Abstract
Patients with locally advanced cervical cancer (LACC) are at risk of para-aortic lymph node (PALN) metastasis. Pelvic concurrent chemoradiotherapy, the current standard treatment for LACC, has a PALN failure rate of 9% according to the Radiation Therapy Oncology Group Trial 90–01, suggesting that it may not completely eliminate all microscopic tumors in the PALNs. To minimize the toxicities associated with conventional prophylactic extended-field radiotherapy, our institute use prophylactic semiextended field radiotherapy that includes only the PALNs below the level of the renal vessels. Use of intensity-modulated radiotherapy (IMRT) is another means of reducing the incidence of toxicity. This study evaluated the safety and efficacy of prophylactic semiextended field IMRT (SEF-IMRT) and concurrent cisplatin chemotherapy in patients with LACC.
We retrospectively assessed survival and toxicity in 76 patients with stage IB2–IVA cervical cancer and negative PALNs who received prophylactic SEF-IMRT and concurrent weekly cisplatin (40 mg/m2) between 2004 and 2013. The region targeted by SEF-IMRT included the PALNs below the level of the renal vessels, and the prescribed dose was 50.4 Gy in 28 fractions. Brachytherapy was administered at a dose of 30 Gy in 6 fractions. Survival outcomes were calculated by using the Kaplan–Meier method, and acute and late toxicities were scored according to the Common Terminology Criteria for Adverse Events, version 3.0.
All patients completed the planned SEF-IMRT, as well as brachytherapy. Acute grade ≥3 gastrointestinal, genitourinary, and hematologic toxicities were observed in 2, 0, and 41 patients, respectively. The median follow-up time after SEF-IMRT was 55 (range, 11–124) months. Eight patients developed out-field distant recurrences without PALN failure, and 1 patient experienced out-field PALN failure with simultaneous distant metastasis. No patients had late genitourinary toxicities, and 3 patients had late grade 3 gastrointestinal toxicities. The 5-year overall survival, disease-free survival, local failure-free survival, regional failure-free survival, PALN failure-free survival, and distant metastasis-free survival rates were 85.0%, 84.4%, 96.0%, 97.3%, 98.6%, and 88.4%, respectively.
For patients with LACC, prophylactic PALN irradiation up to the level of the renal vessels reduced PALN recurrence and resulted in favorable outcomes with few severe toxicities.
Keywords: cervical cancer, extended field radiotherapy, intensity-modulated radiation therapy
1. Introduction
Locally advanced cervical cancer is commonly treated via definitive chemoradiotherapy (CCRT) consisting of pelvic radiotherapy and cisplatin. The incidence of para-aortic lymph node (PALN) metastasis in cervical cancer is 10% to 25%,[1–3] and the pattern of lymphatic spread in cervical cancer—from the low pelvis to the high pelvic lymph nodes (PLNs) and the PALNs—appears orderly.[4,5] In the Radiation Therapy Oncology Group (RTOG) trial 90–01, pelvic CCRT had better 8-year overall survival (OS) and disease-free survival (DFS) rates than did extended field radiotherapy (EFRT) alone.[6] However, the 8-year PALN failure rate was 9% in the pelvic CCRT arm compared with only 4% in the EFRT arm. This finding suggests that pelvic CCRT does not completely eradicate all microscopic tumors in the PALNs, particularly in patients with risk factors. Risk factors for PALN failure after pelvic radiotherapy include International Federation of Gynecology and Obstetrics (FIGO) stage III–IVA, positive PLNs, and a high squamous cell carcinoma antigen (SCC-Ag) level.[7–9]
The RTOG 79–20 trial demonstrated that prophylactic EFRT improved survival and decreased the number of distant metastases compared with pelvic radiotherapy.[10] However, there were more late major gastrointestinal complications in the EFRT arm (8%) than in the pelvic radiotherapy arm (4%), which approached statistical significance (P = 0.06). In the study by Haie et al,[11] severe gastrointestinal complications were significantly more frequent in patients receiving EFRT than in those receiving pelvic radiotherapy, and EFRT did not reduce the number of overall distant metastases or improve local control or survival with no evidence of disease. In addition, the patients in trials in which EFRT was delivered via a conventional technique often experienced severe complications including gastrointestinal toxicities.[10–17] Therefore, the use of prophylactic EFRT in patients without evidence of PALN involvement appears controversial.
To reduce toxicity, our institutions used semiextended field radiotherapy (SEFRT) that excluded the upper parts of the PALN chain in patients with LACC. Intensity-modulated radiotherapy (IMRT) is another means of decreasing toxicity. The aim of this study was to review our results assessing acute and late toxicity and disease outcomes in patients with LACC treated via SEFRT using the IMRT technique (SEF-IMRT).
