Abstract
Rationale:
The pathogenesis and progression of lung cancer is a complicated process in which many genes take part. But molecular gene testing is typically only performed in advanced-stage non-squamous non-small-cell lung cancer (NSCLC). The value of tyrosine kinase inhibitors (TKI) administration is not widely recognized with respect to early-stage NSCLC.
Patient concerns:
Here, we present a case of a man, heavy smoker who initially presented with stage IA lung adenocarcinoma (LADC). Three years after a lung lobectomy, he was diagnosed with advanced lung squamous cell carcinoma (SCC), according to laboratory, imaging, and pathological examinations.
Diagnoses
The case initially had an early-stage LADC with an L858R epidermal growth factor receptor (EGFR) mutation. A subsequent advanced SCC bearing EGFR L858R/T790M mutations occurred 3 years after surgery.
Interventions:
The comprehensive therapy we utilized, including surgical resection for the early-stage lesion and GP chemotherapy and local radiotherapy as the first line therapy along with gefitinib maintenance treatment for the advanced metachronous second primary tumors (MST).
Outcomes:
The synthetical therapy, have resulted in our patient with remaining alive and progression free for 4.5 years.
Lessons:
This case suggests that changes in molecular pathology should be monitored closely throughout cancer progression to guide personalized therapy and improve prognosis. We further review administration of TKI to early-stage NSCLC and to the metachronous second primary tumors (MST) in survivors.
Keywords: early-stage NSCLC, EGFR-TKI, genetic examination, metachronous second primary tumor, therapy
1. Introduction
With the development of modern molecular biology techniques, recent studies have demonstrated that molecular-targeted agents, such as epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) may markedly prolong survival in selected non-small-cell lung cancer (NSCLC) patients based on the presence of driver gene mutations.[1,2] However, these novel therapies have established clinical benefits only in the setting of incurable, advanced NSCLC.[3,4] As a result, molecular gene testing is typically only performed in advanced-stage non-squamous NSCLC based on European Society for Medical Oncology, American Society for Clinical Oncology, and The National Comprehensive Cancer Network guidelines. The value of TKI administration is not widely recognized with respect to early-stage NSCLC.
Surgery remains the standard of care for early-stage NSCLC, but approximately 30% to 75% of completely resected NSCLC patients experience recurrence and death within 5 years.[5] Although the cisplatin-based adjuvant regimen has been suggested, significant toxicity may limit its use.[6] Thus, researchers have begun to explore whether EGFR-TKI can be applied during the perioperative period of the resected NSCLC to improve progression-free survival (PFS) and overall survival (OS). Since the presence of EGFR gene mutation is a known predictive marker for response to EGFR-TKI-targeting therapy, should we support routine EGFR mutation testing on resected NSCLC? This issue remains highly controversial, with conflicting results reported in studies to date.[7–9]
Approximately 5% to 10% of patients will present with metachronous second primary tumor (MST) 2 to 3 years after their initial surgery.[10] No study has explored the relevance between the initially resected tumor and subsequent MST occurrence with respect to the presence of genetic mutations. Whether genetic mutations present in resected tumors are predictive factors for MST's mutation status is unknown. How can TKIs targeting cells with EGFR mutations be appropriately used to deal with this complex issue? We believe that efforts should be made to identify the best treatment strategy.
Here, we report a case of early-stage NSCLC with the L858R mutation and a subsequent MST that occurred 3 years after surgery, at which time double L858R and T790M mutations were identified. For MSTs, cisplatin-gemcitabine (GP) chemotherapy along with local radiotherapy was used as first-line care and then, we chose a subsequent strategy to explore TKI maintenance therapy, expecting improved PFS. Our patient achieved a strikingly long PFS as a result of treatment and maintained good performance status (PS) until now. We hope this report can provide some useful conclusions for identifying the best timing and strategy for TKI administration in early-stage NSCLC patients with EGFR mutations.
