Abstract
The potential value of N-terminal pro-brain natriuretic peptide (NT-proBNP) for contrast-induced acute kidney injury (CI-AKI) in patients with heart failure and mid-range ejection fraction (HFmrEF) is unclear. We investigated whether NT-proBNP is associated with CI-AKI and long-term mortality following elective cardiac catheterization in patients with HFmrEF.
A total of 174 consecutive patients with HFmrEF undergoing elective coronary angiography or intervention were enrolled. The primary endpoint was the development of CI-AKI, defined as an absolute increase of ≥0.3 mg/dL or ≥ 50% from baseline serum creatinine with 48 hours after contrast medium exposure. Receiver-operating characteristic curve analysis was conducted, and Youden index was used to determine the best cutoff NT-proBNP value. Multivariable logistic regression and Cox proportional hazards regression analyses were performed to identify the independent risk factors for CI-AKI and long-term mortality, respectively.
The incidence of CI-AKI was 12.1%. Patients with CI-AKI had higher NT-proBNP values than those without (4373[1561.9–7470.5] vs 1303[625.2–2482.3], P = 0.003). Receiver-operating characteristic curve revealed that NT-proBNP was not significantly different from the Mehran risk score in predicting CI-AKI (area under the curve [AUC] = 0.723 vs 0.767, P = 0.516). The best cutoff NT-proBNP value for CI-AKI was 3299 pg/mL, with 70.6% sensitivity and 83.1% specificity. Multivariable analysis demonstrated that NT-proBNP ≥3299 pg/mL is significantly related to CI-AKI (odds ratio = 12.79; 95% confidence interval, 3.18–51.49; P < 0.001). Cox regression analysis showed that NT-proBNP ≥3299 pg/mL is associated with long-term mortality (adjusted hazard ratio = 11.91; 95%CI, 2.16–65.70; P = 0.004) during follow-up.
In patients with HFmrEF, NT-proBNP ≥3299 pg/mL is associated with CI-AKI and long-term mortality following elective coronary angiography or intervention.
Keywords: contrast-induced acute kidney injury, heart failure and mid-range ejection fraction, mortality, N-terminal pro-brain natriuretic peptide
1. Introduction
Contrast-induced acute kidney injury (CI-AKI) is a common and serious complication of coronary angiography and percutaneous coronary intervention (PCI), which significantly prolongs hospitalization and increases the risk of cardiovascular events and long-term mortality.[1,2] However, as effective treatment for CI-AKI is lacking,[3] risk identification is essential to ensuring that high-risk patients receive appropriate prophylactic measures.[4,5]
The Mehran risk score is a classical risk assessment model for CI-AKI.[6] However, of its 8 variables, those reflecting cardiac function (hypotension, use of intraaortic balloon pump, and New York Heart Association [NYHA] class III/IV heart failure [HF]) do not discriminate between more moderate degrees of cardiac dysfunction such as NYHA class II or relatively normal left ventricular ejection fraction (LVEF), both of which are common in clinical practice.[7] The 2016 European Society of Cardiology guideline for HF highlighted that more attention should be paid to the patients with heart failure and mid-range ejection fraction (HFmrEF; LVEF 40%–49%),[8] whose cumulative prevalence ranges from 10% to 20%[9] and in whom the incidence of acute kidney injury is higher than in HF with severely reduced ejection fraction.[10] Serum creatinine (SCr) level has poor predictive accuracy and is a relatively late marker for renal injury[11]; thus, identification of an early and simple biomarker for CI-AKI risk is essential, particularly for patients with HFmrEF.
N-terminal pro-brain natriuretic peptide (NT-proBNP) is a biomarker of cardiac and renal function. Previous studies and our group's analysis have demonstrated its predictive value for CI-AKI in patients with unselected cardiac function undergoing PCI.[12–14] However, its potential value for CI-AKI and long-term mortality in patients with HFmrEF remains unclear. Therefore, this analysis aimed to identify the association of NT-proBNP with CI-AKI and long-term mortality in patients with HFmrEF undergoing elective coronary angiography or intervention.
2. Methods
2.1. Ethics statement
This study was performed according to the Declaration of Helsinki and the ethics committee of Guangdong General Hospital approved the study protocol. Written informed consent was obtained from the patients involved in the study.