2. Methods and materials
2.1. Patient characteristics
This retrospective study was approved by our institutional review board. Patients with biopsy-confirmed FIGO stage IB–IVA cervical cancers treated via definitive CCRT with a curative aim at our institute between October 2004 and May 2014 were retrospectively reviewed. Patients were included in the study if they had received SEF-IMRT and regular follow-ups, were not pregnant at the time of diagnosis, and had complete medical records. The exclusion criteria were as follows: (a) pathologically proven small cell carcinoma because it has a more aggressive clinical course than cervical squamous cell carcinoma or adenocarcinoma; (b) history of previous malignancy; (c) Eastern Cooperative Oncology Group score ≥2; (d) evidence of PALN metastasis; and (e) previous surgery, chemotherapy, or radiotherapy. The pretreatment workup included comprehensive documentation of the patient's medical history, a gynecologic pelvic examination, cystoscopy, proctoscopy, chest x-ray or computed tomography (CT), abdominopelvic CT, a complete blood cell count, and blood chemistry profiles. Magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) were not routinely used in the workup. Clinical stage was determined by consensus of the gynecologic oncologist, radiologist, and radiation oncologist in a multidisciplinary conference. The lymph nodes were classified as metastatic based on the radiographic findings (>1.0 cm in the short axis dimension) at the time of diagnosis or the radiologist's and oncologist's interpretation of the appearance of the nodes. None of the patients had their PALNs assessed surgically.
2.2. Radiotherapy
All patients underwent CT simulation in the supine position and were immobilized by using alpha cradles. Radiopaque markers were placed on the gross cervical cancer during a gynecologic examination before CT simulation. Planning CT images with a maximum slice thickness of 3 mm were acquired throughout the entire abdomen and pelvis. The gross tumor volume consisted of the primary tumor and regional lymph nodes, as defined by the locations of the radiopaque markers on the primary tumor and CT scans. The clinical target volume (CTV) included the gross disease, cervix, parametrium, uterus, superior third to half of the vagina, presacral region, regional lymph nodes (common, internal, and external iliac lymph nodes), and para-aortic nodes to the level of the renal vessels. A planning target volume (PTV) with a uniform 0.5- to 0.7-cm margin was added to the CTV.
All patients received IMRT consisting of 6 to 9 coplanar fields using 6- or 10-MV photons (Eclipse Treatment Planning System; Varian Medical Systems Inc., Palo Alto, CA). We prescribed a radiation dose of 50.4 Gy in 28 fractions for SEF-IMRT. The target planning constraints were as follows: (a) >95% of the PTV receives >95% of the prescription dose, (b) <1% of the PTV receives <93% of the prescription dose, (c) <10% of the PTV receives >110% of the prescription dose, and (d) the maximum dose to the PTV is <115% of the prescription dose. The normal tissue planning constraints were as follows: (a) <50% of the volume of the rectum receives >45 Gy, (b) <50% of the volume of the bladder receives >45 Gy, (c) <40% of the volume of the non-rectal bowel receives >30 Gy, (d) the spinal cord receives a maximum dose <45 Gy, and (e) the kidney receives a mean dose <16 Gy. No special constraints were applied to the adjacent bone or bone marrow. Following SEF-IMRT, the dose to the involved pelvic lymph nodes was boosted to 59.4 Gy via the IMRT technique.
After adequate tumor regression, high-dose-rate intracavitary brachytherapy was performed using an iridium-192 remote after-loading technique, either concurrently with SEF-IMRT once per week or twice per week followed by an SEF-IMRT course. The standard prescribed dose for each brachytherapy was 5.0 Gy to point A for 6 sessions. We considered reducing the number of fractions per session (n = 5) in patients with grade ≥3 gastrointestinal or genitourinary toxicities or who were >70 years. The total prescribed point A doses (external beam radiotherapy + brachytherapy) of a radiobiological equivalent dose in 2-Gy fractions ranged from 80.8 to 87.1 Gy.
2.3. Chemotherapy
Chemotherapy consisted of weekly cisplatin (40 mg/m2; maximum, 70 mg) delivered concurrently with external beam radiotherapy. Chemotherapy was administered as a half dose if the white blood cell count was < 2.0×109/L, the absolute neutrophil count (ANC) was between 1.0 × 109 and 1.5 × 109/L, or the platelet count was between 5.0 × 107 and 7.5 × 107/L. Chemotherapy was omitted if the ANC was <1.0 × 109/L, the platelet count was <5.0 × 107/L, or creatinine clearance was <50 mL/min. None of the patients underwent platelet transfusions or granulocyte/monocyte colony-stimulating factor treatments.
2.4. Follow-up
During CCRT, the patients received weekly clinical assessments, pelvic examinations, complete blood counts, and renal and liver function tests. After completion of CCRT, a radiation oncologist and a gynecologic oncologist evaluated the patients every 3 months for the first 2 years, and every 6 months thereafter. The 3-month evaluations consisted of a physical and pelvic examination, a Pap smear, and a tumor marker (SCC-Ag) measurement. For the first 2 years, the 6-month evaluations consisted of a blood count and renal and liver function tests. A radiographic examination was carried out every 3 to 6 months. Routine urine and stool examinations were performed every 6 to 12 months to assess the possibility of late complications. Toxicity was assessed at the time of each evaluation according to the Common Terminology Criteria for Adverse Events, version 3.0.
