of inflammatory syndrome (fibrinogenemia = 419 mg/dL, C-reactive protein level = 2.41 mg/dL), minimal hepatic cytolysis syndrome (ALT = 45 IU/L, AST = 40 IU/L), elevated LDH level (282 U/L), minimally increased proteinemia (8.39 g/dL), but a normal proteinogram, moderate isolated increase of gamma-glutamyl transpeptidase level (105 IU/L). Liver and renal function test results were normal, as well as angiotensin convertase and β2 microglobulin levels. Clostridium difficile toxins A and B were absent, as well as the markers for HIV, HBV, and HCV infection. Rheumatoid factor, antinuclear antibodies, and proteinase-3 anti-neutrophil antibody test results were within normal ranges. Colonoscopic evaluation showed the absence of rectal ulcerations, blunting of haustra until the splenic flexure, and the presence of multiple inflammatory pseudopolyps, without other changes (Fig. 2).
Figure 2.
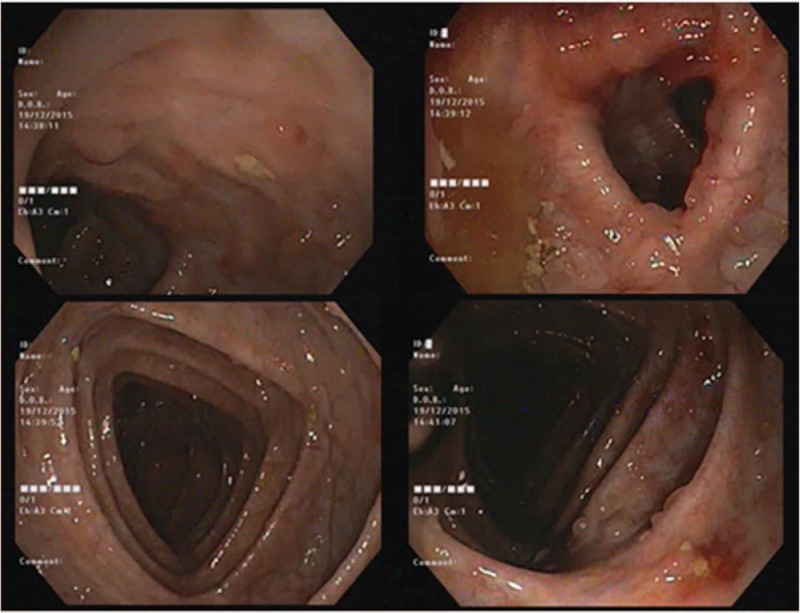
Colonoscopic aspect of the colon at current admission.
Consequently to IFX administration, the patient developed a severe allergic reaction, consisting of skin rash and bronchospasm, which required treatment discontinuation. Considering the adverse effects of IFX therapy, the medication history and the persistence of biological and endoscopic disease activity signs, a switch to adalimumab (ADA) was recommended. Given the respiratory symptoms at presentation and the decision to switch therapy to ADA, pulmonary imaging investigations were required. The chest radiograph highlighted the presence of multiple bilateral micronodules disseminated over the whole pulmonary area and an enlarge mediastinum (Fig. 3).
Figure 3.
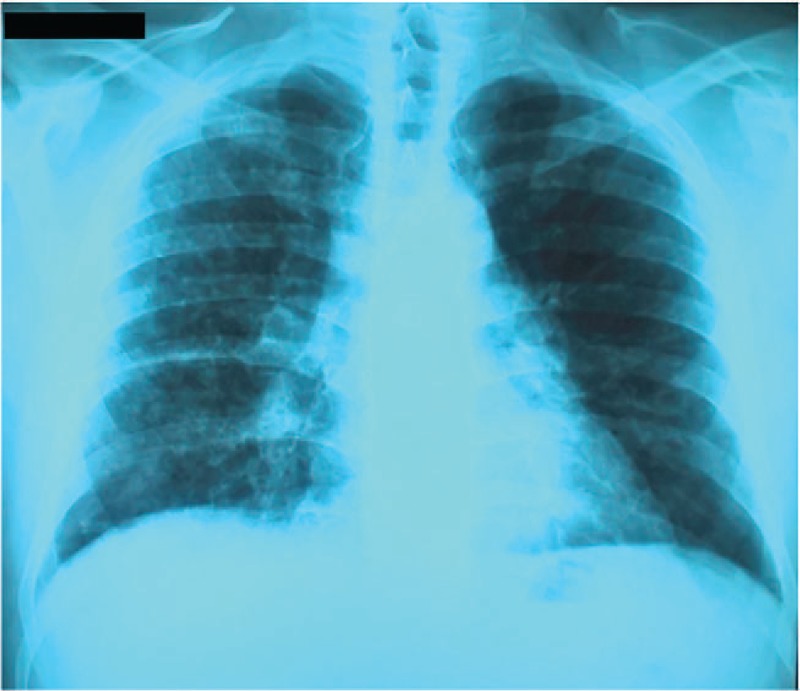
Posteroanterior view chest radiograph showing multiple bilateral micronodules disseminated over the whole pulmonary area and an enlarged mediastinum.