2.2. Study population
This prospective observational analysis is a substudy of Predictive Value of Contrast Volume to Creatinine Clearance Ratio (PRECOMIN, ClinicalTrials.gov Identifier: NCT01400295) and a previous study,[15] which was conducted at Guangdong General Hospital in April 2009 to December 2013. Based on the protocol, patients aged more than 18 years old with LVEF 40% to 49%, NYHA class II–IV, and undergoing elective coronary angiography or intervention were enrolled. Patients who were receiving peritoneal or hemodialysis treatment, with severe heart valve disease or malignancy, underwent cardiovascular surgery, and pregnant were excluded. Moreover, patients who received treatment for nephrotoxicity (eg, glucocorticoids, aminoglycosides) or nephroprotective drugs (eg, N-acetylcysteine) and were exposed to contrast medium (CM) within the perioperative period were excluded.
2.3. Laboratory data collection
NT-proBNP was measured using chemoluminescence immunoassay (Roche Diagnostics, Germany) before the procedure. The detection range was from 5 to 35,000 pg/mL. SCr levels were measured at admission, 24 and 48 hours after contrast administration. The estimated glomerular filtration rate (eGFR) was calculated according to the Modification of Diet in Renal Disease equation.[16] Other biochemical indicators, such as hemoglobin (Hb) A1c, lipid profile, and Hb level, were measured in the morning prior to the procedure. In addition, LVEF was measured by echocardiography examination.
2.4. Cardiac catheterization
Cardiac catheterization was performed according to the standard clinical practice, employing the standard technique, by experienced interventional cardiologists. PCI was performed for patients with significant anatomic (≥50% left main or ≥75% nonleft main coronary artery disease) or physiological coronary artery stenosis (fractional flow reserve ≤0.80). The type of stents was selected according to the interventional cardiologists and the culprit vessels. Nonionic, low-osmolality CM was used for all patients. Patients were hydrated with normal saline 2 to 12 hours preoperatively and 6 to 12 hours postoperatively. Medical therapy for secondary prevention of coronary heart disease was administered according to the patients’ condition.
2.5. End points and follow-up
The primary end-point of this study was the development of CI-AKI, defined as an absolute increase of ≥0.3 mg/dL or ≥50% from baseline SCr level within 48 hours after CM exposure. Additional end-points included all-cause mortality, renal replacement therapy, nonfatal myocardial infarction, revascularization and stroke during in-hospital, and the all-cause mortality during the follow-up.
The patients included in this study were followed up by telephone or office visits at 1, 6, 12, and 24 months after discharge. Adverse events were recorded on the case report form.
2.6. Statistical analysis
All statistical analyses were performed with SPSS software version 22.0 (SPSS Inc, Chicago, IL). Continuous variables were described as mean ± standard deviation or median (interquartile range), and analyzed using Student t test or Wilcoxon rank-sum test. Categorical variables were described as absolute values and percentages, and analyzed by the χ2 test or Fisher exact test. NT-proBNP values were transformed into logarithms since the distribution is skewed. Receiver-operating characteristic curve analysis was conducted, and Youden index was used to determine the best cutoff NT-proBNP value for predicting CI-AKI. The area under the curve (AUC) values between the NT-proBNP and Mehran score were compared by MedCalc statistical software (MedCalc Software, version 11.4, Mariakerke, Belgium). CI-AKI incidence in the higher and lower NT-proBNP values was compared with that in the best cutoff value. Multivariable logistic regression and Cox proportional hazards regression analyses were performed to identify the independent risk factors for CI-AKI and long-term mortality, respectively. Kaplan–Meier method was used to describe the all-cause mortality by log-rank tests. A 2-tailed P < 0.05 was considered statistically significant.
3. Results
3.1. Clinical characteristics and in-hospital events
A total of 174 patients with HFmrEF undergoing elective coronary angiography or intervention were included in the study (mean age 64.7 ± 10.7 years, mean NT-proBNP 1448 [727.8, 3186.5] pg/mL, mean LVEF 44.2% ± 3.0%, and mean eGFR 71.3 ± 23.8 mL/min/1.73 m2), of which 21 patients (12.1%) developed CI-AKI.
The characteristics of patients are listed in Table 1. Compared with patients without CI-AKI, patients with CI-AKI had a lower baseline eGFR and LVEF, were more likely to have chronic kidney disease (CKD; eGFR < 60 mL/min/1.73 m2) and anemia, and had a higher HbA1c level. Furthermore, NT-proBNP and Mehran risk score were higher in patients with CI-AKI. However, demographics, medical history, feature of coronary artery, and perioperative medications were similar.