2.5. Statistical analysis
All statistical analyses were conducted by using Statistical Package for the Social Sciences for Windows software, version 21.0 (IBM, Armonk, NY). Patterns of failure, OS, and DFS were evaluated. The sites of failure were recorded as local, regional, PALN, or distant. In-field failure in SEF-IMRT was defined as disease in the pelvic area and the PALNs within the treatment field, and out-field failure was defined as disease outside the treatment field, especially in the PALNs located above the level of the renal vessels. Survival was defined from the date of SEF-IMRT initiation to the date of the last follow-up or death. Time to recurrence was measured from the date of SEF-IMRT initiation to the date of the first failure. Survival data were analyzed by using the Kaplan–Meier method. A univariate analysis was performed using the log-rank test to identify parameters associated with treatment outcome, and a multivariate analysis was performed using a Cox regression model. A P value ≤0.05 was considered statistically significant.
3. Results
3.1. Patient compliance and treatment-related toxicities
A total of 90 patients fulfilled our inclusion criteria. After exclusion of patients with a pathologically proven small cell carcinoma (n = 5), a previous history of malignancy (n = 5), or an Eastern Cooperative Oncology Group score ≥2 (n = 4), 76 remained eligible for analysis. Patient and tumor characteristics are summarized in Table 1. All patients completed the planned SEF-IMRT and brachytherapy. The acute and late toxicities in the patients are summarized in Table 2. Acute gastrointestinal and genitourinary toxicities of grade ≥3 were observed in 2 and 0 patients, respectively. Forty-one (53.9%) patients developed grade ≥3 hematologic toxicities (n = 31, leukopenia; n = 5, thrombocytopenia; n = 5, anemia).
Table 1.
Characteristics of patients and tumors.
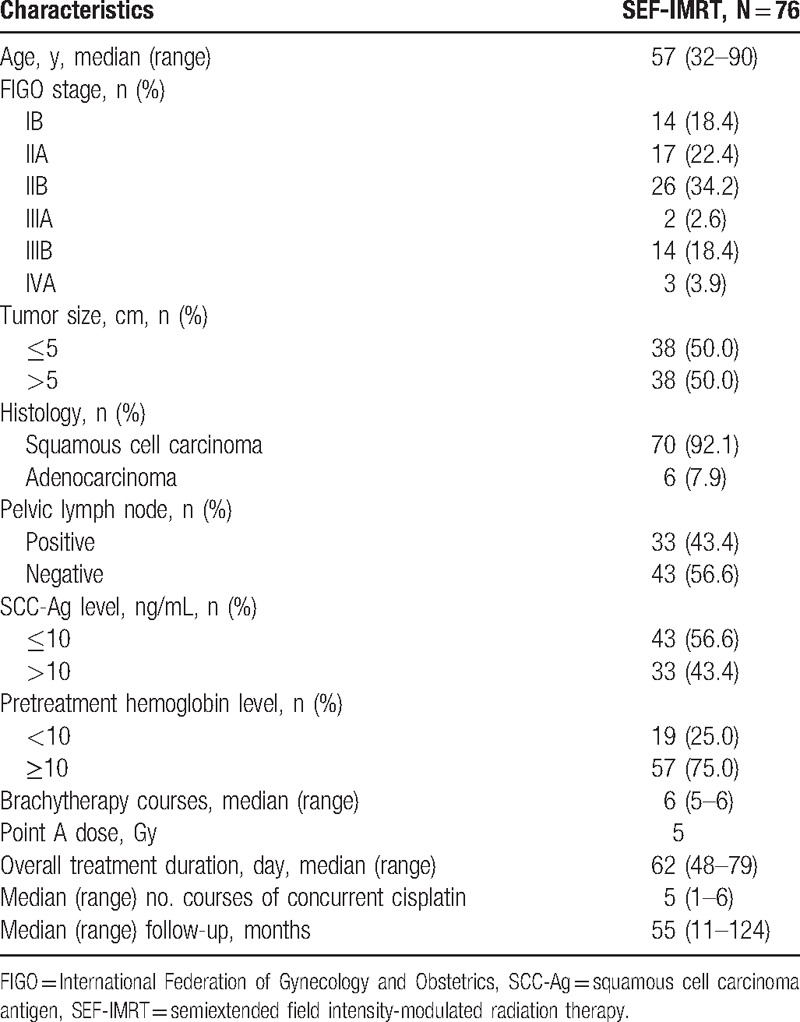
Table 2.
Acute and late toxicity.
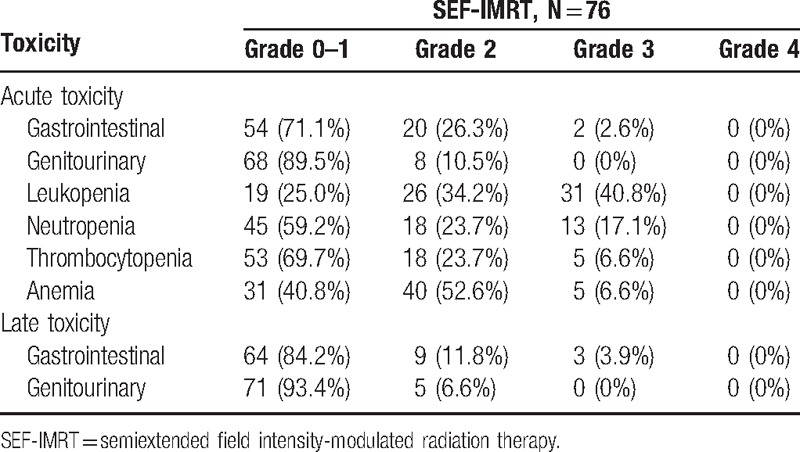
The total length of the treatment including brachytherapy ranged from 48 to 79 (median, 62) days. The median number concurrent cisplatin courses was 5 (range, 1–6). Chemotherapy was omitted because of toxic effects (10 patients), refusal to continue (4 patients), or diminished performance status (3 patients). The chemotherapy dose was reduced in 18 (23%) patients during the treatment course owing to hematologic toxicity. Six patients had ≥2-day breaks in treatments lasting >9 weeks because of acute toxicities (n = 4, diarrhea; n = 2, urinary tract infection). Treatment breaks occurred mainly between 6 and 9 weeks.
