Abstract
Several studies have suggested an association between use of metformin and an increased overall survival in patients diagnosed with pancreatic cancer, however with several important methodological limitations. The aim of the study was to assess the association between overall survival, pancreatic cancer, and metformin use.
A retrospective cohort study of 1111 patients with pancreatic cancer was conducted using data from The Netherlands Comprehensive Cancer Organization (1998–2011). Data were linked to the PHARMO Database Network containing drug-dispensing records from community pharmacies. Patients were classified as metformin user or sulfonylurea derivatives user from the moment of first dispensing until the end of follow up. The difference in overall survival between metformin users and nonusers was assessed, and additionally between metformin users and sulfonylurea derivatives users. Univariable and multivariable parametric survival models were used and use of metformin and sulfonylurea derivatives was included as time-varying covariates.
Of the 1111 patients, 91 patients were excluded because of differences in morphology, 48 patients because of using merely metformin before diagnosis, and 57 metformin-users ever used contemporary sulfonylurea derivatives and were therefore excluded. Lastly, 8 patients with a survival of zero months were excluded. This resulted in 907 patients for the analysis. Overall, 77 users of metformin, 43 users of sulfonylurea derivatives, and 787 nonusers were identified. The adjusted rate ratio for overall survival for metformin users versus nonusers was 0.86 (95% CI: 0.66–1.11; P = 0.25). The difference in overall survival between metformin users and sulfonylurea derivatives users showed an adjusted rate ratio of 0.90 (95% CI: 0.59–1.40; P = 0.67).
No association was found between overall survival, pancreatic cancer, and metformin use. This was in concordance with 2 recently published randomized controlled trials. Future research should focus on the use of adjuvant metformin in other cancer types and the development or repurposing of other drugs for pancreatic cancer.
Keywords: cohort study, metformin, pancreatic cancer, sulfonylurea derivatives, survival
1. Introduction
Pancreatic cancer is the eighth most common cause of cancer deaths in the world.[1] Only 10%–20% of patients with pancreatic cancer qualify for surgery and the prognosis of this disease is poor; median survival for patients undergoing surgery ranges from 20 to 23 months.[2,3] For patients receiving solely chemotherapy, survival approximately ranges from 3 to 11 months.[1,4] With this limited prognosis, there is a high and urgent need for new therapies to improve outcome.
Smoking, obesity, and type 2 diabetes are considered to be important risk factors for the development of pancreatic cancer.[5,6] Metformin is the first-line treatment for patients with type 2 diabetes and is therefore the most prescribed oral glucose-lowering drug (OGLD). The decision to prescribe metformin depends on patient characteristics; metformin use is contraindicated in patients with renal failure, cardiac dysfunction, and hepatic insufficiency.[7] Metformin is a biguanide antihyperglycemic agent and has 3 working mechanisms: it decreases the hepatic glucose production by inhibition of gluconeogenesis and glycogenolysis in muscles, it subsequently improves peripheral insulin sensitivity, and reduces glucose absorption.[7–9] Mouse models suggest that metformin could inhibit the proliferation of xenografted human pancreatic cancer cells within 30 days, but other studies point toward a systemic effect of metformin on cancer by improving the metabolic profile of patients, rather than a direct effect on tumor cells.[9–12]
Recent epidemiologic cohort studies in patients with type 2 diabetes have suggested that patients using metformin have a decreased risk of developing cancer and, possibly, a reduced cancer mortality.[13–16]
Several meta-analysis pointed out that the reduced cancer incidence was not present in all types of cancer; use of metformin seems to be associated with a reduced risk of developing cancer in patients with pancreatic, colorectal, and hepatocellular cancer, with conflicting results for breast cancer, and no association is seen in patients with lung and prostate cancers.[17–21] Preceding epidemiologic studies assessing the effect of metformin on the risk of cancer and survival may have been subject to several time-related biases, for example, misclassifying exposure to metformin, which could have inflated the estimates. Three studies avoiding these biases have found no effect of metformin on cancer incidence.[22] What additionally complicates observational studies on this subject, is that patients using metformin often have other comorbidities supplementary to type 2 diabetes, compared with nonusers. Alternative treatment for type 2 diabetes are sulfonylurea derivatives (SD) which have been used previously as a comparator group in addition to nonusers.[15]
The aim of this study was to assess the association between the use of metformin and overall survival in patients with pancreatic cancer with the use of appropriate methodology, a pitfall of the previous studies. Patients using metformin were compared with nonusers and additionally to patients using SD.