Table 1.
Baseline characteristic of patients with and without contrast-induced acute kidney injury.
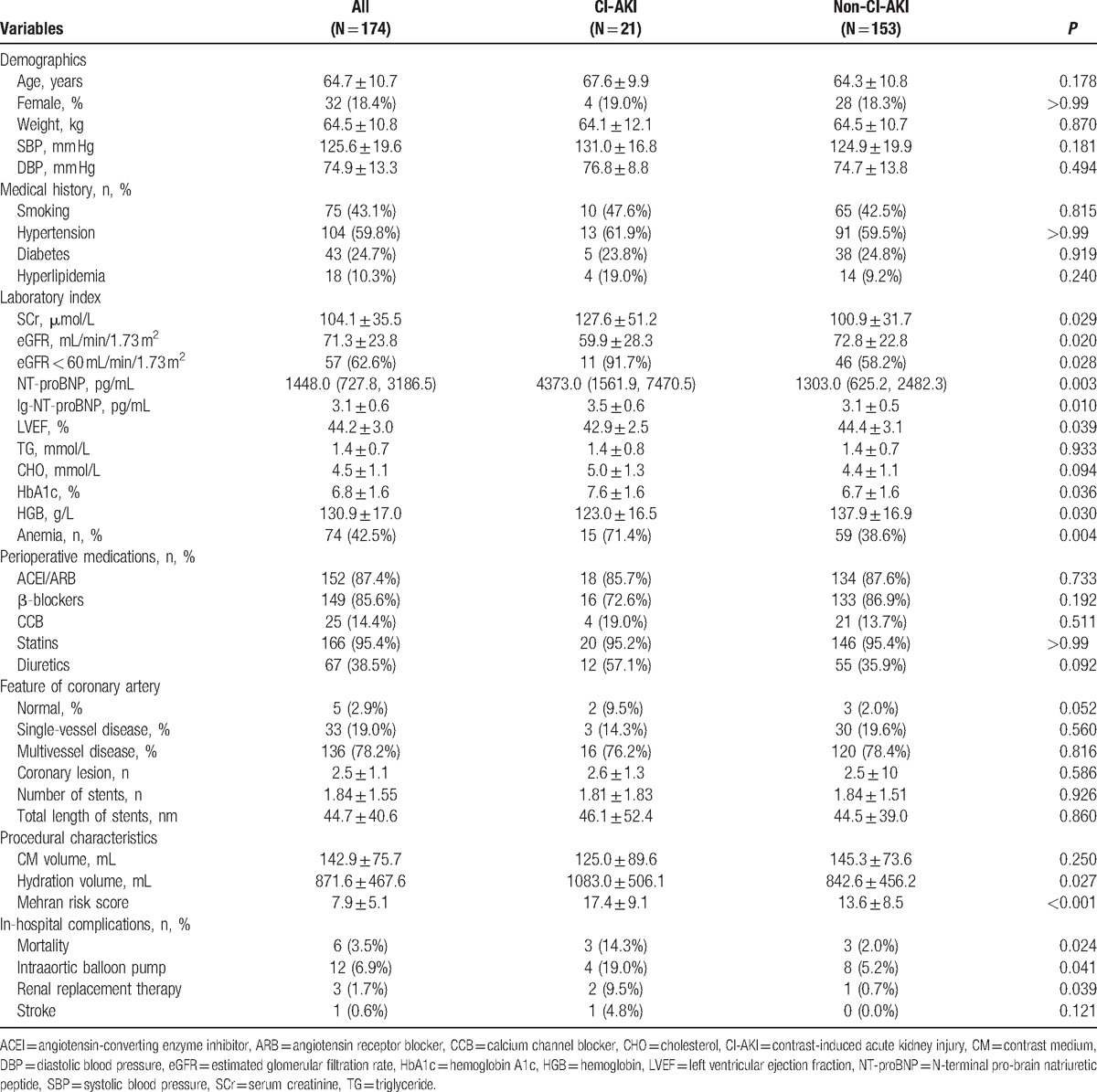
Moreover, patients with CI-AKI had a significantly higher rate of in-hospital mortality (14.3% vs 2.0%, P = 0.024), requirement of intraaortic balloon pump (19.0% vs 5.2%, P = 0.041), and renal replacement therapy (9.5% vs 0.7%, P = 0.039) when compared with patients without CI-AKI (Table 1).