2. Case report
A 52-year-old man with a 30-year history of smoking presented with an abnormal nodule measuring 0.8 × 1.5 cm in the left upper lung lobe imaged through chest computed tomography (CT) scanning in March 2012 in a community hospital. He had previously been well without any additional relevant or abnormal symptoms. Physical examination suggested no significant abnormalities. Laboratory findings were within normal range, except for a carcinoembryonic antigen (CEA) level of 30.25 ng/mL (normal range, 0–5 ng/mL) found in the serum. Subsequently, a positron emission tomography (PET)/CT scan showed a lung lesion with a standardized uptake value of 7.94, which was highly suspected to be a malignant tumor (Fig. 1). Subsequently, he underwent left upper lung lobectomy and lymph node dissection. Postoperative pathological examination revealed an adenocarcinoma (ADC) (Fig. 2A). Immunohistochemistry (IHC) staining results were positive for CK7, TTF-1, p63, and NapsinA and negative for CK 5/6, Syn, cgA, and Ki-67 (20%–30%). The clinical stage was classified as IA2 (pT1bN0M0). Because the tumor was still in an early stage, the patient did not receive adjuvant treatment but continued to be monitored through regular hospital visits every 3 months.
Figure 1.
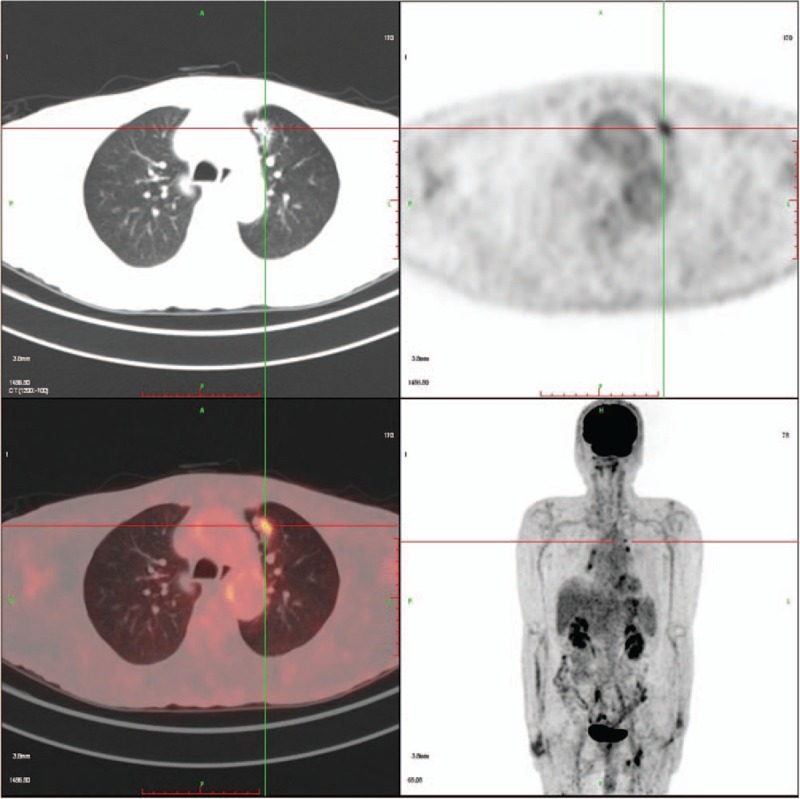
Fluorodeoxyglucose (18F-FDG) position-emission tomography (PET)-CT was used to visualize the lesion, showing the lesion in soft tissue and lung windows. CT = computed tomography.
Figure 2.
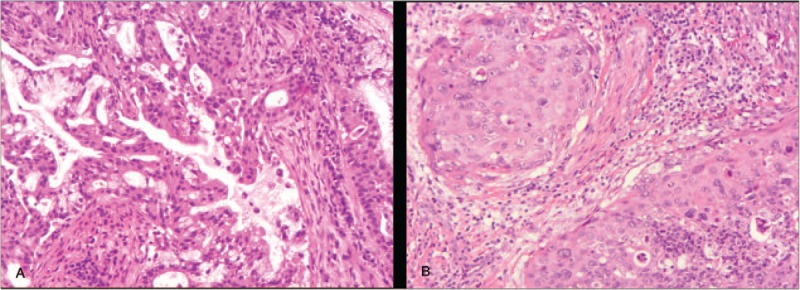
Pathologies of tissues confirmed different types of lung cancers. (A) The first lesion is adenocarcinoma (ACD). (B) The second lesion is squamous cell carcinoma (SCC). H&E staining × 100. H&E = hematoxylin and eosin.