In addition, thoracoabdominal computed tomography was performed, raising the suspicion of mediastinopulmonary sarcoidosis, as multiple pulmonary bilateral disseminated micronodules (1–3 mm) with a tendency to confluence, large, bilateral, symmetrical mediastinal lymphadenopathies, including all mediastinum lymphnode groups (diameters ranging from 15 to 30 mm) and also small retroperitoneal adenopathies (approximately 10 mm diameter), and increased diameters of the liver were described (Fig. 4).
Figure 4.
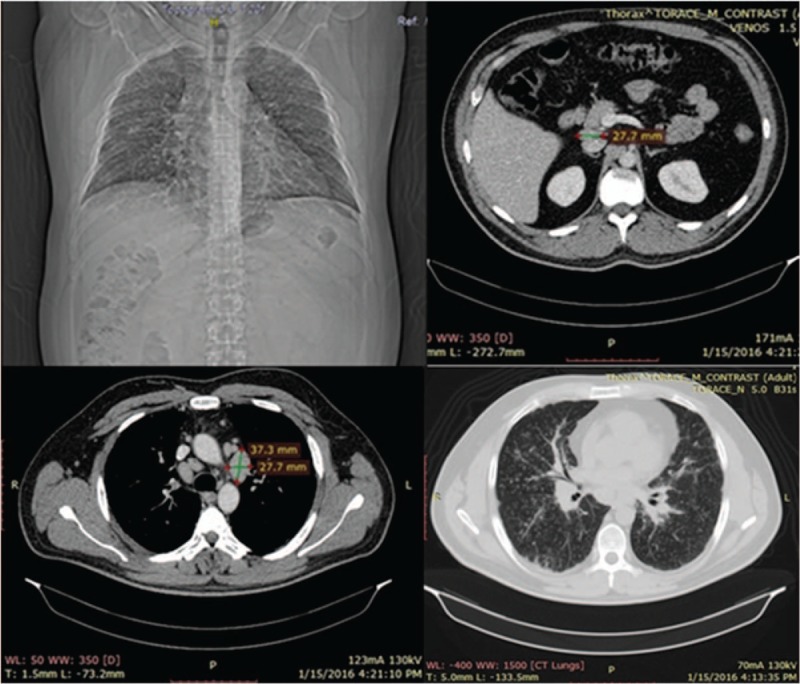
Thoraco-abdominal CT: multiple pulmonary bilateral disseminated micronodules, large, bilateral, symmetrical mediastinal lymphadenopathies retroperitoneal lymphadenopathies. CT = computed tomography.
CMV and EBV serology were evaluated, and QuantiFERON-Test (TB Gold) and fibrobronchoscopy with bronchoalveolar lavage (BAL) were performed. During the BAL, cytological samples for both culture (on common media and Lowenstein-Jensen medium) and immunohistochemical examination were obtained. Serology for EBV and CMV infections was evaluated, with positive Ig G against both EBV (VCA IgG) and CMV, but negative IgM against CMV and EBV. The QuantiFERON test result was negative, cultures on common media and on Lowenstein–Jensen medium were negative, but the flow cytometry immune phenotyping from the BAL sample did not correspond to the criteria for sarcoidosis diagnosis (%Ly>12% and CD4+/CD8+>3.5), showing %Ly = 5.7 and CD4+/CD8+ = 2.4.