2. Methods
2.1. Data
Data from the Eindhoven Cancer Registry (ECR) were linked on patient level to the PHARMO Database Network covering a demographic region in the South-Eastern part of The Netherlands of ∼1.5 million inhabitants.[23] The ECR is maintained by The Netherlands Comprehensive Cancer Organisation and registers newly diagnosed cancer patients from 10 different hospitals located in this region. Patients are informed about this registration and are registered except patients who objected to be registered. The Netherlands Cancer Registry is obliged to work according to the law about protection of privacy data and all procedures to privacy of doctors and patients is fixed in regulations. An independent Committee of Privacy reassures that The Netherlands Cancer Registry is compliant to these regulations. Therefore, informed consent of the patients for this specific study was not applicable.
The PHARMO Database Network is a population-based network of healthcare databases and combines data from different healthcare settings in The Netherlands. For this study, the Outpatient Pharmacy Database is used containing drug-dispensing records from community pharmacies. All dispensed drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification (www.whocc.no/atc_ddd_index), and the records include information on type of product, date, dosage, and quantity.
2.2. Study population
All patients diagnosed with a malignancy of the pancreas (classified according to the International Statistical Classification of Diseases and Related Health Problems – 10th revision code C-25) between 1998 and 2011 were selected from the ECR-PHARMO cohort. To reduce confounding by indication, metformin users were, in addition to nonusers, compared with SD users. Excluded were malignancies with the following morphology: squamous cell carcinoma, epithelial carcinoma, cystic/mucinous/serous carcinoma, or gastrointestinal stromal tumor because of the differences in disease course. The in- and excluded morphology codes can be found in supplementary Table 1.
2.3. Exposure
Patients using metformin (ATC-code: A10BA02) or SD (ATC-code: A10BB) for at least 30 days were defined as users. Users were defined as patients using metformin before and after diagnosis, or only after diagnosis, and not using contemporary SD users. Patients who used metformin solely before diagnosis were excluded from this analysis. Nonusers were defined as patients who never used metformin or SD. SD users were defined as patients who used solely SD.
2.4. Analysis
The period of metformin or SD use was defined from the first dispensing of metformin or SD to the end of the follow-up period. Follow-up time was determined from date of diagnosis (T0) until death or end of the study period at 31 December 2012. Cancer registry data were linked to municipal population registries to obtain vital status. To determine time-dependent exposure, patients were defined as nonusers from T0 to the date of first metformin or SD use. Differences in patient characteristics between metformin users and nonusers and between metformin users and SD users were analyzed using the independent samples t-test and the chi square test.
A Kaplan–Meier survival curve was constructed to compare overall survival between patients using metformin, SD, and nonusers. A parametric survival model with exponential (Poisson) distribution was used to model the effect of metformin use on overall survival, where death of any cause was coded as event. Metformin use and SD use were included as time-varying covariates in the model.
Overall survival between metformin users and nonusers was assessed. Adjustments were made for age, number of comorbidities (0, 1, or ≥2, excluding diabetes mellitus), Tumor-Node-Metastasis (TNM) stage (categorical), year of diagnosis (1998–2003, 2004–2007, and 2008–2011), surgery (yes/no), chemotherapy (yes/no), and radiotherapy (yes/no). Information about comorbidities was available for lung disease, cardiovascular disease, diabetes, disorders of the gastrointestinal tract, urinary tract, nervous system, musculoskeletal system, and a group of other comorbidities. Unknown values were taken into account as categorical variables in the multivariable model. The difference between overall survival in metformin users and SD users was analyzed with the same model that was used for the analysis of metformin versus nonusers. The results of the model should be interpreted as a favorable association with survival when the result shows a rate ratio <1 in relation to the comparison group.
All analyses were performed using Stata version 12 statistical software. Statistical tests were 2-sided and considered significant at the P < 0.05 level.
3. Results
In total, 1111 patients with pancreatic cancer were diagnosed in the period 1998–2011; 91 patients were excluded because of morphology (as described above) and 48 patients were excluded because of using merely metformin before diagnosis (Fig. 1, flow chart of the study population). Overall, 57 metformin users ever used contemporary SD and were therefore excluded. Eight patients with a survival of zero months were excluded from the analysis. This resulted in a study population of 907 patients, of which 77 patients used metformin and 43 used SD as drug for diabetes type 2.
Figure 1.
