Supplemental Digital Content is available in the text
Keywords: anesthetics, anti-inflammatory, xenon
Abstract
Background:
The goal of this study was to investigate the effects of pulmonary static inflation with 50% xenon on postoperative oxygen impairment during cardiopulmonary bypass (CPB) for Stanford type A acute aortic dissection (AAD).
Methods:
This prospective single-center nonrandomized controlled clinical trial included 100 adult patients undergoing surgery for Stanford type A AAD at an academic hospital in China. Fifty subjects underwent pulmonary static inflation with 50% oxygen from January 2013 to January 2014, and 50 underwent inflation with 50% xenon from January 2014 to December 2014. During CPB, the lungs were inflated with either 50% xenon (xenon group) or 50% oxygen (control group) to maintain an airway pressure of 5 cm H2O. The primary outcome was oxygenation index (OI) value after intubation, and 10 minutes and 6 hours after the operation. The second outcome was cytokine and reactive oxygen species levels after intubation and 10 minutes, 6 hours, and 24 hours after the operation.
Results:
Patients treated with xenon had lower OI levels compared to the control group before surgery (P = 0.002); however, there was no difference in postoperative values between the 2 groups. Following surgery, mean maximal OI values decreased by 18.8% and 33.8%, respectively, in the xenon and control groups. After surgery, the levels of interleukin-6 (IL-6), tumor necrosis factor alpha, and thromboxane B2 decreased by 23.5%, 9.1%, and 30.2%, respectively, in the xenon group, but increased by 10.8%, 26.2%, and 26.4%, respectively, in the control group. Moreover, IL-10 levels increased by 28% in the xenon group and decreased by 7.5% in the control group. There were significant time and treatment-time interaction effects on methane dicarboxylic aldehyde (P = 0.000 and P = 0.050, respectively) and myeloperoxidase (P = 0.000 and P = 0.001 in xenon and control groups, respectively). There was no difference in hospital mortality and 1-year survival rate between the 2 groups.
Conclusion:
Pulmonary static inflation with 50% xenon during CPB could attenuate OI decreases at the end of surgery for Stanford type A AAD. Thus, xenon may function by triggering anti-inflammatory responses and suppressing pro-inflammatory and oxidative effects.
1. Introduction
Acute lung injury (ALI) after acute aortic surgery with deep hypothermic circulatory arrest (DHCA) is a serious clinical problem, despite refinements in surgical techniques, avoidance of extracorporeal circulation, and intensive postoperative care. This condition affects postoperative morbidity and mortality and can result in prolonged ventilator support, extended hospitalization and intensive care unit stay, and increases in treatment costs. However, few proven effective preventative or therapeutic interventions for ALI exist. The pathogenesis of ALI after thoracic aortic surgery, particularly in patients who have undergone acute aortic dissection (AAD), is complex and remains unknown. However, studies have indicated that ischemia/reperfusion and inflammation are likely to be underlying risk factors.[1–4] Reactive oxygen species (ROS) and inflammatory factors may cause injury to endothelial cells and increase microvascular permeability, thereby resulting in lung injury with variable degrees of clinical manifestations.[5,6] When treated with a specific neutrophil elastase inhibitor, patients with severe postoperative pulmonary dysfunction following aortic surgery with deep hypothermia had improved outcomes.[7] Therefore, reducing inflammation might be a potential therapeutic strategy for treating ALI.
Xenon has been recognized as an ideal anesthetic for hemodynamic stability[8] and anti-inflammatory effects.[9] It can enhance lipopolysaccharide-induced interleukin-1 beta (IL-1β) expression in microglia by activating extracellular signal-regulated kinase 1/2,[10] inhibiting caspase-3 activation and cytochrome c release from cells,[11] and reducing serum levels of the pro-inflammatory cytokines, such as IL-1, IL-6, and tumor necrosis factor alpha (TNFα) in rats.[12,13] In addition, in recent years, it was shown that xenon had neuroprotective, cardioprotective, and renoprotective effects in different animal models subjected to preconditioning, real-time conditioning, and postconditioning.[14–17] However, the effects of xenon on respiratory function in patients with Stanford type A AAD during cardiopulmonary bypass (CPB) with DHCA remain unclear.
ALI is a mild form of oxygen impairment (oxygenation index [OI] ≤300 mm Hg, positive end-expiratory pressure [PEEP] ≥5 cm H2O) that results in the development of bilateral infiltrates, as can be seen on chest radiographs, but does not cause cardiac failure.[18] In this study, we demonstrated that the potential effects of pulmonary static inflation with 50% xenon during CPB could attenuate postoperative oxygen impairment and improve pulmonary function in patients with Stanford type A AAD following CPB/DHCA.