3.2. Association between NT-proBNP and CI-AKI
Receiver-operating characteristic analysis indicated that the AUC for CI-AKI was 0.723 (95%CI: 0.642–0.795). The Youden index indicated that the best cutoff value of NT-proBNP for CI-AKI was 3299 pg/mL (lg-NT-proBNP: 3.52 pg/mL), with 70.6% sensitivity and 83.1% specificity (Fig. 1). Furthermore, NT-proBNP was not significantly different from Mehran risk score (AUC = 0.723 vs 0.767, P = 0.516). Moreover, CI-AKI incidence was significantly higher in patients with NT-proBNP ≥3299 pg/mL (36.4% vs 4.6%, P < 0.001) (Fig. 2).
Figure 1.

The ROC curve of NT-proBNP for CI-AKI. AUC = area under the curve, CI-AKI = contrast-induced acute kidney injury, NT-proBNP = N-terminal pro-brain natriuretic peptide, ROC = receiver operating characteristic.
Figure 2.
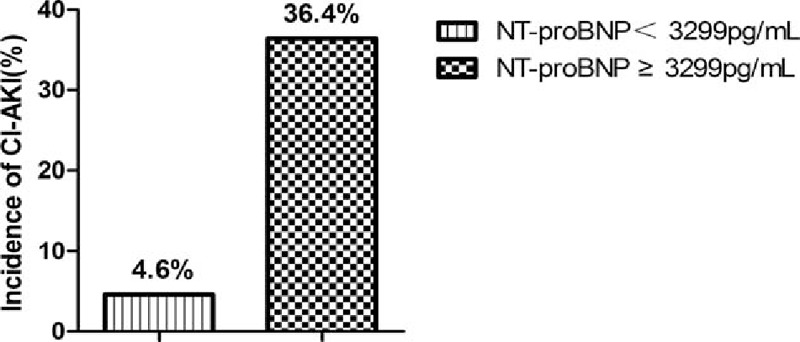
CI-AKI incidence based on the cutoff value of NT-proBNP. CI-AKI = contrast-induced acute kidney injury, NT-proBNP = N-terminal pro-brain natriuretic peptide.
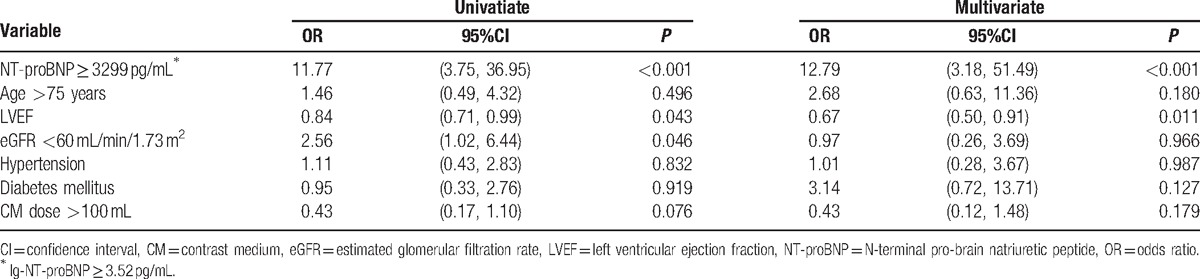
In a univariate logistic regression analysis, NT-proBNP ≥3299 pg/mL was significantly associated with CI-AKI (odds ratio [OR] = 11.77, 95%CI, 3.75–36.95, P < 0.001). Furthermore, LVEF and eGFR < 60 mL/min/1.73 m2 were found to be significant variables. Multivariate logistic regression results revealed that NT-proBNP ≥3299 pg/mL was still related to CI-AKI (OR = 12.79, 95%CI, 3.18–51.49, P < 0.001) after adjustment for potential confounding factors (Table 2).
Table 2.
Univariate and multivariate logistic association for contrast-induced acute kidney injury.