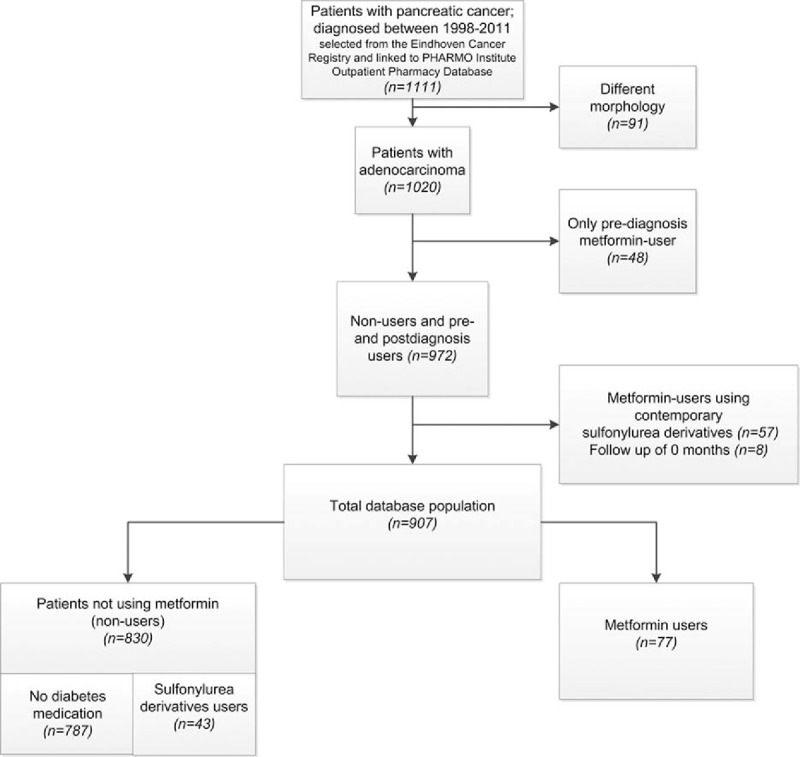
Flowchart of patients selected for analysis.
In total, 863 events were reported. In the metformin group, 64 patients deceased during study period, and in the SD group 41 patients deceased.
Table 1 shows the characteristics of this population. There were no significant differences between the groups concerning TNM stage and treatment (chemotherapy, radiotherapy, and surgery). Metformin users had more additional comorbidities (P < 0.001) compared with both nonusers and SD users. Incidence of diabetes did not differ between the group of metformin users and SD users. Contemporary insulin use was 36% for metformin users versus 33% among patients using SD (P = 0.70). Patients who used metformin were diagnosed in more recent years than SD users or nonusers (P < 0.001). Finally, median survival of metformin users was 5.7 months (interquartile range [IQR]: 2.2–14.7), SD users had a median survival of 6.0 (IQR: 1.6–21.2) months, whereas nonusers had a median survival of 4.0 months (IQR: 1.5–9.2).
Table 1.
Characteristics of the cohort.

Table 2 shows the analysis of overall survival difference between metformin users and nonusers. For all patients with pancreatic cancer, metformin use was associated with an improved overall survival compared with patients not using metformin, rate ratio (RR) 0.76 (95% CI: 0.59–0.98; P = 0.04; Table 2). This association was no longer significant after adjusting for age, number of comorbidities, stage, year of diagnosis, surgery, chemotherapy, and radiotherapy. Multivariable RR for metformin users compared with nonusers was 0.86 (95% CI: 0.66–1.12; P = 0.26).
Table 2.
Time-dependent survival analysis.