The patient's condition had been stable until the onset of left chest pain in April 2015. The patient was transferred to our hospital (a tertiary care hospital) for further treatment. Physical examination suggested a significant tender point in the left chest wall. Laboratory findings showed only a single parameter outside the normal range: the cytokeratin19 fragment antigen 21–1 (CYFRA21–1) level was 17.45 ng/mL in the serum (normal range = 0–3.3 ng/mL). CT and PET/CT scans highlighted the serious involvement of left pleura, showing bilateral lung lesions (Fig. 3A and E). At that point, the clinical stage was upgraded to IVB. After the CT-guided left pleura puncture, pathological IHC analysis showed positive staining for p63, p40, CK 7, and CK 5/6, while TTF-1 and NapsinA staining were absent, supporting a diagnosis of squamous cell carcinoma (SCC) (Fig. 2B). Meanwhile, molecular evaluation confirmed an L858R mutation in exon 21 and a T790M mutation in exon 20 (Fig. 4B and C). Considering the pathology of the SCC was quite distinct compared with that of the originally resected NSCLC specimen, we retrospectively reassessed the surgery specimen to verify our primary diagnosis. The morphologic and IHC results were in complete agreement with the initial diagnoses, but an L858R mutation was identified using the newly added molecular evaluation (Fig. 4A). To relieve the acute pain in the left pleura as quickly as possible, palliative three-dimensional conformal radiotherapy (3D-CRT) for the chest lesion was delivered at a total dose of 42 Gy/14 fractions (f), once daily and 5f/week (Fig. 5). The volume over radiation dose 5 Gy (V5) values of the left, right, and total lungs were 20%, 0%, and 7.8%, respectively; the volume over radiation dose 20 Gy (V20) values were 10%, 0%, and 5%, respectively; the mean lung dose (MLD) was 5 Gy. Meanwhile, considering the MST pathology was that of SCC, the patient received GP chemotherapy (cisplatin 75 mg/m2 d1, gemcitabine 1250 mg/m2 d1, d8, for 21 days as a cycle) for 4 cycles, and the patient achieved partial remission (PR) after 2 cycles, followed by stable disease (SD) after 4 cycles (Fig. 3[B and F] and [C and G]) according to response evaluation criteria in solid tumors (RECIST) 1.1. Then, gefitinib (150 mg qd) was used as maintenance treatment from November 2015 onward. During the treatment, Ibandronate sodium was given monthly for skeletal-related events. The patient is currently under surveillance with SD (Fig. 3D and H) and with PS scores of 0.
Figure 3.
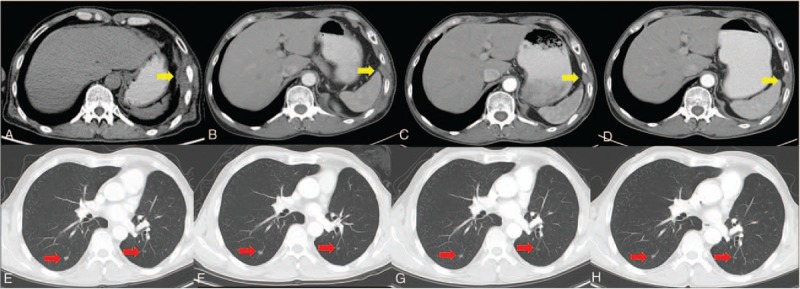
Changes in computed tomography (CT) in the SCC. (A) and (E) show the enhancing masses in left pleura (gold arrow) and bilateral lungs (red arrows) before the treatment, respectively. (B) and (F) reveal the reduction in the lesions after 2 cycles of GP chemotherapy and the radiotherapy for the left pleura. (C) and (G) show stabilization of the lesions after 4 cycles of GP chemotherapy. (D) and (H) reveal lesion size stability after gefitinib maintenance treatment to the present. GP = cisplatin-gemcitabine, SCC = squamous cell carcinoma.
Figure 4.