2. Methods
2.1. Study design
We studied the perioperative clinical variables and serological results from 50 subjects who underwent pulmonary static inflation with 50% oxygen from January 2013 to January 2014, and 50 subjects who underwent pulmonary static inflation with 50% xenon from January 2014 to December 2014 during CPB for Stanford type A AAD in a single-center clinical trial. We were unable to blind researchers to treatments received by the patients, but the patients, inspectors of serum sample, and statistician were blinded to treatments. The clinical trial was registered in the Chinese Clinical Trial Registry (Identifier: ChiCTR-ICR-15006435) and was approved by the Beijing Anzhen Hospital Clinical Research Ethics Committee (Identifier: 2013027). The diagnosis of AAD was confirmed in all of the patients by medical history, chest radiography, transthoracic ultrasound, and contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). Dissection is considered AAD if the time from the onset of symptoms to surgery is 14 days or less. This study only included type A AAD according to the Stanford classification.[19,20] Medical quality 50% xenon was provided by WuHan Newradar Special Gas Co. Ltd (WuHan, China).
2.2. Subjects
Patients between the ages of 18 and 70 years, who were undergoing emergency surgery in our hospital for type A AAD, requiring CPB and DHCA, were enrolled. Exclusion criteria were coronary heart disease; heart failure; severe cardiac tamponade; unstable hemodynamics; nervous system abnormalities; clinically apparent malperfusion,[21] including malperfusion in the lower limb, brain, heart, and kidney; visceral ischemia; and patients who did not provide written informed consent. No patient was prescribed nonsteroidal anti-inflammatory drugs or corticosteroids before or after admission. After written informed consent, 100 patients were blinded to receiving either 50% xenon (xenon group, n = 50) or 50% oxygen (control group, n = 50).
2.3. Trial procedure
Data such as demographics and hemodynamics were recorded immediately after admission. To avoid aortic rupture, systolic blood pressure was controlled between 100 and 120 mm Hg with oral β-blockers and infusion of vasodilators (e.g., nitroprusside) before surgery. In all of the cases, total intravenous anesthesia was performed with a combination of sufentanil and propofol, and the bispectral index (BIS) was maintained at 45% to 55%. After the aorta was cross-clamped and mechanical ventilation was stopped, the lungs were inflated with either 50% xenon (xenon group) or 50% oxygen (control group) to maintain an airway pressure of 5 cm H2O. Pulmonary static inflation with 50% xenon or with 50% oxygen was stopped 15 minutes before aortic de-clamping and cardiac resuscitation. Pulmonary parameters were recorded before the operation, and 10 minutes and 6 hours postoperatively. The OI, respiratory index (RI), and dynamic compliance (Cdyn)/static pulmonary compliance (Cst) were calculated based on a fraction of inspired oxygen (FiO2) of 100%, a PEEP of 5 cm H2O, a tidal volume of 8 mL/kg, an inspiratory/expiratory ratio of 1:2, and a respiratory rate of 10 times/min. All of the patients were followed prospectively with a telephone interview or in our outpatient clinic at 3 and 6 months after cardiac surgery.
2.4. Outcome
The primary outcome was OI value after intubation and 10 minutes and 6 hours after the operation. The second outcome was cytokine and ROS levels after intubation and 10 minutes, 6 hours, and 24 hours after the operation, as well as in-hospital mortality and 1-year survival.
2.5. Cardiac operation
All of the patients underwent CPB with DHCA and unilateral selective antegrade cerebral perfusion (USACP). When the nasopharyngeal temperature reached 20°C to 25°C, circulatory arrest was instituted, a stent graft was implanted, and a 4-branch prosthetic graft was used for arch replacement in the operation. Valve-sparing root resection (aortic valve plasty, Valsalva sinus plasty, or the David procedure) or composite valve graft replacement was performed according to aortic root subtype (subtype A1, A2, or A3).
2.6. Definitions
The perioperative pulmonary function was represented by OI, RI, Cdyn, and Cst.
OI = PaO2/FiO2;
RI = P(A-a)O2/PaO2;
Cdyn (mL/cmH2O) = Vt (tidal volume)/(Ppeak-PEEP); and
Cst (mL/mH2O) = Vt/(Pplat-PEEP)
Hospital mortality included death during hospitalization and death within 30 days of the operation.
2.7. Blood samples
Venous blood (10 mL) was drawn from the central vein catheter immediately after intubation and 10 minutes, 6 hours, and 24 hours after the operation. After rapid centrifugation, plasma was obtained and immediately stored at −70°C for further analysis.
2.8. Enzyme-linked immunosorbent assay
Cytokine and thromboxane B2 (TXB2) concentrations in the plasma were assessed in all of the patients using commercially available enzyme-linked immunosorbent assay (ELISA) kits for TNFα, IL-6, IL-10, and TXB2. Assays were done in duplicate on a microtiter plate reader at a wavelength of 450 nm. Values are reported as pg/mL of the standardized sample (MULTISKAN MK3 automatic microplate reader, Thermo Fisher Scientific; Westminster, MD).
