Abstract
Both sleep disorders and sarcopenia are common among older adults. However, little is known about the relationship between these 2 conditions.
This study aimed to investigate the possible association between sleep duration and sarcopenia in a population of Chinese community-dwelling older adults.
Community-dwelling older adults aged 60 years or older were recruited. Self-reported sleep duration, anthropometric data, gait speed, and handgrip strength were collected by face-to-face interviews. Sarcopenia was defined according to the recommended algorithm of the Asian Working Group for Sarcopenia (AWGS).
We included 607 participants aged 70.6 ± 6.6 years (range, 60–90 years) in the analyses. The prevalence of sarcopenia in the whole study population was 18.5%. In women, the prevalence of sarcopenia was significantly higher in the short sleep duration group (< 6 hours) and long sleep duration group (>8 hours) compared with women in the normal sleep duration group (6–8 hours; 27.5%, 22.2% and 13.9%, respectively; P = .014). Similar results were found in men; however, the differences between groups were not statistically significant (18.5%, 20.6%, and 13.0%, respectively; P = .356). After adjustments for the potential confounding factors, older women having short sleep duration (OR: 4.34; 95% CI: 1.74–10.85) or having long sleep duration (OR: 2.50; 95% CI: 1.05–6.99) had greater risk of sarcopenia compared with women having normal sleep duration. With comparison to men with normal sleep duration, the adjusted OR for sarcopenia was 2.12 (0.96–8.39) in the short sleep duration group and 2.25 (0.88–6.87) in the long sleep duration group, respectively.
A U-shape relationship between self-reported sleep duration and sarcopenia was identified in a population of Chinese community-dwelling older adults, especially in women.
Keywords: older adults, sarcopenia, sleep duration
1. Introduction
Sleep disorders, such as insomnia and poor sleep quality, are very common in older adults. Approximately 50% of older adults have complaints of sleep problems in their daily life.[1] Sleep disorders not only influence the quality of life of older adults but also put them at greater risk of death.[1]
Sarcopenia is a new geriatric syndrome that comprises aging-related declines in skeletal muscle mass, muscle strength, and function.[2] The prevalence of sarcopenia varies across studies due to different diagnostic criteria and the characteristics of study populations. However, the prevalence of sarcopenia increases with advancing age consistently in these studies.[3] Sarcopenia is also related to many adverse health outcomes, such as increased risk of falls, disability, poor quality of life, and mortality.[4]
Both sleep disorders and sarcopenia increase with age, share some risk factors (e.g., lower physical activity), and result in similar adverse health outcomes (e.g., functional impairments, poor quality of life, and death). Therefore, a potential association may exist between sleep disorders and sarcopenia. The evidence regarding the relationship between these 2 conditions is relatively sparse. A previous study demonstrated that long sleep duration was related to skeletal muscle loss in postmenopausal women.[5] In addition, Loprinzi and Loenneke[6] found that engagement in muscular strengthening activities is related to better sleep in 4386 adults aged 20 to 85 years. These findings need to be validated in different populations. Therefore, we conducted this study to investigate the possible association between sleep duration and sarcopenia in a population of Chinese community-dwelling older adults.
2. Methods
2.1. Study population
Data of this study were derived from the Comprehensive Geriatric Assessment and Health Care Service System in Chinese Elderly Project, which has been previously reported.[7] Researchers who are interested in this study can request the raw data from the contact author. The study protocol was approved by the Research Ethics Committee of Sichuan University. Briefly, a cross-sectional study was conducted from October 2010 to August 2012 in 2 cities (Chengdu and Suining) in Sichuan, China. We randomly selected 2 urban communities in Chengdu and 8 rural communities in Suining as the study areas. We included older adults (aged 60 years or older) who signed the informed consent to participant in this project. Individuals with the following comorbidities were excluded from the project: severe cognitive impairment, mental disorders, and severe hearing and eye disorders. A total of 1326 older adults resided in the study areas, and 887 of them were included in this project, generating a participation rate of 67%.