3.3. NT-proBNP value for long-term mortality
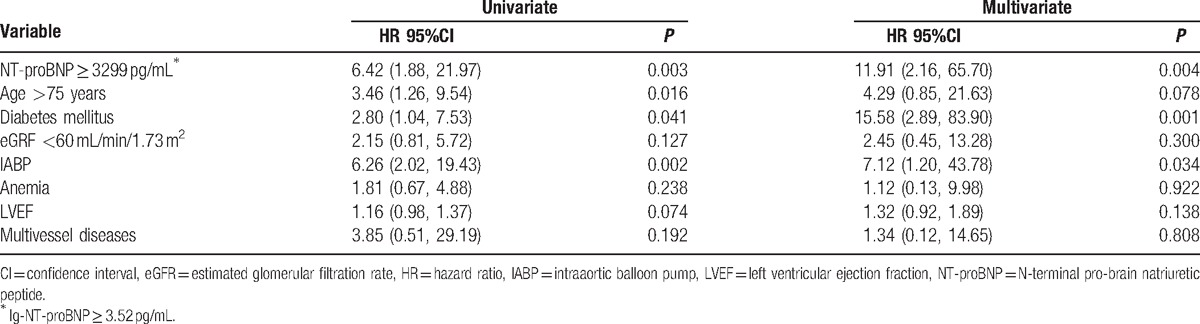
During a mean follow-up of 21.4 months, 11 deaths were reported. Kaplan–Meier analysis indicated that higher NT-proBNP values were associated with higher mortality rate (cumulative all-cause mortality: 28.0% vs 4.3%, P < 0.001) (Fig. 3). After adjusting for the confounders, including age >75 years, diabetes, eGFR < 60 mL/min/1.73 m2, intraaortic balloon pump, anemia, LVEF, and multivessel diseases, multivariate Cox regression showed that NT-proBNP ≥3299 pg/mL remains significantly associated with the long-term mortality (hazard ratio = 11.91, 95%CI, 2.16–65.70, P = 0.004) (Table 3).
Figure 3.
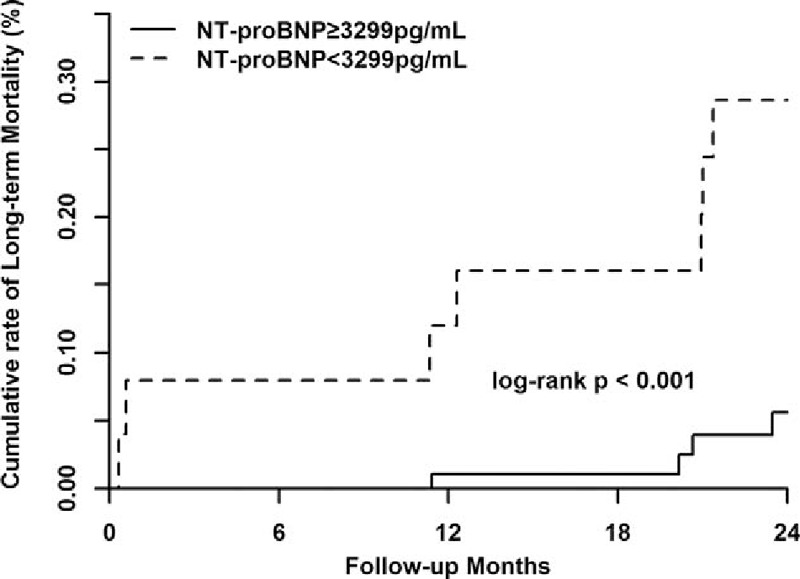
Association between NT-proBNP and long-term mortality. NT-proBNP = N-terminal pro-brain natriuretic peptide.
Table 3.
Multivariate Cox analysis: independent predictors of long-term mortality.
4. Discussion
To our knowledge, this is the 1st study to investigate the preoperative value of NT-proBNP, as a simple and useful biomarker, for CI-AKI and long-term mortality in patients with HFmrEF undergoing elective coronary angiography or intervention. Our data showed that in patients with HFmrEF, NT-proBNP ≥3299 pg/mL is associated with the CI-AKI and long-term mortality following elective coronary angiography or intervention.
Heart failure with reduced ejection fraction (HFrEF; LVEF <40%) is a known risk factor for CI-AKI.[17] In contrast, patients with HFmrEF may receive less attention than those with HFrEF, despite typically being older and thus more likely to have comorbid risk for CI-AKI, such as hypertension, diabetes, anemia, and renal insufficiency.[18] Furthermore, recent studies indicate that the CI-AKI incidence in patients with LVEF ≥40% is 5.2% to 7.8%,[19,20] ranging up to 12.0% in the subgroup with CKD.[20] However, these studies included patients with unselected cardiac function and it is well established that, the incidence of renal insufficiency and other adverse events is higher in patients with HF than in those without HF.[9] In this study, the incidence of CI-AKI was 12.1% in all patients and 19.3% in patients with CKD, consistent with the higher risk associated with HFmrEF. Similar to previous studies, patients with CI-AKI had poor outcomes.[1] Therefore, effective preprocedural identification of patients at high-risk of CI-AKI is vital.