2.9. ROS measurement
Methane dicarboxylic aldehyde (MDA), myeloperoxidase (MPO), total antioxidation capacity (TAOC), and total superoxide dismutase (TSOD) were evaluated by ultraviolet spectrophotometry (UV-2000 UV Spectrophotometer, Unico Instrument Co., Ltd; Shanghai, China).
2.10. Statistical analysis
The sample size was selected to detect differences in postoperative OI values between the 2 groups. With a 5% (2-sided) type I error rate, based on preliminary experiments, 45 patients per treatment group were needed to detect a 22% difference between treatment groups, assuming a standardized effect size of 0.51 (expected effect size divided by standard deviation [SD] of OI). The power was from 73% to 78% as an autocorrelation from 0.6 to 0.5 accordingly. Assuming a 10% dropout rate, the final sample size was 100 patients (50 patients/group) (Power Analysis and Sample Size, PASS V11.0, NCSS, LLC; Kaysville, UT). All of the data analyses were performed using a commercially available statistical software package (SPSS for Windows, V.18.0; Chicago, IL). Quantitative variables were presented as mean ± SD or median (interquartile range [IQR]), and categorical variables were presented as frequencies or percentages. The normally distributed continued variables were compared by the 2-tailed Student's t test. When parametric data were not normally distributed, Wilcoxon signed-rank sum tests were used for intergroup comparison. The categorical data were compared using Pearson's chi-squared test or Fisher's exact, as appropriate. Correlations were assessed using Pearson correlation or Spearman's rank correlation. OI was analyzed using repeated measures analysis of variance (ANOVA) with treatment and control as grouping factors and time as a within-subject factor. Perioperative RI, Cdyn, Cst, IL-6, IL-10, TNF, TXB2, values of ROS (MDA, MPO, TSOD, TAOC), perioperative hemodynamics, and detection of serum biomarkers (i.e., white blood cells [WBCs], hemoglobin [Hb], platelet [PLT], glutamine [Glu], and lactate [LAC]) were examined in a similar way. Nonparametric and parametric tests were applied to demographics (age, sex, body mass index [BMI], smoking history, hypertension, diabetes), clinical variables (hemodynamics, onset of symptoms to surgery, aortic diameter, left ventricular ejection fraction [LVEF], left ventricular end diastolic diameter (LVEDd), EuroSCORE), surgical variables (surgery time, duration of surgery, intravenous colloid and crystalloid and transfusion), and postoperative factors (intravenous fluids [colloid or crystalloid], blood transfusions, urine volume, seroma drainage, mechanical ventilation time, in-hospital mortality). The Kaplan–Meier method was used to estimate the survival time of patients from the date of operation to last follow-up or death and to demonstrate the effects of xenon on survival at 1 year. Patient data were censored at the date when the patient was last known to be alive. A log-rank test was used to evaluate survival differences between groups. The missing values were less than 10% and were displaced by means according to intention-to-treat rules. P values less than 0.05 were considered statistically significant.
3. Results
A total of 100 patients undergoing Stanford type A AAD in our hospital were screened for eligibility; 50 patients received 50% xenon and 50 received 50% oxygen. All of the patients fulfilled the evaluation criteria. There were no significant differences between groups regarding patient demographics, pre-operative cardiac function and hemodynamics, operative data, time to extubation, or in-hospital mortality (Tables 1 and 2). More patients in the xenon group needed their blood pressure to be controlled before surgery (P = 0.009) by oral β-blockers and/or infusion of vasodilators. The median EuroSCORE value was 5 (5, 6) in the xenon group and 5 (5, 5) in the control group (P = 0.021) prior to surgery.
Table 1.
Subject demographics. Data are given in numbers, percentage, mean ± standard deviation, and median (interquartile range, IQR).

Table 2.
Subject perioperative characteristics. Data are given as numbers, percentage, mean ± standard deviation, or median (interquartile range, IQR).

3.1. Effects of xenon on OI and RI values
Patients treated with xenon had lower OI and RI values than the control group before surgery (P = 0.002 and P = 0.004, respectively); however, there was no difference in postoperative values between the 2 groups (Table 3). Postoperative OI values decreased in both groups during the study period, with significant time (P < 0.001) and treatment effects (P = 0.012), but without a significant difference in the treatment–time interaction (P = 0.129) (Appendix Fig. 2A). In the first fraction (from preoperative to postoperative 10 minutes), there was a mean maximal decrease in OI of 18.8% and 33.8% in the xenon and control groups, respectively, with a significant difference between groups (P = 0.021). In paired comparisons, the median RI value in the first fraction increased (P = 0.005 for the xenon group; P = 0.000 for the control group) by 34.5% and 130% in the xenon and control groups, respectively, with a significant difference between groups (P = 0.000) (Appendix Fig. 2B). Perioperative Cdyn and Cst values were similar in both groups. There was a significant time effect for Cdyn (P = 0.002), but no effect of time or treatment for Cst (Appendix Fig. 2C and D).