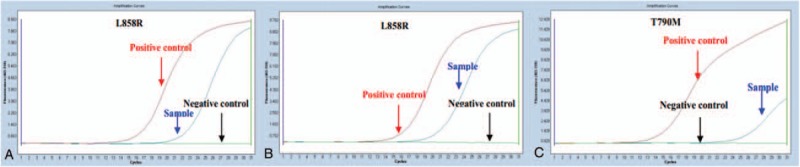
ARMS-PCR identifying EGFR mutations in tumor specimens. (A) Presence of the L858R mutation in the ACD lesion. Band C shows both L858R exon 21 mutation (B) and a de novo T790M mutation (C) are present in the SCC. ARMS-PCR: amplification-refractory mutation system (ARMS) mutant-enriched polymerase chain reaction (PCR) method. ACD = adenocarcinoma, EGFR = epidermal growth factor receptor, SCC = squamous cell carcinoma.
Figure 5.
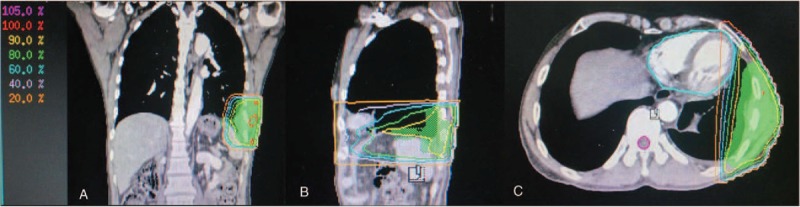
PTV of the three-dimensional conformal radiotherapy (3DCRT). The PTV (green shadows) shown in coronal (A), sagittal (B), and cross-sectional (C) images, respectively. PTV = planning target volume.
3. Discussion
The 5-year survival rate for stage IA NSCLC patients after complete tumor resection is 68.5%[11]; however, more than one-third of these patients experience tumor recurrence, MST, or distant metastasis within 5 years. In this case report, we explored the therapy regimens of early-stage NSCLC with respect to whether EGFR mutation testing should be performed earlier to determine whether to begin TKI treatment and the strategy of TKI administration for those survivors to present with “EGFR mutant” MST.
3.1. The potential predictive value of EGFR status in early-stage NSCLC
Early and advanced NSCLCs usually exhibit divergent biological characteristics; for example, EGFR pathway mutations may not have a primary role in stage I/II NSCLC.[12] Early and advanced NSCLCs are typically considered separately when evaluating the efficacy of EGFT-TKIs. However, many studies have suggested that EGFR evaluation could be predictive of survival benefit following TKI therapy in patients with NSCLC harboring EGFR mutations. Given the evidence for TKI efficacy in advanced NSCLC, the poor survival of patients with completely resected NSCLC, the modest improvements with adjuvant chemotherapy, and TKI's oral route of administration and acceptable toxicity profile, we considered that EGFR-TKIs could be efficacious in early resected NSCLCs. Several trials have been conducted to explore the applications of TKIs in early resectable NSCLCs.[13,14] In a phase III BR.19 trial,[13] 503 patients with completely resected and pathologically confirmed stage IB/II/IIIA NSCLC were analyzed. No significant benefits or differences on OS and PFS were detected between gefitinib and placebo groups. Similarly, there was no disease-free survival (DFS) or OS benefit from gefitinib for the 15 patients with EGFR mutation-positive tumors. We suspect the poor results may have resulted from the low rate of EGFR mutations present in these patients (only 15 positive tumors, 3.0%, 15/503). Therefore, appropriate selection of patients with EGFR mutations is likely necessary to avoid misleading conclusions. Subsequently, further studies based on the “EGFR mutant” populations have been conducted. In a retrospective study from the Memorial Sloan Kettering Cancer Center (MSKCC)[14] involving 167 patients with resected NSCLC harboring EGFR activating mutations (70% of them with stage I disease), patients treated with either neoadjuvant or adjuvant TKI therapy (gefitinib or erlotinib) had a 2-year DFS rate of 89%, compared with 72% for those who did not receive an EGFR TKI (P = 0.06). A slight benefit in 2-year overall survival was also observed, but this was not significant (96% versus 90%; P = 0.296). The SELECT trial is the first to prospectively test the efficacy of adjuvant erlotinib in EGFR-mutant NSCLCs.[15] With a sample size of 100 patients, the study was powered to demonstrate a primary endpoint of 2-year DFS (>85%). As a result, with a median follow-up of 3 years, the 2-year DFS was 90% (97% stage I), significantly higher than the historical control of 76% in resected early-stage EGFR-mutant NSCLC. However, median DFS and OS have not yet been reached. Overall, the results of these studies indicate that adjuvant TKI treatment is safe and feasible; however, several limitations of these studies that may impact the validity of the data exist: the study in MSKCC could not avoid inherent defects in retrospective research, and as a single-arm clinical trial, the SELECT study is not persuasive in the absence of comparison with chemotherapy. Thus, whether adjuvant TKI therapy can benefit EGFR-mutated patients is still not known with certainty. Moreover, the following additional questions remain to be addressed: what is the optimal duration of therapy? What is the optimal TKI dose? Does the type of EGFR mutation matter? Taking these limitations and questions into account, ongoing randomized adjuvant trials (CTONG1104 in China and IMPACT in Japan) are being conducted that should provide convincing evidence for the effect of adjuvant TKI therapy in early resected EGFR-mutated NSCLC patients.