NT-proBNP is a widely available and useful biomarker for triage and diagnosis of patients with dyspnea.[21] This biomarker is associated not only with systolic and diastolic HF,[22] but also with advanced age, CKD, anemia, and diabetes,[23–25] all of which have been included in the Mehran risk score for CI-AKI.[6] In this study, the NT-proBNP value and Mehran risk score were higher in patients with CI-AKI (P < 0.01). Furthermore, the predictive value of NT-proBNP was similar to that of Mehran risk score (P = 0.561). However, the Mehran risk score includes 8 variables; some of which are present only in a minority of critically unwell patients (use of IABP), or can only be determined postprocedure (CM volume). In contrast, NT-proBNP appears to be a promising simple and sensitive preoperative biomarker of CI-AKI risk in patients undergoing elective coronary angiography or interventions.
Our findings are consistent with previous work associating NT-proBNP with the development of CI-AKI. A substudy of HORIZONS-AMI,[14] including a total of 979 patients, found an independent predictive value of brain natriuretic peptide (BNP) for CI-AKI after PCI. Similarly, Moltrasio et al[26] and Akgul et al[27] also identified BNP level at admission as being associated with CI-AKI and its severity. Although BNP level correlates with NT-proBNP level, NT-proBNP levels are more stable and sensitive than BNP levels because of a longer half-life.[28] A recent large prospective analysis by Goussot et al[12] further confirmed the value of NT-proBNP level for CI-AKI. However, all of these studies included patients with acute coronary syndrome, low LVEF, and non-HF. Therefore, the results cannot be extended to patients with HFmrEF undergoing elective procedures. In contrast, our present study is the 1st study to analyze the association between NT-proBNP and CI-AKI in this common patient group.
Few studies have examined the best NT-proBNP cutoff value for predicting CI-AKI. An observational study by Kurtul et al[29] including 436 patients with acute coronary syndrome demonstrated that preoperative NT-proBNP ≥2149 pg/mL was an independent predictor of CI-AKI after CM exposure. However, a total of 101(23.17%) patients with LVEF <40% were included, and patients with NYHA class ≥III were excluded in the analysis. Two more studies conducted by our group also indicated the best cutoff value of NT-proBNP for CI-AKI[13,20]; however, the number of patients with HFmrEF was limited in these studies. Therefore, the present results are the useful supplements to this work.
The physiopathology of NT-proBNP and CI-AKI remains incompletely understood. Nevertheless, several mechanisms might be involved in the process. Pressure or volume overload and myocardial ischemia or infarction increase NT-proBNP level,[28,30] which inhibits myocardial contractility, reduces cardiac output, and affects hemodynamics. Therefore, higher NT-proBNP level in HF patients may contribute to renal hypoperfusion and kidney injury. Renal hypoperfusion also triggers renin–angiotensin system and sympathetic nervous system activation, which plays a significant role in the development of CI-AKI.[31] In contrast, NT-proBNP can oppose this neurohormonal activation through vasodilation, natriuresis, and diuresis.[32] A high NT-proBNP level thus reflects adverse hemodynamic parameters, myocardial dysfunction, and activation of the neurohormonal system, all of which potentiate CI-AKI. It is likely that it is this position at the center of the cardiac-renal axis contributes to NT-proBNPs utility as a predictor of CI-AKI.
Finally, previous studies have indicated that the elevated NT-proBNP is strongly associated with poor long-term outcomes in patients with acute coronary syndrome and HF.[33,34] Similarly, our study demonstrated that NT-proBNP ≥3299 pg/mL is associated with long-term mortality. Therefore, early identification of patients at high risk of CI-AKI and long-term mortality by NT-proBNP may assist in directing treatment and resource use where it will be of greatest benefit.
5. Limitations
We acknowledge several limitations of our study. First, this prospective observational study was conducted at a single center and included a relatively small number of patients with HFmrEF. Second, the NT-proBNP level was measured in the clinical laboratory, with a detection range from 5 to 35,000 pg/mL. NT-proBNP level can also be measured by a point-of-care testing device, with a detection range from 60 to 9000 pg/mL. Therefore, the conclusions cannot be extended to point-of-care testing devices. Third, as the NT-proBNP level was not measured during the follow-up, we were unable to identify the association between temporal change in NT-proBNP and outcomes.