CA125 level was normal (9.83 IU/mL), whereas CA15–3 was elevated (82.8 IU/mL), supporting the hypothesis of pulmonary damage in the interstitium, with fibroblast activity and progression to fibrosis. Additionally, the mediastinoscopy with sampling of a paratracheal lymph node was performed. Histopathological examination highlighted the presence of multiple giant epithelioid granulomas confined by fibrous elements, corresponding to sarcoid granulomas (Fig. 5).
Figure 5.
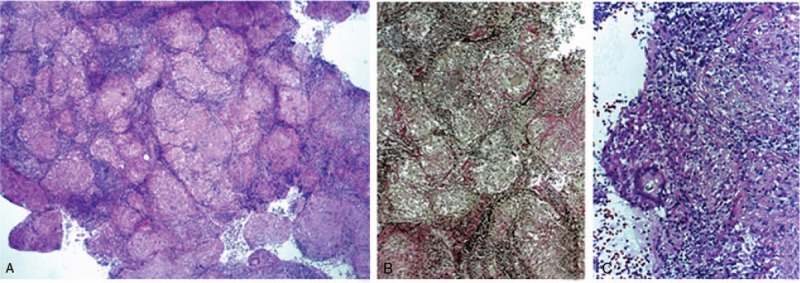
Histopathological exam of paratracheal lymph node: (A and B) multiple giant epithelioid granulomas confined by fibrous elements; (C) multinucleate giant cell, with Schaumann body.
Following the computed tomography (CT) scan results, the pneumological evaluation classified the disease as stage II pulmonary sarcoidosis and recommended initiation of corticotherapy (prednisone, 30 mg/day).
After a 3-month course of steroid treatment, with improvement of dyspnea and cough, but persistence of nocturnal sweating, a second CT scan was performed, showing improvement of the pulmonary and mediastinal changes, with persistence of abdominal adenopathies and hepatomegaly (Fig. 6). The pneumological reevaluation recommended progressive decline of corticotherapy dose and further gastroenterological monitoring for UC. Considering the clinical and biological remission of the UC, the patient is currently under 16 mg prednisone/day, in progressive decline of corticotherapy dose.
Figure 6.
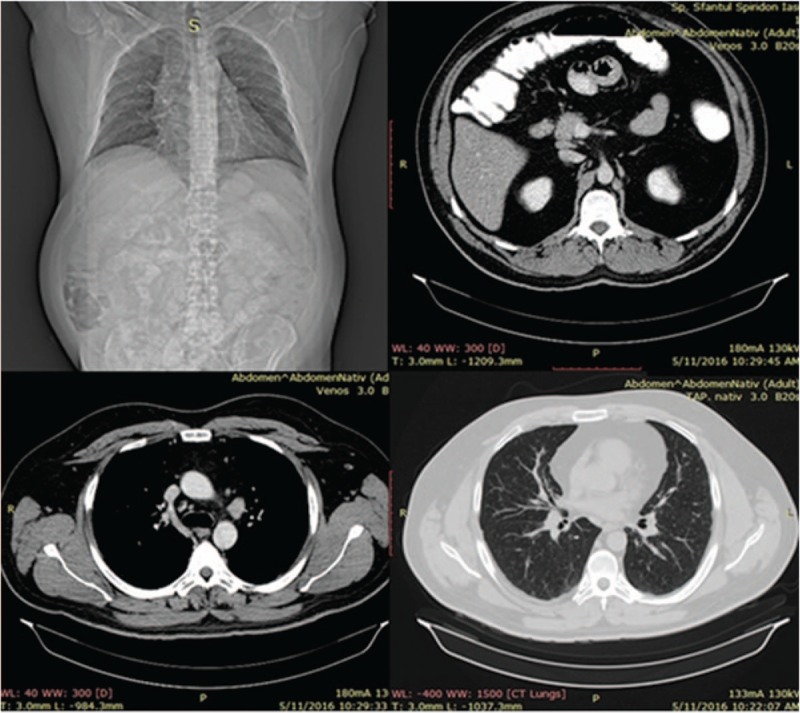
Thoracoabdominal CT after 3 months of corticotherapy. CT = computed tomography.