After a median follow-up duration of 55 (range, 11–124) months, 3 patients had late grade 3 gastrointestinal complications, and no patients had a late grade 3 genitourinary toxicity. Among the former, 1 patient had a small bowel perforation at 56.9 months, 1 had a stricture of the rectosigmoid junction at 41.8 months, and 1 had proctitis at 8.9 months after completion of CCRT; all complications required surgical intervention.
3.2. Treatment outcomes
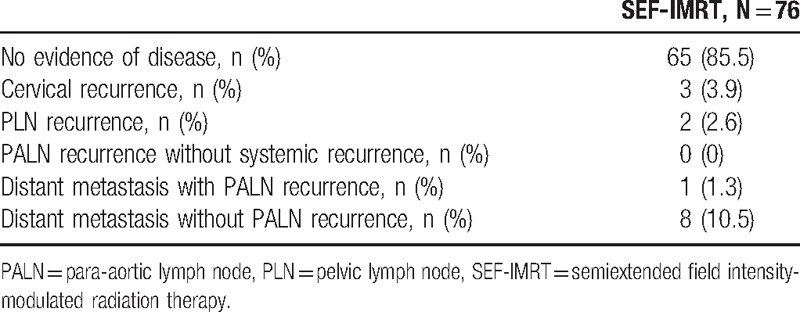
The median follow-up time for surviving patients was 55 (range, 11–124) months. The 5-year OS, DFS, local failure-free survival, regional failure-free survival, PALN failure-free survival, and distant metastasis-free survival (DMFS) rates were 85.0%, 84.4%, 96.0%, 97.3%, 98.6%, and 88.4%, respectively. The Kaplan–Meier plots of the 5-year OS and DFS rates are shown in Fig. 1. During the follow-up period, 65 (85.5%) patients had no recurrence, and 11 (14.5%) patients had treatment failure at the time of analysis as follows: 3 (3.9%), in-field local recurrence; 2 (2.6%), in-field regional recurrence; and 9 (11.8%), out-field distant metastasis (Table 3). Only 1 patient experienced out-field PALN failure, and this patient also had simultaneous distant metastasis (mediastinal and left supraclavicular lymph nodes). No patients had in-field PALN failure.
Figure 1.
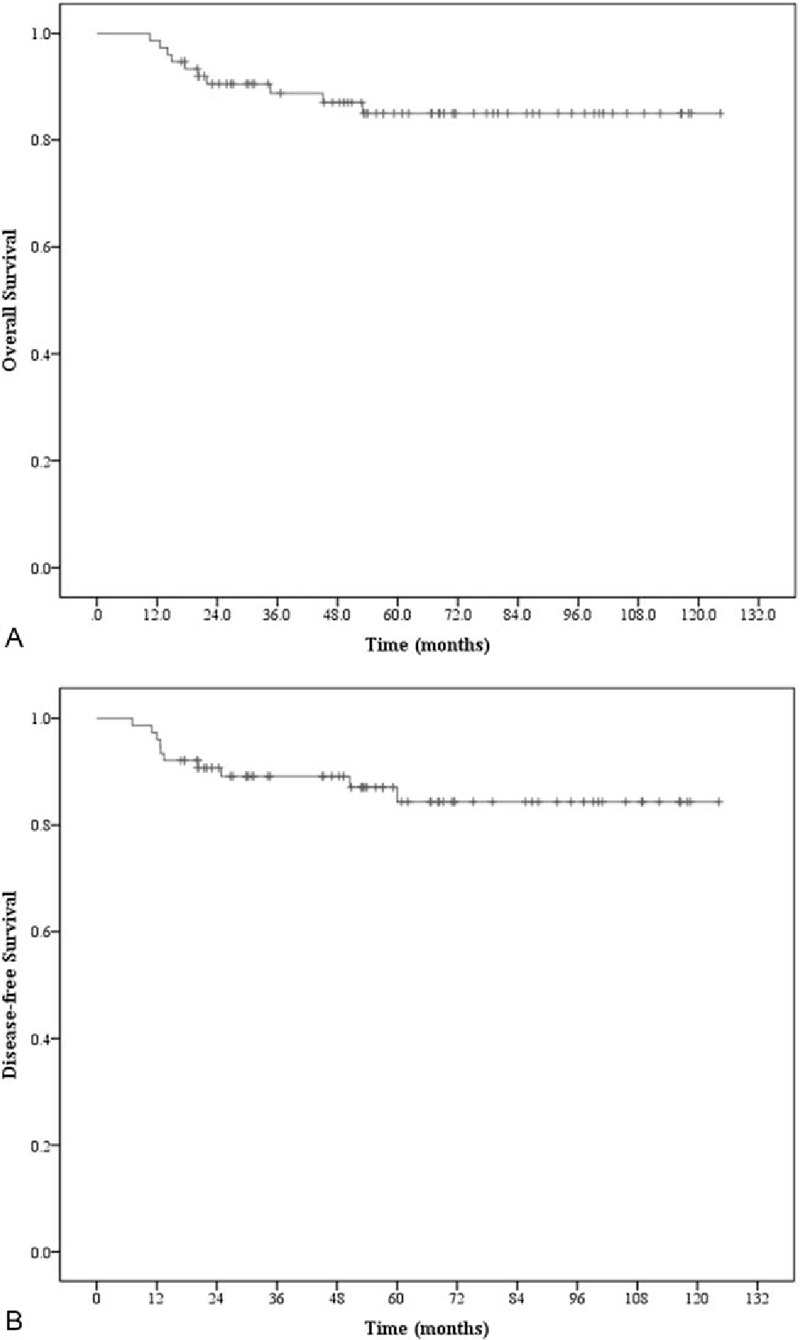
Kaplan–Meier survival curves for the 76 patients analyzed in this study. (A) Overall survival. (B) Disease-free survival.