2.2. Data collection
Face-to-face interviews were performed to collect the data from all of the study participants. All the study staff were trained using investigation manuals, multimedia materials, and simulated study participants. All interviewers were required to pass a test before starting the formal investigation.
2.3. Evaluation of self-reported sleep duration and sleep quality
Each participant was asked the time they went to sleep at night and the time they woke up in the previous 7 days. The mean sleep duration was calculated accordingly. Thereafter, we divided the participants into 3 groups: short sleep duration (<6 hours of sleep); normal sleep duration (6–8 hours of sleep); and long sleep duration (>8 hours of sleep). We also assessed self-reported sleep quality using the following question: “What was your level of nighttime sleep quality in the past 7 days?” The answers included 3 options: “Good,” “Fair,” and “Poor.”
2.4. Evaluation of sarcopenia
The recommended diagnostic algorithm of the Asia Working Group for Sarcopenia (AWGS)[8] was applied to assess sarcopenia in this study. According to the AWGS recommendation, study participants with a low muscle mass plus a low handgrip strength (HS) and/or a low gait speed (GS) were regarded as having sarcopenia.
In this study, we estimated the muscle mass by the appendicular skeletal muscle mass (ASM) using the following equation which was previously validated in a Chinese population[9]:
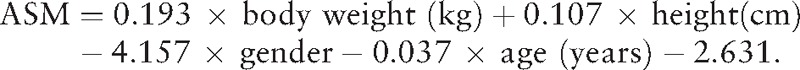 |
In this equation, for gender, value 1 represented men, and value 2 represented women. Body weight and height were measured using a wall-mounted stadiometer and a digital floor scale to the nearest 0.1 cm and 0.1 kg, respectively. Using dual x-ray absorptiometry (DXA) as the gold standard, the adjusted R2 of the equation was 0.90, and the standard error of estimate (SEE) was 1.63 kg.[9]
Thereafter, we calculated the muscle mass index (SMI) using the ASM divided by the square of the height in meters (SMI = ASM/height2). According to previous studies,[10–12] the cut-off of the SMI used in this study was based on the 20% lowest percentile of the study population; as a result, an SMI less than 6.89 kg/m2 in men and less than 4.91 kg/m2 in women was classified as a “low muscle mass.”
Furthermore, well-experienced technicians measured handgrip strength using a handheld dynamometer based on strain gauge sensors (EH101, Xiangshan Inc., Guangdong, China) to the nearest 0.1 kg. Three readings were obtained for each hand, and the highest value in either hand was applied for the analyses. A preliminary study was conducted to assess the reliability of the HS test using the intraclass correlation coefficient (ICC). The results showed an excellent test–retest reliability of the HS test (ICC = .85; n = 115). According to the AWGS recommendation, low HS referred to <26 kg for men and <18 kg for women.[8]
The participants’ usual gait speed (in m/s) was measured in a 20-meter walking test. Walkers or canes were allowed if necessary. Timing commenced when the study participants started foot movement and stopped when 1 foot contacted the ground after completely crossing the 20-m mark.[13] Based on the AWGS suggestion, low gait speed was defined as less than 0.8 m/s.[8]
2.5. Other measurements of covariates
The following information was obtained from the face-to-face interviews: age, gender, smoking status, alcohol drinking status, education level, physical activity, cardiovascular disease, respiratory disease, central nervous system disease, diabetes, osteoarthritis, tumor of any type, bone fracture, falls in the previous 12 months, and the use of hypnotics in the past month. The morbidities mentioned above were also confirmed according to local medical records databases.