Table 3.
Subject intraoperative and postoperative data concerning lung function.

3.2. Effects of xenon on inflammatory mediators
Patients treated with xenon had higher levels of IL-6 and TXB2 compared to the control group before surgery (P = 0.000, respectively). Moreover, postoperative 6 and 24 hours, xenon patients had slightly higher serum IL-10 levels (P = 0.008 and P = 0.001, respectively). At postoperative 10 minutes and 24 hours, TXB2 levels were lower in the xenon group than in the control group (P = 0.001 and P = 0.019, respectively). There were significant time and treatment-time effects for IL-6 (P = 0.000 and P = 0.000, respectively), IL-10 (P = 0.000 and P = 0.001, respectively), TNFα (P = 0.012 and P = 0.025, respectively), and TXB2 (P = 0.000 and P = 0.001, respectively) (Appendix Fig. 3A–D). In the first fraction, IL-6, TNFα, and TXB2 levels decreased by 23.5%, 9.1%, and 30.2%, respectively, in the xenon group, but levels increased by 10.8%, 26.2%, and 26.4%, respectively, in the control group. In the first fraction, IL-10 expression increased by 28% in the xenon group and decreased by 7.5% in the control group. In the second (postoperative 10 minutes to postoperative 6 hours) and third fractions (postoperative 6 to 24 hours), IL-6, IL-10, TNFα, and TXB2 levels were similar in both groups. In addition, the perioperative serum level of prostaglandin I2 (PGI2) was relatively stable, with no significant differences between groups. These data are summarized in Table 4.
Table 4.
Subject intraoperative and postoperative data regarding hemodynamics, cytokines, and reactive oxygen species.

3.3. Effects of xenon on ROS levels
Xenon patients had higher serum levels of MDA and MPO (P = 0.002 and P = 0.005 respectively) before surgery, and higher serum TSOD levels towards the end of follow-up (treatment effect, P = 0.000). There was a significant time and treatment–time interaction effect for MDA (P = 0.000 and P = 0.050, respectively) and MPO (P = 0.000 and P = 0.001, respectively) (Appendix Fig. 4A and B). The perioperative value of TAOC and TSOD increased in both groups during the study period, with a significant time effect (P = 0.000 and P = 0.000, respectively), but without a significant difference in treatment–time interaction effects (P = 0.757 and P = 0.134, respectively) (Appendix Fig. 4C and D). These data are summarized in Table 4.
3.4. Effects of xenon on survival
Among the 100 subjects, 9 died after surgery in the hospital and 9 patients were lost to follow-up. A complete 12-month follow-up was performed in the remaining 82 patients discharged from the hospital during outpatient clinic visits and/or by telephone. In the control group, 5 patients died in the hospital (2 by perioperative hemorrhage and 3 by severe multiple organ failure) and 1 died due to gastrointestinal hemorrhage after 3 months. In the xenon group, 4 patients died in the hospital because of severe multiple organ failure and 1 died due to ventricular fibrillation after 6 months. The Kaplan–Meier survival curve showed that there was no difference in survival rates at 1 year between the 2 groups (86.7 ± 5.1% in the control group; 89.1 ± 4.6 in the xenon group, P = 0.954) (Fig. 1).
Figure 1.