Table 3.
Failure patterns of patients with cervical cancer receiving SEF-IMRT.
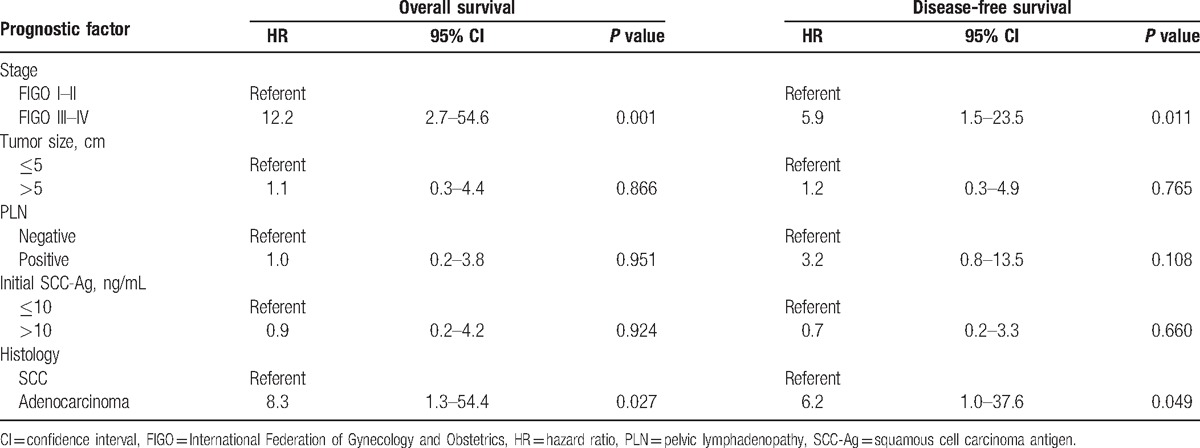
The results of the univariate analysis are shown in Table 4. The risk factors associated with death and disease relapse were examined via multivariate analysis (Table 5). The independent risk factors for disease relapse were stage III–IVA disease (P = 0.011; hazard ratio, 5.9; 95% confidence interval, 1.5–23.5). The 5-year DFS rate differed significantly between patients with stage I–II versus stage III–IV disease (92.1% vs 57.9%, respectively; P = 0.003).
Table 4.
Univariate analysis of prognostic factors on overall survival, disease-free survival, distant metastasis free survival and para-aortic metastasis free survival.
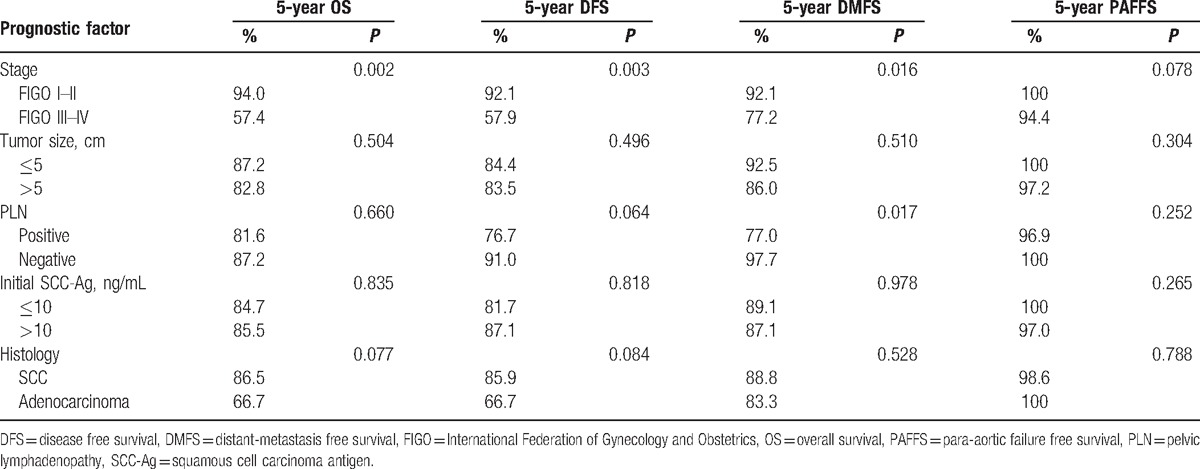
Table 5.