3. Discussion
Infliximab represents a chimeric recombinant TNF-α IgG monoclonal antibody, which can induce inflammatory cell lysis, through activating complement and antibody-mediated cytotoxicity and promoting T-cell response, consequently inhibiting the inflammatory cascade.[1]
Anti-TNFα agents are a valuable therapeutic tool for various inflammatory diseases, including UC, although there are several risks associated with biological therapies, such as various infections, hepatosplenic T-cell lymphoma, or other neoplasia.
However, there are several other unknown or underreported side effects, such as abnormal immunologic responses. In spite of a good safety profile, TNF antagonists may induce paradoxical inflammation, which can be considered a class-effect, rather than a drug-specific reaction, being reported not only for IFX therapy, but also for ADA and etanercept; this type of unexpected response can appear in the form of leucocytoclastic vasculitis, psoriasis, or sarcoidosis. This represent previously underrecognized phenomena during immunomodulatory treatment, initiated for inflammatory arthritis or inflammatory bowel diseases, increasingly reported as side-effects of this therapy. Among the autoimmune pathologies possibly triggered by the anti-TNFα agents used for ulcerative colitis treatment have been reported cases of autoimmune hepatitis, Lewis–Sumner syndrome, Takayasu arteritis, Guillian–Barre syndrome.[2,3]
Sarcoidosis and other paradoxical inflammatory responses may represent emerging complications of anti-TNF agents. Paradoxical inflammatory responses can be considered a possible class-effect of anti-TNFα therapy, since they have been reported not only for IFX, but also for ADA and etanercept.[4–6] Most of the reported cases in literature correspond to emergence of sarcoidosis after anti-TNF agents targeting rheumatological pathologies.[7]
In the reported case, it was particularly important to define the pulmonary disease developed during biological therapy, since the symptoms were highly nonspecific, and there were several other entities which could have had a major impact on patient prognosis, such as a T-cell lymphoma, but also tuberculosis. In patients under biological therapy, who develop respiratory symptoms, it is important to exclude tuberculosis or other infections (such as CMV and EBV infections), since their emergence or reactivation during anti-TNFα therapy are clearly stated.[8]
This is particularly important for IFX, which appears to be responsible for higher risk of developing infections than other anti-TNFα agents.[9] Since the serology was negative for EBV, CMV infection, and there is a strong association between biological therapy and Mycobacterium tuberculosis reactivation/ infection, several tests were performed in order to exclude tuberculosis.
Taking into account the patient's characteristics (male of young age) and clinical landmarks (hepatomegaly, fever, and night sweats), an important differential diagnosis for the presented case was the hepatosplenic T-cell lymphoma (HSTCL). Although a rare entity, it is important to be recalled, since it represents an aggressive extranodal form of non-Hodgkin's lymphoma, with a poor prognosis and its association with a history of immunosuppression (AZA) and biological therapy (IFX) is clearly stated in literature.[10] However, HSTCL was excluded as a possible diagnosis in the presented case, since the hematological parameters of the patient were within normal ranges, there were no splenic infiltrates, but several adenopathies identified on the CT scan; moreover, the later histopathological examination identified features compatible with other entities.
In order to identify the associated pulmonary pathology, after excluding a possible infection, the hypothesis of pulmonary damage in the interstitium was also taken into account by evaluating the CA 15–3 level (which was elevated) and performing a paratracheal lymph node sampling followed by histopathological examination, which together with suggestive CT images lead to the diagnosis of sarcoidosis. Drug-related pulmonary disease in UC is poorly reported, since the pulmonary manifestations in IBD remain nonspecific and tend to improve upon the withdrawal of the drug, with occasional requirement of steroid treatment.[11]
According to the literature, this medical condition improves with cessation of IFX and/or corticoid treatment,[12] strategy which was also applied for the presented case, with a favorable outcome. An important issue to be further considered is the evolution of UC after cessation of IFX, considering that the patient is currently in clinical remission, but a mild inflammatory status is present. According to literature, patients who discontinued IFX while being in clinical remission, but with elevated CRP level had higher risk of relapse, probably indicating an underlying asymptomatic inflammation and the lack of deep remission in this subgroup.[13] Thus, a thorough strategy should be assembled in the case of relapse; a possible therapeutic choice might be switching to ADA, since the other therapeutical options included in the step-up strategy were applied, without ability to induce and maintain remission of UC. However, a back-up option to be considered is surgery, but this alternative must be weighed up, taking into account patient's quality of life and- not least-consent for this invasive approach.