6. Conclusion
Our experience is consistent with other studies indicating that the elevated NT-proBNP, a simple and useful biomarker, is associated with the CI-AKI and long-term mortality in patients with HFmrEF following elective coronary angiography or intervention. In this population, NT-proBNP ≥3299 pg/mL was the best cut-off value, with 70.6% sensitivity and 83.1% specificity for CI-AKI, and was significantly associated with long-term mortality. The utility of NT-proBNP, both alone and in concert with CI-AKI risk models, warrants further evaluation in large-scale multicenter clinical trials.
Acknowledgments
The authors thank the efforts of statistical consultant, An Fan, MD, Guangzhou, 510515 Guangdong, China.
Footnotes
Abbreviations: AUC = area under the curve, CI-AKI = contrast-induced acute kidney injury, CKD = chronic kidney disease, CM = contrast medium, eGFR = estimated glomerular filtration rate, HF = heart failure, HFmrEF = heart failure and mid-range ejection fraction, LVEF = left ventricular ejection fraction, NT-proBNP = N-terminal pro-brain natriuretic peptide, NYHA = New York Heart Association, PCI = percutaneous coronary intervention, SCr = serum creatinine.
KW, H-lL, L-lC, and W-jB contributed equally to this work.
Funding/support: This study was supported by Guangdong Provincial Cardiovascular Clinical Medicine Research Fund (Grant no: 2009X41 to YL and NT), Science and Technology Planning Project of Guangdong Province (PRECOMIN study to YL in 2011 and study grant no. 2008A030201002 to JY-C), and Guangdong Cardiovascular Institute; and also supported by Progress of Science and Technology Project in Guangdong Province (Grant number: 2013b031800025, 2016b020215130).
The authors have no conflicts of interest to disclose.
References
- [1].Tsai TT, Patel UD, Chang TI, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv 2014;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Parfrey P. The clinical epidemiology of contrast-induced nephropathy. Cardiovasc Intervent Radiol 2005;28(Suppl 2):S3–11. [DOI] [PubMed] [Google Scholar]
- [3].Sudarsky D, Nikolsky E. Contrast-induced nephropathy in interventional cardiology. Int J Nephrol Renovasc Dis 2011;4:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 2011;21:2527–41. [DOI] [PubMed] [Google Scholar]
- [5].Stacul F. Reducing the risks for contrast-induced nephropathy. Cardiovasc Intervent Radiol 2005;28(Suppl 2):S12–8. [DOI] [PubMed] [Google Scholar]
- [6].Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention. J Am Coll Cardiol 2004;44:1393–9. [DOI] [PubMed] [Google Scholar]
- [7].Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J 2006;151:444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;w128. [Google Scholar]
- [9].Lam CSP, Solomon SD. The middle child in heart failure: heart failure with mid-range ejection fraction (40–50%). Eur J Heart Fail 2014;16:1049–55. [DOI] [PubMed] [Google Scholar]
- [10].Sweitzer NK, Lopatin M, Yancy CW, et al. Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am J Cardiol 2008;101:1151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dennen P, Parikh CR. Biomarkers of acute kidney injury: can we replace serum creatinine? Clin Nephrol 2007;68:269–78. [DOI] [PubMed] [Google Scholar]
- [12].Goussot S, Mousson C, Guenancia C, et al. N-Terminal fragment of Pro B-type natriuretic peptide as a marker of contrast-induced nephropathy after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am J Cardiol 2015;116:865–71. [DOI] [PubMed] [Google Scholar]
- [13].Liu Y, He YT, Tan N, et al. Preprocedural N-terminal pro-brain natriuretic peptide (NT-proBNP) is similar to the Mehran Contrast-Induced Nephropathy (CIN) Score in predicting CIN following elective coronary angiography. J Am Heart Assoc 2015;4:e1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jarai R, Dangas G, Huber K, et al. B-type natriuretic peptide and risk of contrast-induced acute kidney injury in acute ST-segment-elevation myocardial infarction: a substudy from the HORIZONS-AMI trial. Cir Cardiovasc Interv 2012;5:813–20. [DOI] [PubMed] [Google Scholar]