Furthermore, the cognitive function of each participant was evaluated using the previous validated Chinese version of the Mini-Mental Status Examination (MMSE).[14] The diagnostic cut-off points for cognitive impairment are as follows: a score of ≤17 for illiterates, ≤20 for primary school graduates, and ≤24 for high school graduates or those with higher education.[15] Depression was assessed using the Chinese version of the 30-item Geriatric Depression Scale (GDS-30); a score of ≥ 11 indicates depression.[16] The nutrition status of each participants was evaluated using the previously validated Mini Nutritional Assessment Short-Form (MNA-SF) [17]; a score between 8 and 11 indicates that the study participants are “at risk of malnutrition,” whereas a score of ≤ 7 indicates that the study participants suffer from malnutrition.[17]
2.6. Statistical analyses
We performed all of the statistical analyses using the SPSS software version 20.0 (SPSS Inc., Chicago, IL). The categorical and continuous data were presented as percentages (%) and the mean ± standard deviation, respectively. Demographic and clinical characteristics were compared according to the sleep duration category. The between-group differences were tested by ANOVA or the Pearson chi-squared test. The statistical tests were 2-tailed and a P<0.05 was considered to be statistically significant. The crude and adjusted odd ratios (OR) and 95% confidence intervals (CI) for sarcopenia by sleep duration were estimated using logistic regression models. Model 1 was adjusted for age and gender. Model 2 was adjusted for age, gender, body mass index (BMI), education level, marital status, smoking status, alcohol drinking status, physical activities, self-reported sleep quality, the use of hypnotics, nutrition status, cognitive status, and depression. In these models, the age and BMI were treated as continuous data, whereas the other variables were treated as categorical data. Collinearity among the covariates was checked by estimating Spearman correlation coefficients. We performed the Hosmer–Lemeshow test to assess the goodness of fit for each model. We also stratified the data by gender, because gender difference was identified in the association between sleep and sarcopenia in a previous study.[18]