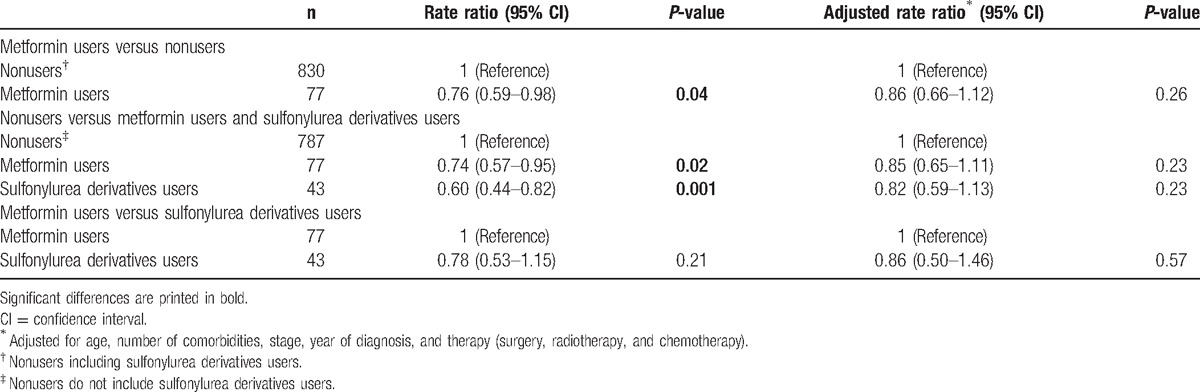
Overall survival was additionally assessed between the following 3 groups: patients not using OGLD, metformin users, and SD users (Fig. 2, Table 2). Overall survival for metformin users versus nonusers was RR 0.74 (95% CI: 0.57–0.95; P = 0.02). After adjusting for potential confounders, the RR for overall survival of metformin users versus nonusers was 0.85 (95% CI: 0.65–1.11; P = 0.23). Overall survival for SD users compared with nonusers was RR = 0.60 (95% CI: 0.44–0.82; P = 0.001), and multivariable analysis showed an RR = 0.82 (95% CI: 0.59–1.13; P = 0.23).
Figure 2.

Kaplan–Meier estimation of survival curves in patients with pancreatic cancer grouped according to medication use.
Lastly, Table 2 shows the comparison of metformin users versus SD users. SD use was not associated with overall survival compared with metformin users. RR was 0.78 (95% CI: 0.53–1.15; P = 0.21) and adjusted RR was 0.86 (95% CI: 0.50–1.46; P = 0.57).
4. Discussion
This retrospective, observational cohort study showed no association between the use of metformin and overall survival in patients with pancreatic cancer.
These results are in concordance with the results of 2 retrospective cohort studies. These studies found no association between the use of metformin and survival in patients with (advanced) pancreatic cancer; however, these studies were only done in respectively 44 and 516 patients with type 2 diabetes mellitus.[24,25] Similarly, 2 recently published randomized controlled trials – carried out in 121 patients in the Netherlands and 60 patients in Italy – also showed no effect of metformin on survival.[26,27] Despite differences in design, such as cancer stage, chemotherapy regime, blinding, and use of placebo, both trials show a consistent no effect of metformin on survival in patients with advanced pancreatic cancer.
The results of the randomized controlled trials and our study are in conflict with numerous other observational studies describing a beneficial effect of metformin, not only in pancreatic cancer.[15,19,28–32] Mortality decrease for patients with pancreatic cancer using metformin was consistently 27%–40% in previous studies; however, these cohorts were smaller than the cohort that was analyzed in this study, with respectively 302, 764, and 349 patients who were analyzed.[28–30] This discrepancy could be partly explained by the difference in methodology.[8] Suissa and Azoulay[22] studied the effect of time-related biases in observational drug studies of metformin on cancer. Authors state that using time-varying techniques prevents misclassification of metformin exposure. There have been several publications claiming to account for these biases. These studies could not demonstrate any association between metformin and cancer incidence or the observed incidence reduction was considerably smaller than previous results.[22,33,34] Another explanation could be the high number of patients with irresectable pancreatic cancer (89%) in our study, whereas other studies found a survival benefit in patients with resectable pancreatic cancer.[29,32]
In addition, differences in patient characteristics could partly explain the results of our study. Patients using SD had less comorbidities than metformin users. Before 2006, the guidelines recommended prescription of metformin for patients with a body mass index (BMI) >27.[35,36] It could therefore be possible that a majority of the patients who were prescribed metformin (35%) before 2006 were overweight. Obese patients have a worse prognosis and overall survival, partly because of a higher risk surgical risk.[37] This observation could also partly explain the observed trend toward a better survival for patients using SD, however no information on BMI was available in the present dataset.
For the direct action of metformin on cancer cells, the effective drug concentrations achieved in neoplastic tissue are crucial.[38] It is possible that the required concentration in the target tissue is not attained with the present dose of metformin. Because of the retrospective nature of this study, no information is available about sufficient concentrations of the effect on tumor cells.[38] The Dutch randomized controlled trial has also addressed this aspect, where an effect on survival was found in a subset of patients reaching adequate insulin level decrease.[26]
Our study has some notable strengths. First, this is one of the largest cohorts so far to analyze the association between the use of metformin and survival in patients with pancreatic cancer. Second, our data are linked through 2 validated databases—ECR and PHARMO—preventing both recall and information bias. Third, this is the first observational study adressing the association between the use of metformin and overall survival in patients with pancreatic cancer, taking into account the time between the beginning of the follow up and the first drug prescription, which prevents time-related biases. Not including the time-varying covariate in our model also revealed a highly significant survival benefit for metformin users. Additionally, metformin users were compared with SD users, a group of patients with a more similar baseline prognosis. Lastly, previous articles focused on the effect of metformin on overall survival limited to patients with pancreatic cancer and type 2 diabetes, whereas this study included all patients with pancreatic cancer.