Multivariate analysis of prognostic factors on overall survival and disease-free survival.
4. Discussion
To our knowledge, this is the first study reporting the safety and outcomes of SEF-IMRT for patients with LACC. This retrospective study found that patients receiving SEF-IMRT along with weekly cisplatin had favorable treatment compliance and tolerable toxicities. The outcomes were also favorable, and the main failure sites of SEF-IMRT were distant.
The lymphatic spread of cervical cancer is contiguous. The disease initially involves the lower PLNs, and then progresses to the upper PLNs followed by the PALNs.[5] Patients with LACC are at risk of PALN metastasis, and the current standard treatment for these patients is pelvic CCRT. The reported rate of PALN failure for pelvic CCRT in the RTOG 90–01 trial was 9% (95% confidence interval, 4–13%),[6] which suggests that pelvic CCRT might not completely eliminate all microscopic tumors in the PALNs, particularly in high-risk subjects.[6,8,9] Beadle et al[18] described detailed patterns of regional failure; the most common site of regional recurrence was marginal, usually just above the superior boundary of the pelvic field. Hence, modification of the treatment field might improve treatment outcomes without significantly increasing the occurrence of severe toxicities.
Previous studies of EFRT using conventional techniques reported conflicting data for OS and incidence of distant metastases and significant increases in the incidence of severe toxicities.[10,11] Kuku et al[19] found that EFRT was a significant predictor of severity and chronicity of ongoing disease in patients who developed radiation-induced bowel injuries after treatment for cervical cancer. Given the severe toxicities, the use of EFRT in patients without evidence of PALN involvement remains controversial. Based on the results of this study, we suggest that modifying the radiation field to exclude the PALNs above the level of the renal vessels could reduce toxicity without compromising treatment outcomes.
IMRT is a promising modality for reducing toxicity and improving the therapeutic ratio. Jensen et al[20] studied the effects of IMRT on 21 patients, 14 and 20 of whom had positive PALNs and PLNs, respectively. In that study, the reported probabilities of acute grade ≥3 hematologic, gastrointestinal, and genitourinary toxicities were 57.1%, 19.0%, and 0%, respectively, and the 18-month cumulative incidence of any late grade ≥3 toxicity was 4.8%. In a prospective study evaluating extended field IMRT with concurrent cisplatin in 32 patients with stage IB2–IIIB cervical cancer with positive PLNs and negative PALNs, acute grade ≥3 gastrointestinal, genitourinary, and hematologic toxicities occurred in 2 (6.3%), 1 (3.1%), and 18 (56.3%) patients, respectively.[21] One (3.1%) patient had a late grade 3 gastrointestinal complication, and 1 (3.1%) experienced genitourinary toxicity. The 3-year actuarial OS, DFS, and DMFS rates were 87%, 82%, and 79%, respectively. These findings show that EF-IMRT is a safe and effective modality. When the radiation field of SEF-IMRT excludes the upper part PALNs, as done in the above-mentioned studies, less toxicity with comparable treatment outcomes can be expected.
Prophylactic SEFRT using a conventional technique had favorable PALN control and survival outcomes in a retrospective study by Choi et al[22] of patients with cervical cancer and PALN metastasis. In that study, a prescribed dose of 45 Gy in 25 fractions was delivered by using a 4-field (anterior–posterior, posterior–anterior, and 2 lateral fields) box technique. Seventy-seven (74.8%) patients received SEFRT with concomitant chemotherapy and 26 (25.2%) patients received SEFRT alone. The 5-year OS and recurrence-free survival rates were 82% and 76%, respectively, and the rate of late grade 3 gastrointestinal complications was 2.9%. Comparisons between the study by Choi et al[22] and our study are not straightforward because some patients in our study were PLN-negative and all patients received IMRT at a prescribed dose of 50.4 Gy in 28 fractions and concurrent weekly cisplatin.
In our study, the 5-year OS, DFS, local failure-free survival, regional failure-free survival, PALN failure-free survival, and DMFS rates were 85.0%, 84.4%, 96.0%, 97.3%, 98.6%, and 88.4%, respectively. The patterns of failure were similar to those in previous EFRT and SEFRT studies, with most distant failures.[20–22] No patients had late genitourinary complications, and 3 (3.9%) had late grade 3 gastrointestinal toxicities. Although PLN status has been frequently reported as a major risk factor for death and disease relapse in patients with LACC,[1,7–9,23] our study does not support this premise. If the patients in our study were stratified according to risk of LACC, as done by Liang et al,[9] the 5-year PALN failure-free survival rates would be 100% and 97.4% (P = 0.34), and the 5-year DMFS rates would be 100% and 87.2% (P = 0.01), for low and high risk, respectively. The results of our study show that SEF-IMRT with concurrent cisplatin eliminates microscopic tumors in the lower PALNs and provides favorable treatment outcomes with acceptable acute and late toxicities. The predominant failures are distant, and thus studies investigating the effectiveness of intensified systemic therapy are warranted.