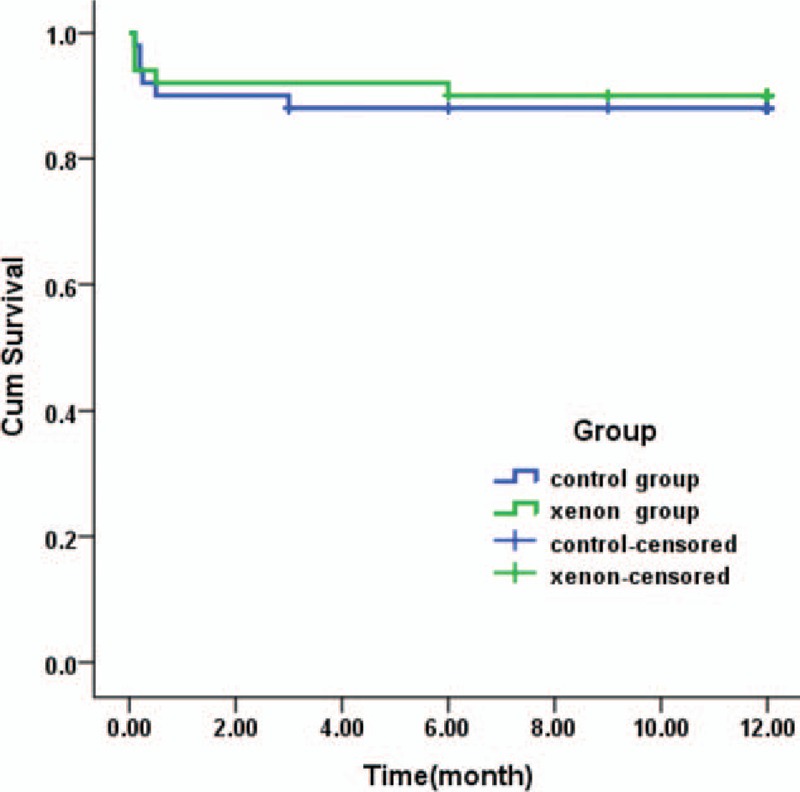
Kaplan–Meier curves showing 1 year survival of subjects requiring 50% xenon inflation of the lung during CPB vs patients who received 50% oxygen. CPB = cardiopulmonary bypass.
3.5. Effects of xenon on additional variables
There was no overall treatment effect between groups regarding hemodynamics, WBC and PLT counts, and Glu and LAC levels. However, time-dependent changes in WBC and PLT counts, and blood concentrations of Glu and LAC were seen in both groups.
4. Discussion
This is the first study to highlight the effects of pulmonary static inflation with 50% xenon on postoperative oxygen impairment during CPB for treatment of Stanford type A AAD. Although perioperative OI and RI values were not significantly different between the xenon and control groups, xenon treatment attenuated the decreased OI value and increased RI at varying degrees at the end of surgery. Our data also demonstrate that pulmonary static inflation with 50% xenon during CPB triggered anti-inflammatory responses and suppressed pro-inflammatory and oxidative effects compared to pulmonary static inflation with 50% oxygen in the complex clinical setting of AAD surgery. However, the exposure time with 50% xenon was approximately 100 minutes in our study, and there was limited impact on lung protection, which may account for the lack of difference in OI values and inflammation 6 hours after the operation between groups.
The mechanisms underlying AAD are complex,[19,22,23] and aortic dissection is the end stage of different pathological processes that stress the aortic wall. Recent studies have shown that inflammation plays an important role in aortic dissection.[24–26] After the onset of AAD, proinflammatory cytokines are secreted from leukocytes and infiltrate the aortic wall, causing a systemic inflammatory response. The pulmonary microcirculation is a natural reservoir for leukocytes,[27] which leads to the accumulation of cytokines, toxic mediators, and alveolar fluid.[28] Inflammatory responses and ischemia/reperfusion injury occurs during cardiac surgery requiring CPB.[29] Thus, the 2-hit hypothesis of ALI indicates that the initial insult by AAD and a second insult by the CPB result in an exaggerated response after surgery.
Recently, a randomized controlled clinical trial demonstrated the feasibility and safety of balancing xenon anesthesia for coronary artery bypass graft surgery during CPB[30]; however, the mechanisms underlying xenon function remain unknown. According to the 2-hit hypothesis of ALI, xenon plays an important role in postconditioning for the first-bit and pre-conditioning for the second-bit. Furthermore, the combination of xenon and mild hypothermia seems to be synergistic for neuroprotection.[31–33] Other studies have been conducted showing that atelectasis is produced to a much larger extent after CPB, resulting in increases of intrapulmonary shunting and postoperative hypoxia.[34,35] Thus, positive airway pressure methods during CPB, such as pulmonary static inflation, improve postoperative gas exchange variables.[36] The possibility that xenon insufflation simply prevented alveolar collapse better than oxygen, is quite likely because solubility, and therefore, absorption is very limited as opposed to oxygen. Finally, pulmonary static inflation with 50% xenon was processed during the cooling period in DHCA, which enhanced the properties of xenon and improved lung function after surgery.