3. Results
3.1. Description of the study population
We included 607 participants in our analyses; 280 individuals were excluded due to missing data for gait speed, handgrip strength, and/or sleep duration. All of the participants were Han Chinese. The age of the included participants ranged from 60 years to 90 years. There were no significant differences between the included and excluded participants with respect to age (70.6 ± 6.6 years vs 71.2 ± 7.2 years), gender (58.6% vs 53.9%), and BMI (22.9 ± 3.2 vs 23.1 ± 2.9).
3.2. Characteristics of participants with or without sarcopenia
The prevalence of sarcopenia in all participants was 18.5%. Women appeared to be more prone to sarcopenia than men, but the difference was not statistically significant (19.9% vs 16.3%; P = .259). Table 1 shows the characteristics of men and women with or without sarcopenia. In both men and women, participants with sarcopenia were older than those without sarcopenia (men: 70.8 ± 6.4 years vs 72.6 ± 7.0 years, P = 0.005; women: 69.1 ± 6.2 years vs 72.3 ± 7.2 years, P < 0.001).
Table 1.
Characteristics of participants with or without sarcopenia.
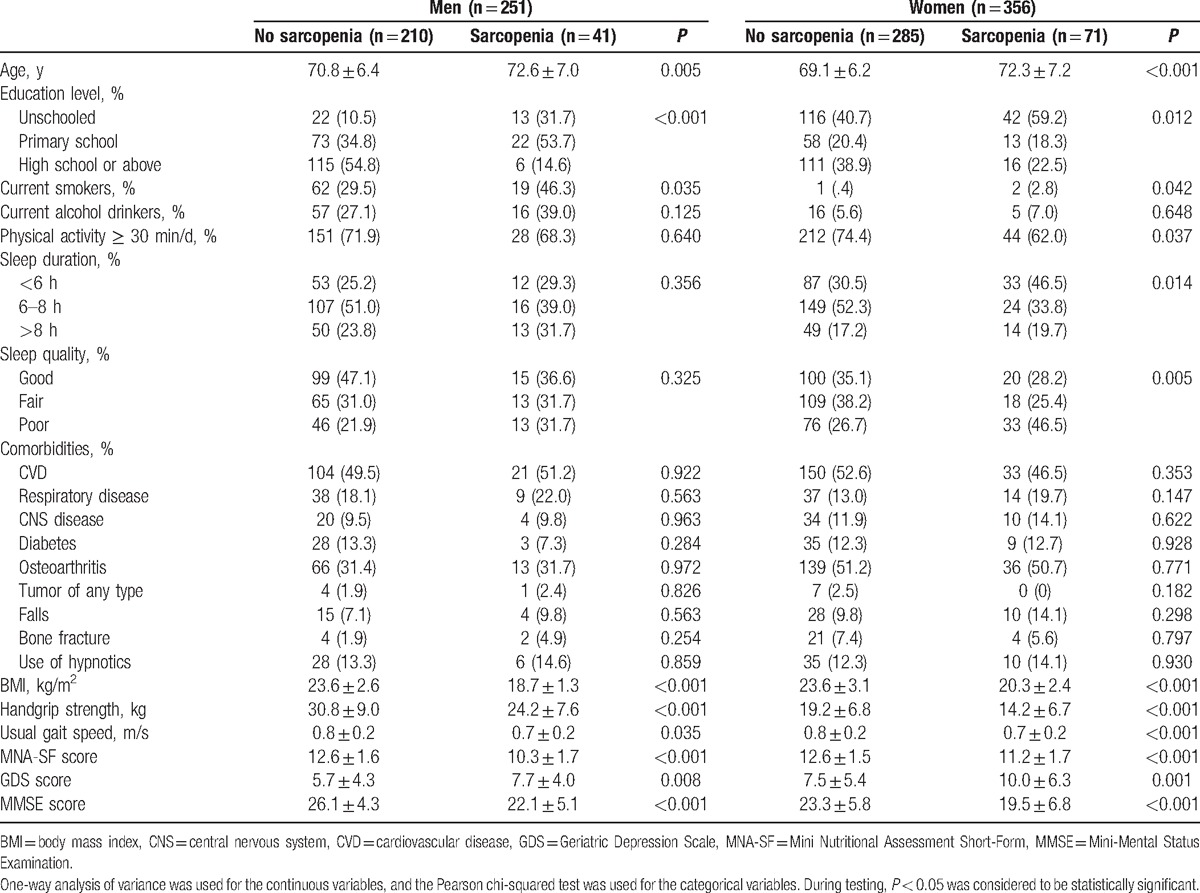
Sarcopenic women were more likely to have short or long sleep duration and poor sleep quality compared with women without sarcopenia. Similar results were identified in men, but the differences were not statistically significant (Table 1).
Furthermore, in both men and women, the sarcopenic individuals achieved lower MNA-SF score and MMSE score but higher GDS score, which indicated that they might have worse nutrition status and cognitive function but higher prevalence of depression.
3.3. Characteristics of participants according to sleep duration
Table 2 shows the characteristics of men and women according to sleep duration. In women, the prevalence of sarcopenia was significantly higher in the short sleep duration group (27.5%) and long sleep duration group (22.2%) compared with those in the normal sleep duration group (13.9%, P = 0.014). Similar results were found in men; however, the differences between groups were not statistically significant (18.5%, 20.6%, and 13.0%, respectively; P = 0.356).
Table 2.
Characteristics of participants according to sleep duration.