The present study had some limitations. This study excluded patients with small cell carcinoma of the uterine cervix because it has a more aggressive clinical course and the number of patients was low. Hence, the impact of the radiation field on treatment in patients with small cell carcinoma could not be elaborated in the present study and needs further investigation.
The major weakness of this study is that lymph node status was mainly evaluated via CT rather than FDG-PET, which is a useful tool for assessing metastatic nodes.[23,24] However, pretreatment FDG-PET performed to detect tumor-containing lymph nodes cannot exclude the existence of micrometastases in the PALNs because of the limited resolution of the gamma camera.[25,26] In addition, a prospective study reported that detection of PALNs via FDG-PET had no survival benefits, although FDG-PET decreased the need for extended-field CCRT.[27,28] In that study, the PALN failure rate for patients (n = 63) receiving pelvic CCRT but not pretreatment FDG-PET was 15.9% compared with 1.3% in the present study. FDG-PET might have revealed PLNs and PALNs in some of the patients in our study, and the treatment outcomes and policies might have differed if pretreatment FDG-PET had been performed in all patients. Thus, future SEF-IMRT studies evaluating the treatment outcomes of LACC patients with PLNs detected via FDG-PET are needed.
In conclusion, our results suggest that prophylactic SEF-IMRT reduces PALN recurrence and provides favorable treatment outcomes with acceptable acute and late toxicities in patients with LACC without PALN involvement, and is hence the recommended treatment for patients at risk of PALN metastasis.
Footnotes
Abbreviations: ANC = absolute neutrophil count, CCRT = concurrent chemoradiotherapy, CT = computed tomography, CTV = clinical target volume, DFS = disease-free survival, DMFS = distant metastasis-free survival, EFRT = extended field radiotherapy, FDG-PET = 18F-fluorodeoxyglucose positron emission tomography, FIGO = International Federation of Gynecology and Obstetrics, IMRT = intensity-modulated radiotherapy, LACC = locally advanced cervical cancer, OS = overall survival, PALN = para-aortic lymph node, PLN = pelvic lymph nodes, PTV = planning target volume, RTOG = Radiation Therapy Oncology Group, SCC-Ag = squamous cell carcinoma antigen, SEF-IMRT = semiextended field intensity-modulated radiotherapy, SEFRT = semiextended field radiotherapy.
JL conceived and designed the study, collected, analyzed, and interpreted the data, prepared the draft, and gave final approval of the version to be submitted. JBL and FJS collected the data, undertook data analysis and interpretation, and performed the statistical analysis. YJC, CLC, and YTJ also performed the statistical analysis and carried out clinical revision of the data. MHW critically reviewed the intellectual content and gave final approval of the version to be submitted. All authors read and approved the final manuscript.
The authors have no conflicts of interest to disclose
References
- [1].Gouy S, Morice P, Narducci F, et al. Nodal-staging surgery for locally advanced cervical cancer in the era of PET. Lancet Oncol 2012;13:e212–20. [DOI] [PubMed] [Google Scholar]
- [2].Gouy S, Morice P, Narducci F, et al. Prospective multicenter study evaluating the survival of patients with locally advanced cervical cancer undergoing laparoscopic para-aortic lymphadenectomy before chemoradiotherapy in the era of positron emission tomography imaging. J Clin Oncol 2013;31:3026–33. [DOI] [PubMed] [Google Scholar]
- [3].Vandeperre A, Van Limbergen E, Leunen K, et al. Para-aortic lymph node metastases in locally advanced cervical cancer: comparison between surgical staging and imaging. Gynecol Oncol 2015;138:299–303. [DOI] [PubMed] [Google Scholar]
- [4].Berman ML, Keys H, Creasman W, et al. Survival and patterns of recurrence in cervical cancer metastatic to periaortic lymph nodes (a Gynecologic Oncology Group study). Gynecol Oncol 1984;19:8–16. [DOI] [PubMed] [Google Scholar]
- [5].Liu Z, Hu K, Liu A, et al. Patterns of lymph node metastasis in locally advanced cervical cancer. Medicine 2016;95:e4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol 2004;22:872–80. [DOI] [PubMed] [Google Scholar]
- [7].Hong JH, Tsai CS, Lai CH, et al. Risk stratification of patients with advanced squamous cell carcinoma of cervix treated by radiotherapy alone. Int J Radiat Oncol, Biol Phys 2005;63:492–9. [DOI] [PubMed] [Google Scholar]
- [8].Huang EY, Wang CJ, Chen HC, et al. Multivariate analysis of para-aortic lymph node recurrence after definitive radiotherapy for stage IB–IVA squamous cell carcinoma of uterine cervix. Int J Radiat Oncol Biol Phys 2008;72:834–42. [DOI] [PubMed] [Google Scholar]