3.2. Assessment of EGFR mutations in MST
Given increasing duration of early-stage NSCLC survivorship, the incidence and mortality from MST are likely to rise, especially because some factors can elevate the risk of developing MST, including genetic syndromes[16] and common tobacco or alcohol exposures.[17] In particular, EGFR mutations are associated with a “never-smoked” status,[18] whereas the lack of smoking history is unusual for patients with SCC (1%–3% reported in studies).[19,20] Accordingly, when the MST is diagnosed as an SCC, the existence of EGFR mutations is generally not considered. Interestingly, in this report, a patient with a heavy smoking history was diagnosed with lung SCC (LSCC) and possessed EGFRL858R/T790M mutations 3 years after the operation of the lung ADC (LADC). How do we explain this phenomenon? Rekhtman et al[21] has suggested, that when EGFR mutations are found in small biopsies, a so-called pure SCC should be excluded. And in our report, we cannot determine whether ADC component in it considering the small diagnostic biopsies and the LADC history, although the specimens from the patient were morphologically and histologically shown to be pure SCC. The diagnostic criteria of the World Health Organization classification of lung carcinomas includes examination of the entire tumor.[22] However, in “real-world” situations, very few cases obtain large surgical specimens, and so the classification of most lung carcinomas is performed using small biopsies or cytology-type samples. We hope this case can be a reference to aid in avoiding misdiagnosis and to serve as an example that if one has been diagnosed with LADC, EGFR assessment should be performed for subsequent MSTs, particularly in cases where EGFR mutation was identified in the initially resected tumor. Although there is still no exact report to favor our suggestion, the use of histology and gene mutations of the resected tumors as predictive factors can play a key role in the EGFR assessment of MST with related research going forward.
3.3. Administering treatment to “EGFR mutant” MST
Several studies have reported successful TKI treatment of LADC/LSCC patients containing sensitive EGFR mutations at different rates.[23,24] EGFR TKIs have been recognized as an option to treat “EGFR mutant-sensitive” MST, irrespective of histologic type. Conversely, many resistance mutations cause sterichindrance and prevent the binding of EGFR TKIs. For example, the EGFR T790M point mutation in exon 20 is most common and was confirmed to be half of the reason for drug resistance.[25] The EGFR T790M mutation has been identified prior to treatment in 25% of lung cancer patients also harboring sensitive EGFR mutations.[26] How can we address these patients with both sensitive and T790M mutations? AZD9291, a third generation TKI, is selective for both T790M and sensitive EGFR mutations and is highly active in tumors with both T790M and L858R mutations.[27] Unfortunately, this drug has not yet been approved for patients in China. Beyond that, second-generation irreversible EGFR TKIs (e.g., afatinib and dacomitinib) and first-generation EGFR TKIs (e.g., gefitinib and erlotinib) are all ineffective in inhibiting the physiologic activity or emergence of T790M.[28,29] In instances when molecular characteristics cannot guide the EGFR-TKI therapies, histology plays an important role in determining treatment responses in the advanced disease setting. Due to the results from ECOG 1594[30]and JMDB trials,[31] GP chemotherapy, reported to yield clinical benefits in LSCC patients, was given as the first-line treatment. In our patient, the disease reached PR after 2 cycles of GP chemotherapy and SD after 4 cycles. The first-generation TKIs have proven efficacy in maintenance therapy in non-progressing advanced stage NSCLC patients who had received a platinum doublet (SATURN).[32] Moreover, both the subset analysis of SATURN and our report propose that a maintenance treatment regimen including TKIs for these “EGFR mutant” patients will result in a dramatically longer PFS than “EGFR wild type” patients. To our knowledge, this report is the first integrated approach using GP chemotherapy and gefitinib maintenance treatment for MST with EGFRL858R/T790M mutations. To date, the patient has reached 15 months PFS with a PS score of 0.