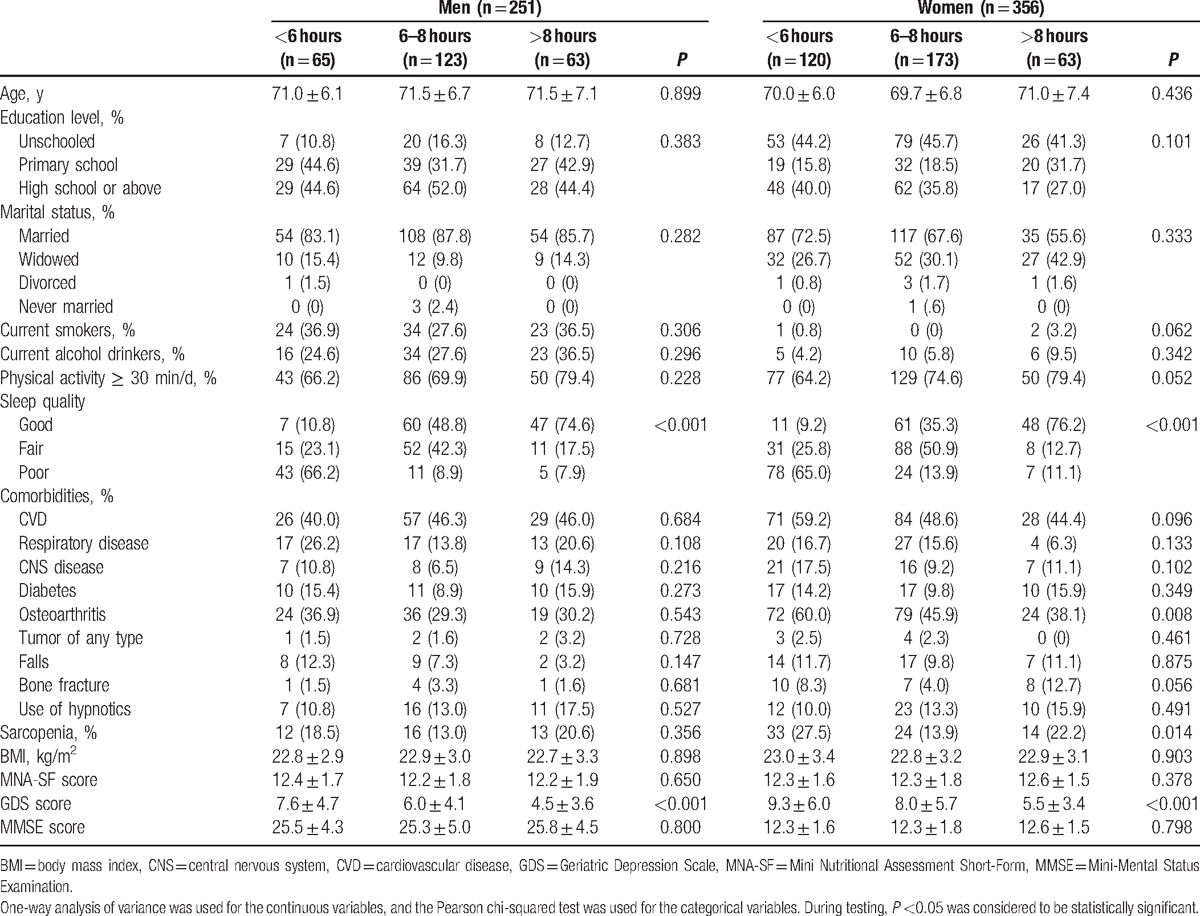
Furthermore, in both men and women, short sleepers and long sleepers were more likely to have poor sleep quality compared with the elders with normal sleep duration.
3.4. Association between sleep duration and sarcopenia
Table 3 shows the results of different models derived from the multiple logistic regression analyses. In the whole study population, with comparison to the elders with normal sleep duration, short sleepers had an over 4-fold increased likelihood of sarcopenia (adjusted odds ratio, OR: 4.24; 95% CI 1.75–10.30), whereas long sleepers had an over 3-fold increased likelihood of sarcopenia (adjusted OR: 3.50; 95% CI: 1.39–8.80).
Table 3.
Association between sarcopenia and sleep duration according to logistic regression models.