Higher serum proinflammatory cytokine levels indicate higher morbidity in preoperative ALI in AAD,[4,37] whereas the primary biological function of serum IL-10 is to attenuate inflammatory responses and prevent impairment of endothelial dysfunction.[38] TXB2 is the stable metabolite of TXA2,[39,40] a pro-inflammatory mediator. After the onset of AAD, the production of TXB2 by aggregated PLTs was increased and further activated by surgery. In this study, we compared the inflammatory profile between the xenon and control groups. Interestingly, we found that IL-6, TNFα, and TXB2 levels were significantly reduced and anti-inflammatory IL-10 levels were significantly increased after 50% xenon inflation compared to 50% oxygen inflation during CPB. Measuring the serum levels of MDA, MPO, TAOC, and TSOD is a reliable method for assessing the balance between pro-oxidants and antioxidants in the blood. Higher levels of MDA and MPO are indicative of oxidative stress. In our study, when compared to baseline, MDA and MPO levels decreased in the xenon group and increased in the control group 10 minutes postoperatively, demonstrating that xenon treatment attenuates oxidative stress.[41]
Previous experiments have demonstrated that xenon can induce impairment of neural function after CPB combined with cerebral air embolism in rats.[42] However, Casey and colleagues[43] reported that xenon does not generate more gaseous microemboli when compared to air or oxygen in an in vitro CPB circuit. In a clinical trial for delivering xenon to patients undergoing coronary artery bypass graft surgery during CPB, there were no clinical neurologic injuries and no differences in serum levels of S100-β and total number of emboli or temporal distribution between the 2 groups.[30] During anesthesia, CPB, and surgery, the generation of intravenous gas bubbles was limited. In addition, in our study, up to 1–2 L xenon was used per patient for pulmonary static inflation, only a little of which was delivered into the gas inflow of the CPB circuit. Moreover, the xenon bubbles were completely reabsorbed in the absence of a xenon source in the range of 10 to 15 minutes.[44] Thus, we discontinued xenon delivery before 15 minutes of de-clamping to avoid undesirable effects of xenon.
By the retrospective examination, 78.49% of patients undergoing treatment for Stanford type A AAD were found to have pre-operative hypoxemia, and only 70% of patients underwent the surgical procedure within 48 hours whereas it was within 21 days for the others.[3] The method of pulmonary static inflation with 50% xenon during CBP is easy to operate and effectively attenuates decreased OI and increased RI at the end of surgery, which may save more lives.
4.1. Study limitations
The present study had several limitations. One major limitation of this study was that it was not a randomized clinical trial, and as such, was susceptible to certain types of bias. In addition, it was conducted at only 1 university center, the number of cases was small, and the patients were followed up for a relatively short period time. Therefore, it may not have been adequately powered for the measurements performed. Furthermore, because only subjects with Stanford type A AAD were included, biased effects due to the variable effects of AAD-related medication cannot be excluded, although they are not expected. Thus, differences between groups regarding baseline cytokines levels have to be regarded as a reactivity of the variable extent of AAD, which was unknown at grouping. Moreover, the noble gas xenon was only administered during the cooling period of CPB. This was a short time period, so any potential advantages of xenon with respect to organ protection characteristics could have been masked during surgery or with postoperative interventions. Finally, although 50% xenon was used to inflate pulmonary pressure to 5 cm H2O during CPB, the effective intra-alveolar concentration of xenon was not assessed. Additional studies are needed to evaluate the relationship between this concentration and inhaled xenon concentration during CPB.
5. Conclusions
Pulmonary static inflation with 50% xenon during CPB attenuated the decreased OI and increased RI values at the end of surgery for Stanford type A AAD. The mechanism underlying the role of xenon function may be to trigger anti-inflammatory responses and suppress pro-inflammatory and oxidative effects. However, the advantages of xenon could have been underestimated due to limited exposure time.
Acknowledgments
The authors thank Prof. Jing Liu from the Department of Epidemiology, Beijing Anzhen Hospital, Capital Medical University, Beijing Institute of Heart Lung and Blood Vessel Diseases, Beijing, China, for his kind assistance during the statistical analyses and for his editorial contributions.
Supplementary Material
Footnotes
Abbreviations: AAD = acute aortic dissection, ALI = acute lung injury, CPB = cardiopulmonary bypass, CT = contrast-enhanced computed tomography, DHCA = deep hypothermic circulatory arrest, ERK = extracellular signal-regulated kinase, FiO2 = inspired oxygen fraction, IL-10 = interleukin-10, IL-6 = interleukin-6, ITT = intention-to-treat, MDA = methane dicarboxylic aldehyde, MPO = myeloperoxidase, MRI = magnetic resonance imaging, OI = oxygenation index, PaO2 = arterial oxygen tension, PEEP = positive end-expiratory pressure, PETCO2 = end-tidal carbon dioxide pressure, RI = respiratory index, ROS = oxygen species, ROS = reactive oxygen species, SD = standard deviation, TAOC = total antioxidation capacity, TNFα = tumor necrosis factor alpha, TSOD = total superoxide dismutase, TXB2 = thromboxane B2.
Clinical trial Registration. Chinese Clinical Trial Registry (http://www.chictr.org.cn). Identifier: ChiCTR-ICR-15006435.
Authorship: MJ, JL, ZZ, and WC contributed to the concept, design, data analysis, and writing. YY and XP performed the serum measurement and follow-up studies. All of the authors critically revised the manuscript and have approved the final version.