- [9].Liang JA, Chen SW, Chang WC, et al. Risk stratification for failure in patients with advanced cervical cancer after concurrent chemoradiotherapy: another way to optimise treatment results. Clin Oncol (Royal College of Radiologists (Great Britain)) 2008;20:683–90. [DOI] [PubMed] [Google Scholar]
- [10].Rotman M, Pajak TF, Choi K, et al. Prophylactic extended-field irradiation of para-aortic lymph nodes in stages IIB and bulky IB and IIA cervical carcinomas. Ten-year treatment results of RTOG 79-20. JAMA 1995;274:387–93. [PubMed] [Google Scholar]
- [11].Haie C, Pejovic MH, Gerbaulet A, et al. Is prophylactic para-aortic irradiation worthwhile in the treatment of advanced cervical carcinoma? Results of a controlled clinical trial of the EORTC radiotherapy group. Radiother Oncol 1988;11:101–12. [DOI] [PubMed] [Google Scholar]
- [12].Malfetano JH, Keys H, Cunningham MJ, et al. Extended field radiation and cisplatin for stage IIB and IIIB cervical carcinoma. Gynecol Oncol 1997;67:203–7. [DOI] [PubMed] [Google Scholar]
- [13].Grigsby PW, Heydon K, Mutch DG, et al. Long-term follow-up of RTOG 92-10: cervical cancer with positive para-aortic lymph nodes. Int J Radiat Oncol Biol Phys 2001;51:982–7. [DOI] [PubMed] [Google Scholar]
- [14].Sood BM, Gorla GR, Garg M, et al. Extended-field radiotherapy and high-dose-rate brachytherapy in carcinoma of the uterine cervix: clinical experience with and without concomitant chemotherapy. Cancer 2003;97:1781–8. [DOI] [PubMed] [Google Scholar]
- [15].Chung YL, Jian JJ, Cheng SH, et al. Extended-field radiotherapy and high-dose-rate brachytherapy with concurrent and adjuvant cisplatin-based chemotherapy for locally advanced cervical cancer: a phase I/II study. Gynecol Oncol 2005;97:126–35. [DOI] [PubMed] [Google Scholar]
- [16].Uno T, Mitsuhashi A, Isobe K, et al. Concurrent daily cisplatin and extended-field radiation therapy for carcinoma of the cervix. Int J Gynecol Cancer 2008;18:80–4. [DOI] [PubMed] [Google Scholar]
- [17].Ring KL, Young JL, Dunlap NE, et al. Extended-field radiation therapy with whole pelvis radiotherapy and cisplatin chemosensitization in the treatment of IB2-IIIB cervical carcinoma: a retrospective review. Am J Obstet Gynecol 2009;201:109.e101–6. [DOI] [PubMed] [Google Scholar]
- [18].Beadle BM, Jhingran A, Yom SS, et al. Patterns of regional recurrence after definitive radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2010;76:1396–403. [DOI] [PubMed] [Google Scholar]
- [19].Kuku S, Fragkos C, McCormack M, et al. Radiation-induced bowel injury: the impact of radiotherapy on survivorship after treatment for gynaecological cancers. Brit J Cancer 2013;109:1504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jensen LG, Hasselle MD, Rose BS, et al. Outcomes for patients with cervical cancer treated with extended-field intensity-modulated radiation therapy and concurrent cisplatin. Int J Gynecol Cancer 2013;23:119–25. [DOI] [PubMed] [Google Scholar]
- [21].Liang JA, Chen SW, Hung YC, et al. Low-dose, prophylactic, extended-field, intensity-modulated radiotherapy plus concurrent weekly cisplatin for patients with stage IB2–IIIB cervical cancer, positive pelvic lymph nodes, and negative para-aortic lymph nodes. Int J Gynecol Cancer 2014;24:901–7. [DOI] [PubMed] [Google Scholar]
- [22].Choi J, Yoon HI, Lee J, et al. Optimal extent of prophylactic irradiation of paraaortic lymph nodes in patients with uterine cervical ancer. PloS One 2015;10:e0145158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kidd EA, Siegel BA, Dehdashti F, et al. Lymph node staging by positron emission tomography in cervical cancer: relationship to prognosis. J Clin Oncol 2010;28:2108–13. [DOI] [PubMed] [Google Scholar]
- [24].Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol 2001;19:3745–9. [DOI] [PubMed] [Google Scholar]
- [25].Tsai CS, Chang TC, Lai CH, et al. Preliminary report of using FDG-PET to detect extrapelvic lesions in cervical cancer patients with enlarged pelvic lymph nodes on MRI/CT. Int J Radiat Oncol Biol Phys 2004;58:1506–12. [DOI] [PubMed] [Google Scholar]
- [26].Sakurai H, Suzuki Y, Nonaka T, et al. FDG-PET in the detection of recurrence of uterine cervical carcinoma following radiation therapy—tumor volume and FDG uptake value. Gynecol Oncol 2006;100:601–7. [DOI] [PubMed] [Google Scholar]
- [27].Tsai CS, Lai CH, Chang TC, et al. A prospective randomized trial to study the impact of pretreatment FDG-PET for cervical cancer patients with MRI-detected positive pelvic but negative para-aortic lymphadenopathy. Int J Radiat Oncol Biol Phys 2010;76:477–84. [DOI] [PubMed] [Google Scholar]
- [28].Lin SY, Tsai CS, Chang YC, et al. The role of pretreatment FDG-PET in treating cervical cancer patients with enlarged pelvic lymph node(s) shown on MRI: a phase 3 randomized trial with long-term follow-up. Int J Radiat Oncol Biol Phys 2015;92:577–85. [DOI] [PubMed] [Google Scholar]