4. Conclusions
The strategies for managing IBD in the young are still under debate, with possibility to apply either the step-up, or the top-down strategy. However, regularly monitoring patients is important, no matter the therapeutic approach, but thorough monitoring is particularly important for patients undergoing biological therapy, considering the risk for developing infections or paradoxical inflammatory responses. Moreover, optimizing the further therapeutic strategy in patients with IBD under biological therapy, with clinical remission is based on individual characteristics. It is certain that UC patients require continuous care throughout lifetime, having as main goals preserving the quality of life, maintaining remission, and preventing complications.
Footnotes
Abbreviations: 5-ASA = 5-aminosalicylates, ADA = adalimumab, ALT = alanine aminotransferase, Anti-TNFα = antitumor necrosis factor alpha, AST = aspartate aminotransferase, BAL = bronchoalveolar lavage, CMV = cytomegalovirus, CRP = C-reactive protein, CT = computed tomography, EBV = Epstein–Barr virus, HBV = hepatitis B virus, HCV = hepatitis C virus, HIV = human immunodeficiency virus, HSTCL = hepatosplenic T-cell lymphoma, IBD = inflammatory bowel disease, IFX = infliximab, IgG = immunoglobulin G, Ly = lymphocytes, UC = ulcerative colitis.
Declaration: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-chief of this journal.
Disclosure: This case report has not been published and is not under consideration for publication elsewhere.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Iskandar H, Dhere T, Farraye F. Ulcerative colitis: update on medical management. Curr Gastroenterol Rep 2015;17:44. [DOI] [PubMed] [Google Scholar]
- [2].Alvarez-Lario B, Prieto-Tejedo R, Colazo-Burlato M, et al. Severe Guillain–Barré syndrome in a patient receiving anti-TNF therapy. Consequence or coincidence. A case-based review. Clin Rheumatol 2013;32:1407–12. [DOI] [PubMed] [Google Scholar]
- [3].Parra RS, Ribeiro Feitosa M, Foresto Machado Vanessa, et al. Infliximab-associated fulminant hepatic failure in ulcerative colitis: a case report. J Med Case Rep 2015;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kanellopoulou T, Filiotou Anna, Kranidioti H, et al. Sarcoid-like granulomatosis in patients treated with anti-TNFα factors. A case report and review of the literature. Clin Rheumatol 2011;30:581–3. [DOI] [PubMed] [Google Scholar]
- [5].Kudrin A, Chilvers ER, Ginawi A, et al. Sarcoid-like granulomatous disease following etanercept treatment for RA. J Rheumatol 2007;34:648–9. [PubMed] [Google Scholar]
- [6].Farah RE, Shay MD. Pulmonary sarcoidosis associated with etanercept therapy. Pharmacother 2007;27:1446–8. [DOI] [PubMed] [Google Scholar]
- [7].Ishiguro T, Takayanagi N, Kurashima K, et al. Development of sarcoidosis during etanercept therapy. Intern Med 2008;47:1021–5. [DOI] [PubMed] [Google Scholar]
- [8].Long R, Gardam M. Tumor necrosis factor-alpha inhibitors and the reactivation of latent tuberculosis infection. CMAJ 2003;168:1153–6. [PMC free article] [PubMed] [Google Scholar]
- [9].Murdaca G, Spanò F, Contatore M, et al. Infection risk associated with anti-TNF-α agents: a review. Expert Opin Drug Saf 2015;14:571–82. [DOI] [PubMed] [Google Scholar]
- [10].Thai Anne, Prindiville T. Hepatosplenic T-cell lymphoma and inflammatory bowel disease. J Crohn Colitis 2010;4:511–22. [DOI] [PubMed] [Google Scholar]
- [11].Orchard T. Extraintestinal complications of inflammatory bowel disease. Curr Gastroenterol Rep 2003;5:512–7. [DOI] [PubMed] [Google Scholar]
- [12].Cleynen I, Vermeire S. Paradoxical inflammation induced by anti-TNF agents in patients with IBD. Nat Rev Gastroenterol Hepatol 2012;9:496–503. [DOI] [PubMed] [Google Scholar]
- [13].Shomron B-H, Ren M, Minhu C. Optimizing biologic treatment in IBD: objective measures, but when, how and how often? BMC Gastroenterol 2015;15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]