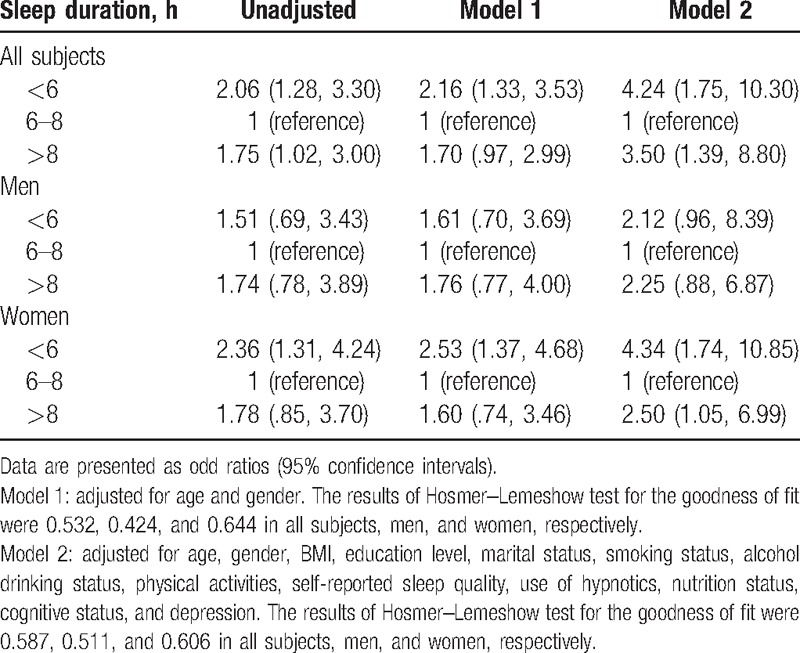
The gender-specific analyses revealed that women with less than 6 hours of sleep (adjusted OR: 4.34, 95% CI: 1.74–10.85) and women with more than 8 hours of sleep (adjusted OR: 2.50, 95% CI: 1.05–6.99) had a greater risk of sarcopenia compared to women with 6 to 8 hours of sleep. However, in comparison to men with normal sleep duration, the adjusted OR for sarcopenia was 2.12 (0.96–8.39) in the short sleep duration group and 2.25 (0.88–6.87) in the long sleep duration group, respectively. The results of Hosmer–Lemeshow test for goodness of fit indicated that both model 1 and model 2 fit our data well in each study population (Table 3).
4. Discussion
This study demonstrates that sarcopenia is more prevalent in the community-dwelling elders with short sleep durations and those with long sleep durations compared to those with normal sleep durations. The elders with short or long sleep durations are at an increased risk of having sarcopenia after adjustments for the potential confounding factors. These findings are significant in women, but not in men.
The association between sleep duration and sarcopenia in older adults has not been well studied in previous studies. This study identified a U-shape pattern between self-reported sleep duration and sarcopenia in a population of Chinese community-dwelling older adults. This finding was consistent with a recent study conducted in Taiwan.[18] In their study, Chien and colleagues recruited 488 community-dwelling older adults and also found a U-shape pattern between sleep duration and sarcopenia. However, they defined sarcopenia only based on low muscle mass which was measured by bioelectrical impedance analysis (BIA). Although a unique diagnostic criterion of sarcopenia has not yet been achieved, 6 major diagnostic criteria of sarcopenia are currently available.[19] All these diagnostic criteria recommend that sarcopenia should be defined by combining low muscle mass and low muscle function. In addition, as we stated above, sleep was related to skeletal muscle loss and function in previous studies.[5,6] All these findings imply that there is the possible association between sleep and sarcopenia.