4. Conclusions
We present a case of early-stage NSCLC with an initial L858R EGFR mutation and a subsequent advanced MST bearing EGFRL858R/T790M mutations, occurring 3 years after surgery. The comprehensive therapy we utilized, including surgical resection for the early-stage lesion and GP chemotherapy and local radiotherapy as the first line therapy along with gefitinib maintenance treatment for the advanced MST, has resulted in our patient remaining alive and progression free for more than 4.5 years. Actually, due to the limitations of understanding in 2012, we didn’t test the initial tumor's EGFR mutation status until EGFR mutations were confirmed in the MST in 2015. So, this phenomenon really reminds that EGFR assessment is quite necessary to avoid missed diagnosis for patients with EGFR-mutated even in early NSCLC who have more potential to be benefit from the TKI treatment. Moreover, it is still unclear whether some relationships exist between the MST and the initially resected tumor at the molecular level. However, molecular evaluation can effectively distinguish both similarities and differences between MST and the initially which may aid in identifying any relationship that exists between them. In conclusion, this case demonstrates that diagnosis and treatment based on histology combined with molecular evaluation is becoming inevitable. Further, investigation is still needed to select the optimal patient subgroup that should receive TKI therapy.
Footnotes
Abbreviations: CEA = carcinoembryonic antigen, CT = computed tomography, CYFRA21–1 = cytokeratin19 fragment antigen 21–1, EGFR = epidermal growth factor receptor, GP = cisplatin-gemcitabine, IHC = immunohistochemistry, LADC = lung adenocarcinoma, LSCC = lung squamous carcinoma, MLD = mean lung dose, MST = metachronous second primary tumor, NSCLC = non-small-cell lung cancer, NSE = neuron specific enolase, OS = overall survival, PET = positron emission tomography, PFS = progression-free survival, PR = partial remission, PS = performance status, RECIST = response evaluation criteria in solid tumors, SD = stable disease, SOC = standard of care, TKI = tyrosine kinase inhibitors.
Ethical approval: Institutional review board (IRB) of Shandong Cancer Hospital has confirmed that it is not necessary to achieve IRB approval for this case report because it is a retrospective review of patient's records and related images, and does not involve patient's privacy, rights, and interests. The patient has consented for the publication of the present case report.
Grant support: This work is supported by the National Natural Science Foundation of China (81472810), the Science and Technology Development Plans of Shandong Province (2014GSF118138), and the special fund for Scientific Research in Public Interest (201402011).
The authors declare that they have no conflicts of interest.