Funding: The study was supported by grants from the Beijing Municipal Science & Technology Commission (Nos. Z141107002514135, Z151100004015133, Z161100000513067), the Research Special Fund for Public Welfare Industry of Health (No. 201402009), and the National Key Technology R&D Program (NO. Z141107002514031). The funding institution had no influence on the design, analysis, or publication of this study.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Han S, Mallampalli RK. The acute respiratory distress syndrome: from mechanism to translation. J Immunol 2015;194:855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Luo F, Zhou XL, Li JJ, et al. Inflammatory response is associated with aortic dissection. Ageing Res Rev 2009;8:31–5. [DOI] [PubMed] [Google Scholar]
- [3].Wang Y, Xue S, Zhu H. Risk factors for postoperative hypoxemia in patients undergoing Stanford A aortic dissection surgery. J Cardiothorac Surg 2013;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Komukai K, Shibata T, Mochizuki S. C-reactive protein is related to impaired oxygenation in patients with acute aortic dissection. Int Heart J 2005;46:795–9. [DOI] [PubMed] [Google Scholar]
- [5].Apostolakis E, Filos KS, Koletsis E, et al. Lung dysfunction following cardiopulmonary bypass. J Cardiac Surg 2010;25:47–55. [DOI] [PubMed] [Google Scholar]
- [6].Hasegawa Y, Ishikawa S, Ohtaki A, et al. Impaired lung oxygenation in acute aortic dissection. J Cardiovasc Surg 1999;40:191–5. [PubMed] [Google Scholar]
- [7].Morimoto K, Nishimura K, Miyasaka S, et al. The effect of sivelestat sodium hydrate on severe respiratory failure after thoracic aortic surgery with deep hypothermia. Ann Thorac Cardiovasc Surg 2011;17:369–75. [DOI] [PubMed] [Google Scholar]
- [8].Law LS, Lo EA, Gan TJ. Xenon anesthesia: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg 2016;122:678–97. [DOI] [PubMed] [Google Scholar]
- [9].Winkler DA, Thornton A, Farjot G, et al. The diverse biological properties of the chemically inert noble gases. Pharmacol Ther 2016;160:44–64. [DOI] [PubMed] [Google Scholar]
- [10].Fahlenkamp AV, Coburn M, Haase H, et al. Xenon enhances LPS-induced IL-1beta expression in microglia via the extracellular signal-regulated kinase 1/2 pathway. J Mol Neurosci 2011;45:48–59. [DOI] [PubMed] [Google Scholar]
- [11].Spaggiari S, Kepp O, Rello-Varona S, et al. Antiapoptotic activity of argon and xenon. Cell Cycle (Georgetown, TX) 2013;12:2636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhao H, Watts HR, Chong M, et al. Xenon treatment protects against cold ischemia associated delayed graft function and prolongs graft survival in rats. Am J Transplant 2013;13:2006–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhao H, Luo X, Zhou Z, et al. Early treatment with xenon protects against the cold ischemia associated with chronic allograft nephropathy in rats. Kidney Int 2014;85:112–23. [DOI] [PubMed] [Google Scholar]
- [14].Maze M. Preclinical neuroprotective actions of xenon and possible implications for human therapeutics: a narrative review. Can J Anaesth 2016;63:212–26. [DOI] [PubMed] [Google Scholar]
- [15].Lavaur J, Lemaire M, Pype J, et al. Neuroprotective and neurorestorative potential of xenon. Cell Death Dis 2016;7:e2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].De Deken J, Rex S, Monbaliu D, et al. The efficacy of noble gases in the attenuation of ischemia reperfusion injury: a systematic review and meta-analyses. Crit Care Med 2016;44:e886–896. [DOI] [PubMed] [Google Scholar]
- [17].Roehl AB, Funcke S, Becker MM, et al. Xenon and isoflurane reduce left ventricular remodeling after myocardial infarction in the rat. Anesthesiology 2013;118:1385–94. [DOI] [PubMed] [Google Scholar]
- [18].Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526–33. [DOI] [PubMed] [Google Scholar]
- [19].Golledge J, Eagle KA. Acute aortic dissection. Lancet 2008;372:55–66. [DOI] [PubMed] [Google Scholar]
- [20].Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation 2003;108:628–35. [DOI] [PubMed] [Google Scholar]
- [21].Yan TD, Tian DH, Lemaire SA, et al. Standardizing clinical end points in aortic arch surgery: a consensus statement from the International Aortic Arch Surgery Study Group. Circulation 2014;129:1610–6. [DOI] [PubMed] [Google Scholar]
- [22].Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J 2012;33:26–35b. [DOI] [PubMed] [Google Scholar]