- [15].Tan N, Liu Y, Zhou YL, et al. Contrast medium volume to creatinine clearance ratio: a predictor of contrast-induced nephropathy in the first 72 hours following percutaneous coronary intervention. Catheter Cardiovasc Interv 2012;79:70–5. [DOI] [PubMed] [Google Scholar]
- [16].Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–70. [DOI] [PubMed] [Google Scholar]
- [17].Rosenstock JL, Gilles E, Geller AB, et al. Impact of heart failure on the incidence of contrast-induced nephropathy in patients with chronic kidney disease. Int Urol Nephrol 2010;42:1049–54. [DOI] [PubMed] [Google Scholar]
- [18].Lam CS, Teng TH. Understanding heart failure with mid-range ejection fraction. JACC Heart Fail 2016;4:473–6. [DOI] [PubMed] [Google Scholar]
- [19].Koo HM, Doh FM, Ko KI, et al. Diastolic dysfunction is associated with an increased risk of contrast-induced nephropathy: a retrospective cohort study. BMC Nephrol 2013;14:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu YH, Liu Y, Zhou YL, et al. Association of N-terminal pro-B-type natriuretic peptide with contrast-induced nephropathy and long-term outcomes in patients with chronic kidney disease and relative preserved left ventricular function. Medicine (Baltimore) 2015;94:e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Christ M, Mueller C. Use of natriuretic peptide assay in dyspnea. Dtsch Arztebl Int 2008;105:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nishikimi T. Do plasma levels of brain natriuretic peptide (BNP) and N-Terminal proBNP (NT-proBNP) increase in diastolic dysfunction as well as in systolic dysfunction? Cir J 2012;76:2540–1. [DOI] [PubMed] [Google Scholar]
- [23].Matsumoto M, Tsujino T, Naito Y, et al. Anemia as a factor that elevates plasma brain natriuretic peptide concentration in apparently healthy subjects. Int Heart J 2008;49:577–86. [DOI] [PubMed] [Google Scholar]
- [24].Su H. Levels of B-type natriuretic peptide in chronic heart failure patients with and without diabetes mellitus. Exp Ther Med 2012;5:229–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schaub JA, Coca SG, Moledina DG, et al. Amino-terminal Pro-B-type natriuretic peptide for diagnosis and prognosis in patients with renal dysfunction: a systematic review and meta-analysis. JACC Heart Fail 2015;3:977–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Moltrasio M, Cabiati A, Milazzo V, et al. B-type natriuretic peptide and risk of acute kidney injury in patients hospitalized with acute coronary syndromes∗. Crit Care Med 2014;42:619–24. [DOI] [PubMed] [Google Scholar]
- [27].Akgul O, Uyarel H, Pusuroglu H, et al. High BNP level as risk factor for acute kidney injury and predictor of all-cause mortality in STEMI patients. Herz 2014;39:507–14. [DOI] [PubMed] [Google Scholar]
- [28].Liang J, Dietz B, Stokes N. Cardiopulmonary laboratory biomarkers in the evaluation of acute dyspnea. Open Access Emer Med 2016;8:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kurtul A, Duran M, Yarlioglues M, et al. Association between N-terminal pro-brain natriuretic peptide levels and contrast-induced nephropathy in patients undergoing percutaneous coronary intervention for acute coronary syndrome. Clin Cardiol 2014;37:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Weber M, Dill T, Arnold R, et al. N-terminal B-type natriuretic peptide predicts extent of coronary artery disease and ischemia in patients with stable angina pectoris. Am Heart J 2004;148:612–20. [DOI] [PubMed] [Google Scholar]
- [31].Azzalini L, Spagnoli V, Ly HQ. Contrast-induced nephropathy: from pathophysiology to preventive strategies. Can J Cardiol 2016;32:247–55. [DOI] [PubMed] [Google Scholar]
- [32].Hall C. Essential biochemistry and physiology of (NT-pro)BNP. Eur J Heart Fail 2004;6:257–60. [DOI] [PubMed] [Google Scholar]
- [33].Kragelund C, Grønning B, Køber L. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. ACC Curr J Rev 2005;14:22–3. [DOI] [PubMed] [Google Scholar]
- [34].Huang YT, Tseng YT, Chu TW, et al. N-terminal pro b-type natriuretic peptide (NT-pro-BNP)-based score can predict in-hospital mortality in patients with heart failure. Sci Rep 2016;6:29590. [DOI] [PMC free article] [PubMed] [Google Scholar]