A limitation to this observational study could be the small number of patients who received OGLD in a large cohort, which also complicated a subgroup analysis, for example, in patients who underwent a resection. Only 43 patients were solely using SD, and because of this small number of users, no robust statements can be made. However, because of the wide interest in a growing field, this remains a relevant study, complementing the existing evidence. Moreover, because of the retrospective nature of the study, the amount of information available is limited. There is no detailed information about the population such as smoking, BMI, glycemic control, or cause of death. It could hypothetically be possible that the nonsignificant relative risk reduction of 15% found in the adjusted analysis was not significant due to a lack of power. However, in the randomized controlled trials recently published, no effect of metformin on survival of patients with pancreatic cancer could be proven, and these trials were of course designed with a power calculation.
This observational study contributes to the mounting evidence against an association between improved survival in patients with pancreatic cancer using metformin.[24,26,27,39] These findings could discourage new trials to be designed for metformin as adjuvant therapy in pancreatic cancer, as this disease is generally discovered in an advanced stage were the anti-tumor effect of metformin will not be able to sufficiently inhibit tumor growth.[40] However, this study does not exclude the opportunity that metformin could be a valuable adjuvant therapy in other cancer types or only in patients with resectable, early stage pancreatic cancer. Nowadays, new oncology drugs are very expensive and drug repurposing is an attractive strategy to offer more effective options for patients with cancer.[41]
Footnotes
Abbreviations: ATC = anatomical therapeutic chemical, ECR = Eindhoven Cancer Registry, IQR = interquartile range, OGLD = oral glucose-lowering drug, RR = rate ratio, SD = sulfonylurea derivatives.
M.H.S. is an employee of the PHARMO Institute for Drug Outcomes Research. This independent research institute performs financially supported studies for government and related health care authorities and for pharmaceutical companies. However, this study was not financially supported by a pharmaceutical company.
The other authors have no conflicts of interest to disclose.
References
- [1].Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Reprint of: Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2015;51:1201–2. [DOI] [PubMed] [Google Scholar]
- [2].Zill OA, Greene C, Sebisanovic D, et al. Cell-free DNA next-generation sequencing in pancreatobiliary carcinomas. Cancer Discov 2015;5:1040–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Malik NK, May KS, Chandrasekhar R, et al. Treatment of locally advanced unresectable pancreatic cancer: a 10-year experience. J Gastrointest Oncol 2012;3:326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].National Comprehensive Cancer Network, Guidelines Pancreatic Adenocarcinoma. Version 2.2015, Guideline 2015, 2015. [Google Scholar]
- [5].Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 1995;273:1605–9. [PubMed] [Google Scholar]
- [6].Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008;134:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Song R. Mechanism of Mmtformin: a tale of two sites. Diabetes Care 2016;39:187–9. [DOI] [PubMed] [Google Scholar]
- [8].Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov 2012;2:778–90. [DOI] [PubMed] [Google Scholar]
- [9].Pernicova I, Korbonits M. Metformin—mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol 2014;10:143–56. [DOI] [PubMed] [Google Scholar]
- [10].Martin M, Marais R. Metformin: a diabetes drug for cancer, or a cancer drug for diabetics? J Clin Oncol 2012;30:2698–700. [DOI] [PubMed] [Google Scholar]
- [11].Kisfalvi K, Eibl G, Sinnett-Smith J, et al. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res 2009;69:6539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cifarelli V, Lashinger LM, Devlin KL, et al. Metformin and rapamycin reduce pancreatic cancer growth in obese prediabetic mice by distinct microRNA-regulated mechanisms. Diabetes 2015;64:1632–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009;32:1620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Landman GW, Kleefstra N, van Hateren KJ, et al. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care 2010;33:322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Currie CJ, Poole CD, Jenkins-Jones S, et al. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012;35:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wu L, Zhu J, Prokop LJ, et al. Pharmacologic therapy of diabetes and overall cancer risk and mortality: a meta-analysis of 265 studies. Sci Rep 2015;5:10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Noto H, Goto A, Tsujimoto T, et al. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One 2012;7:e33411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Soranna D, Scotti L, Zambon A, et al. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist 2012;17:813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ye XF, Wang J, Shi WT, et al. Relationship between aspirin use after diagnosis of colorectal cancer and patient survival: a meta-analysis of observational studies. Br J Cancer 2014;111:2172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee MS, Hsu CC, Wahlqvist ML, et al. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang P, Li H, Tan X, et al. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol 2013;37:207–18. [DOI] [PubMed] [Google Scholar]
- [22].Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 2012;35:2665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].van Herk-Sukel MP, van de Poll-Franse LV, Lemmens VE, et al. New opportunities for drug outcomes research in cancer patients: the linkage of the Eindhoven Cancer Registry and the PHARMO Record Linkage System. Eur J Cancer 2010;46:395–404. [DOI] [PubMed] [Google Scholar]
- [24].Hwang AL, Haynes K, Hwang WT, et al. Metformin and survival in pancreatic cancer: a retrospective cohort study. Pancreas 2013;42:1054–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ambe CM, Mahipal A, Fulp J, et al. Effect of metformin use on survival in resectable pancreatic cancer: a single-institution experience and review of the literature. PLoS One 2016;11:e0151632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kordes S, Pollak MN, Zwinderman AH, et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 2015;16:839–47. [DOI] [PubMed] [Google Scholar]
- [27].Reni M, Dugnani E, Cereda S, et al. (Ir)relevance of metformin treatment in patients with metastatic pancreatic cancer: an open-label, randomized phase 2 trial. Clin Cancer Res 2015;22:1076–85. [DOI] [PubMed] [Google Scholar]
- [28].Sadeghi N, Abbruzzese JL, Yeung SC, et al. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res 2012;18:2905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jo A, Kim Y, Kang S, et al. The effect of metformin use and mortality among those with pancreatic cancer and type 2 diabetes mellitus: findings from a Nationwide Population Retrospective Cohort Study. Value Health 2015;18:A439. [Google Scholar]
- [30].Choi Y, Kim TY, Oh DY, et al. The impact of diabetes mellitus and metformin treatment on survival of patients with advanced pancreatic cancer undergoing chemotherapy. Cancer Res Treat 2015;48:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Franciosi M, Lucisano G, Lapice E, et al. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One 2013;8:e71583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kozak MM, Anderson EM, von Eyben R, et al. Statin and metformin use prolongs survival in patients with resectable pancreatic cancer. Pancreas 2016;45:64–70. [DOI] [PubMed] [Google Scholar]
- [33].Kowall B, Stang A, Rathmann W, et al. No reduced risk of overall, colorectal, lung, breast, and prostate cancer with metformin therapy in diabetic patients: database analyses from Germany and the UK. Pharmacoepidemiol Drug Saf 2015;24:865–74. [DOI] [PubMed] [Google Scholar]
- [34].Gandini S, Puntoni M, Heckman-Stoddard BM, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res 2014;7:867–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bouma M, Rutten GE, de Grauw WJ, et al. Summary of the practice guideline ‘Diabetes mellitus type 2’ (second revision) from the Dutch College of General Practitioners. Ned Tijdschr Geneeskd 2006;150:2251–6. [PubMed] [Google Scholar]
- [36].Wiersma TJ, Heine RJ, Rutten GE. [Summary of the practice guideline ’Diabetes mellitus type 2’ (first revision) of the Dutch College of General Practitioners]. Ned Tijdschr Geneeskd 1999;143:1688–91. [PubMed] [Google Scholar]
- [37].Noun R, Riachy E, Ghorra C, et al. The impact of obesity on surgical outcome after pancreaticoduodenectomy. JOP 2008;9:468–76. [PubMed] [Google Scholar]
- [38].Christensen MM, Brasch-Andersen C, Green H, et al. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genomics 2011;21:837–50. [DOI] [PubMed] [Google Scholar]
- [39].Suissa S, Azoulay L. Metformin and cancer: mounting evidence against an association. Diabetes Care 2014;37:1786–8. [DOI] [PubMed] [Google Scholar]
- [40].Yang YX, Rustgi AK. Impact of metformin on advanced pancreatic cancer survival: too little, too late? Clin Cancer Res 2015;22:1031–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bertolini F, Sukhatme VP, Bouche G. Drug repurposing in oncology-patient and health systems opportunities. Nat Rev Clin Oncol 2015;12:732–42. [DOI] [PubMed] [Google Scholar]