- [23].Wu D, Shen YH, Russell L, et al. Molecular mechanisms of thoracic aortic dissection. J Surg Res 2013;184:907–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ranasinghe AM, Bonser RS. Biomarkers in acute aortic dissection and other aortic syndromes. J Am Coll Cardiol 2010;56:1535–41. [DOI] [PubMed] [Google Scholar]
- [25].Wen D, Du X, Dong JZ, et al. Value of D-dimer and C reactive protein in predicting in hospital death in acute aortic dissection. Heart 2013;99:1192–7. [DOI] [PubMed] [Google Scholar]
- [26].Del Porto F, di Gioia C, Tritapepe L, et al. The multitasking role of macrophages in Stanford type A acute aortic dissection. Cardiology 2014;127:123–9. [DOI] [PubMed] [Google Scholar]
- [27].Kuebler WM, Goetz AE. The marginated pool. Eur Surg Res 2002;34:92–100. [DOI] [PubMed] [Google Scholar]
- [28].Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–49. [DOI] [PubMed] [Google Scholar]
- [29].Engels M, Bilgic E, Pinto A, et al. A cardiopulmonary bypass with deep hypothermic circulatory arrest rat model for the investigation of the systemic inflammation response and induced organ damage. J Inflamm (London) 2014;11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lockwood GG, Franks NP, Downie NA, et al. Feasibility and safety of delivering xenon to patients undergoing coronary artery bypass graft surgery while on cardiopulmonary bypass: phase I study. Anesthesiology 2006;104:458–65. [DOI] [PubMed] [Google Scholar]
- [31].Fries M, Brucken A, Cizen A, et al. Combining xenon and mild therapeutic hypothermia preserves neurological function after prolonged cardiac arrest in pigs. Crit Care Med 2012;40:1297–303. [DOI] [PubMed] [Google Scholar]
- [32].Lobo N, Yang B, Rizvi M, et al. Hypothermia and xenon: novel noble guardians in hypoxic-ischemic encephalopathy? J Neurosci Res 2013;91:473–8. [DOI] [PubMed] [Google Scholar]
- [33].Saravanan P, Exley AR, Valchanov K, et al. Impact of xenon anaesthesia in isolated cardiopulmonary bypass on very early leucocyte and platelet activation and clearance: a randomized, controlled study. Br J Anaesth 2009;103:805–10. [DOI] [PubMed] [Google Scholar]
- [34].Magnusson L, Zemgulis V, Wicky S, et al. Atelectasis is a major cause of hypoxemia and shunt after cardiopulmonary bypass: an experimental study. Anesthesiology 1997;87:1153–63. [DOI] [PubMed] [Google Scholar]
- [35].Rodrigues RR, Sawada AY, Rouby JJ, et al. Computed tomography assessment of lung structure in patients undergoing cardiac surgery with cardiopulmonary bypass. Braz J Med Biol Res 2011;44:598–605. [DOI] [PubMed] [Google Scholar]
- [36].Loeckinger A, Kleinsasser A, Lindner KH, et al. Continuous positive airway pressure at 10 cm H(2)O during cardiopulmonary bypass improves postoperative gas exchange. Anesth Analg 2000;91:522–7. [DOI] [PubMed] [Google Scholar]
- [37].Girdauskas E, Kuntze T, Borger MA, et al. Acute respiratory dysfunction after surgery for acute type A aortic dissection. Eur J Cardiothorac Surg 2010;37:691–6. [DOI] [PubMed] [Google Scholar]
- [38].Shao Y, Cheng Z, Li X, et al. Immunosuppressive/anti-inflammatory cytokines directly and indirectly inhibit endothelial dysfunction- a novel mechanism for maintaining vascular function. J Hematol Oncol 2014;7:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fontana P, Zufferey A, Daali Y, et al. Antiplatelet therapy: targeting the TxA2 pathway. J Cardiovasc Translat Res 2014;7:29–38. [DOI] [PubMed] [Google Scholar]
- [40].Vieira-de-Abreu A, Campbell RA, Weyrich AS, et al. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin Immunopathol 2012;34:5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhao H, Huang H, Ologunde R, et al. Xenon treatment protects against remote lung injury after kidney transplantation in rats. Anesthesiology 2015;122:1312–26. [DOI] [PubMed] [Google Scholar]
- [42].Jungwirth B, Gordan ML, Blobner M, et al. Xenon impairs neurocognitive and histologic outcome after cardiopulmonary bypass combined with cerebral air embolism in rats. Anesthesiology 2006;104:770–6. [DOI] [PubMed] [Google Scholar]
- [43].Casey ND, Chandler J, Gifford D, et al. Microbubble production in an in vitro cardiopulmonary bypass circuit ventilated with xenon. Perfusion 2005;20:145–50. [DOI] [PubMed] [Google Scholar]
- [44].Sta Maria N, Eckmann DM. Model predictions of gas embolism growth and reabsorption during xenon anesthesia. Anesthesiology 2003;99:638–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