References
- [1].Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866–74. [DOI] [PubMed] [Google Scholar]
- [2].Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141–51. [DOI] [PubMed] [Google Scholar]
- [3].Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42. [DOI] [PubMed] [Google Scholar]
- [4].Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–22. [DOI] [PubMed] [Google Scholar]
- [5].Goldstraw P, Crowley J, Chansky K, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706–14. [DOI] [PubMed] [Google Scholar]
- [6].Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351–60. [DOI] [PubMed] [Google Scholar]
- [7].Janjigian YY, Park BJ, Zakowski MF, et al. Impact on disease-free survival of adjuvant erlotinib or gefitinib in patients with resected lung adenocarcinomas that harbor EGFR mutations. J Thorac Oncol 2011;6:569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].D’Angelo SP, Janjigian YY, Ahye N, et al. Distinct clinical course of EGFR-mutant resected lung cancers: results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J Thorac Oncol 2012;7:1815–22. [DOI] [PubMed] [Google Scholar]
- [9].Kim YT, Seong YW, Jung YJ, et al. The presence of mutations in epidermal growth factor receptor gene is not a prognostic factor for long-term outcome after surgical resection of non-small-cell lung cancer. J Thorac Oncol 2013;8:171–8. [DOI] [PubMed] [Google Scholar]
- [10].Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi89–98. [DOI] [PubMed] [Google Scholar]
- [11].Pfannschmidt J, Muley T, Bulzebruck H, et al. Prognostic assessment after surgical resection for non-small cell lung cancer: experiences in 2083 patients. Lung Cancer 2007;55:371–7. [DOI] [PubMed] [Google Scholar]
- [12].Zhai H, Zhong W, Yang X, et al. Neoadjuvant and adjuvant epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) therapy for lung cancer. Transl Lung Cancer Res 2015;4:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med 2005;353:133–44. [DOI] [PubMed] [Google Scholar]
- [15].Pennell NA, Neal JW, Chaft JE, et al. SELECT: a multicentre phase II trial of adjuvant erlotinib in resected early-stage EGFR mutation-positive NSCLC [abstract]. J Clin Oncol 2014;32:5s. [Google Scholar]
- [16].Tiwari AK, Roy HK, Lynch HT. Lynch syndrome in the 21st century: clinical perspectives. QJM 2016;109:151–8. [DOI] [PubMed] [Google Scholar]
- [17].Rabbani F, Grimaldi G, Russo P. Multiple primary malignancies in renal cell carcinoma. J Urol 1998;160:1255–9. [PubMed] [Google Scholar]
- [18].Kang SM, Kang HJ, Shin JH, et al. Identical epidermal growth factor receptor mutations in adenocarcinomatous and squamous cell carcinomatous components of adenosquamous carcinoma of the lung. Cancer 2007;109:581–7. [DOI] [PubMed] [Google Scholar]
- [19].Lee SY, Kim MJ, Jin G, et al. Somatic mutations in epidermal growth factor receptor signaling pathway genes in non-small cell lung cancers. J Thorac Oncol 2010;5:1734–40. [DOI] [PubMed] [Google Scholar]
- [20].Sakurai H, Asamura H, Watanabe S, et al. Clinicopathologic features of peripheral squamous cell carcinoma of the lung. Ann Thorac Surg 2004;78:222–7. [DOI] [PubMed] [Google Scholar]
- [21].Rekhtman N, Paik PK, Arcila ME, et al. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res 2012;18:1167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization classification of tumors of the thymus: continuity and changes. J Thorac Oncol 2015;10:1383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tanaka K, Hata A, Kida Y, et al. Gefitinib for a poor performance status patient with squamous cell carcinoma of the lung harboring EGFR mutation. Intern Med 2012;51:659–61. [DOI] [PubMed] [Google Scholar]
- [24].Hata A, Katakami N, Yoshioka H. How sensitive are epidermal growth factor receptor-tyrosine kinase inhibitors for squamous cell carcinoma of the lung harboring EGFR gene-sensitive mutations? J Thorac Oncol 2014;9:e20. [DOI] [PubMed] [Google Scholar]
- [25].Heuckmann JM, Rauh D, Thomas RK. Epidermal growth factor receptor (EGFR) signaling and covalent EGFR inhibition in lung cancer. J Clin Oncol 2012;30:3417–20. [DOI] [PubMed] [Google Scholar]
- [26].Lee Y, Lee GK, Lee YS, et al. Clinical outcome according to the level of preexisting epidermal growth factor receptor T790M mutation in patients with lung cancer harboring sensitive epidermal growth factor receptor mutations. Cancer 2014;120:2090–8. [DOI] [PubMed] [Google Scholar]
- [27].Ku BM, Bae YH, Koh J, et al. AZD9291 overcomes T790 M-mediated resistance through degradation of EGFR (L858R/T790M) in non-small cell lung cancer cells. Invest New Drugs 2016;34:407–15. [DOI] [PubMed] [Google Scholar]
- [28].Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528–38. [DOI] [PubMed] [Google Scholar]
- [29].Zhou W, Ercan D, Chen L, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 2009;462:1070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92–8. [DOI] [PubMed] [Google Scholar]
- [31].Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–51. [DOI] [PubMed] [Google Scholar]
- [32].Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521–9. [DOI] [PubMed] [Google Scholar]