The finding that short sleep duration was associated with sarcopenia is understandable. Although the potential mechanisms that mediate this association are not fully understand yet, several mechanisms might contribute to it. First, it is well known that sleep exerts a significant modulatory effect not only on metabolism, but also on hormones.[20] Short sleep duration or sleep deprivation reduced the secretion of growth hormone (GH), insulin-like growth factor-1 (IGF-1), and testosterone, [20] and increased cortisol level.[21] The reduction of GH/IGF-1 and testosterone may decrease muscle protein synthesis and enhance muscle protein degradation through decreasing IGF/P13K/Akt and mTOR activity and increasing myostatin expression.[22] On the other hand, cortisol causes muscle atrophy by increasing muscle degradation and reduces protein synthesis[22]; therefore, the increased cortisol may result in sarcopenia. Additionally, short sleep duration has been shown to be associated with insulin resistance.[23] Insulin can improve muscle anabolism and inhibit muscle degradation, and insulin resistance has been proven to be related to sarcopenia.[24] Second, short sleep duration could induce low grade inflammation,[25] which has been proven to be related to sarcopenia through oxidative and proteolytic pathways.[26] Oxidative stress has been proven to contribute to age-related reduction in skeletal muscle function,[27] and genetically enhancing mitochondrial antioxidant activity was found to improve skeletal muscle function in aged mice.[28]
The finding that long sleep duration was associated with sarcopenia was similar with the results of Chien's study.[18] Another study also demonstrated that postmenopausal women with long sleep durations were at risk of reduced skeletal muscle.[5] Long sleep duration has also been proven to be related to other adverse health outcomes (e.g., frailty and death). For example, a recent systematic review of 16 prospective studies indicated that long sleep duration was associated with a greater risk of death.[29] However, no studies published to date have revealed the possible mechanism mediating the association of long sleep duration and sarcopenia. One possible explanation of this association was that long sleep duration might suggest underling health conditions, frailty, fatigue, or low level of physical activity, which were previously related to sarcopenia.
Recently, a large investigation study demonstrated that both shorter (≤6 h) and longer sleep (≥10 h) durations were related to higher risks of dyslipidemia (hypercholesterolemia and hyper-low-density lipoprotein cholesterolemia) in Chinese women but not in men.[30] On the other hand, another study in Korea indicated that elderly men with sarcopenia obesity was associated with a higher risk for dyslipidemia compared with those having sarcopenia or obesity alone.[31] Putting these findings together, although the gender difference was identified in the results of the both studies, dyslipidemia might play a potential role in the relationship between sleep duration and sarcopenia. In future studies addressing the association between sleep and sarcopenia, dyslipidemia may be considered as a potential confounder.
Currently, there is no well-established evidence for drug therapies for sarcopenia yet.[8] The validated interventions for sarcopenia are limited to lifestyle interventions, such as nutrition therapy and physical activities.[32] Therefore, exploring and understanding other lifestyle factors that are associated with sarcopenia (e.g., sleep) are important, as it may provide potential targets for preventing and treating sarcopenia. This study identified the possible relationship between self-reported sleep duration and sarcopenia in Chinese elderly. Prospective studies are warranted to detect whether the improvement of sleep could serve as a possible intervention for sarcopenia, or vice versa.
In our study, gender-specific analyses indicated that the elderly women with short or long sleep durations had a greater risk of sarcopenia. Although a similar trend was identified in the older men, the results were not statistically significant. The possible reason for the gender difference in the association of sleep duration and sarcopenia was the smaller sample size in men than in women (251 vs 356). In addition, both our study and Chien's study[18] were conducted in Chinese population, the external validity of our findings needs to be studied in other ethnic groups.
This study has some limitations. First, sleep duration information was obtained from retrospective self-reporting instead of actigraphy; therefore, a recall bias might be induced. Difference between self-reported and actigraphy-measured sleep time has been reported; the concordance between these 2 measurements was up to 66% to 78% of old adults in a previous study.[33] Second, the muscle mass was estimated with a previously validated anthropometric equation, rather than the BIA or DXA as recommended by the AWGS.[8] Some experts argued that anthropometric measures are a poor marker of muscle mass.[34] However, the cut-off points of BIA for defining low muscle mass in Chinese older people have not been established yet.[35] DXA is expensive and not suitable for community studies. Furthermore, a recent study indicated that the muscle mass (estimated by anthropometric equations) combined with the handgrip and gait speed comprised an important alternative to DXA to improve the diagnosis of sarcopenia and reduced costs.[10] Fourth, the AWGS suggested using a 6-meter walking test to measure gait speed.[8] In this study, we applied a 20-meter walking test; therefore, the walking distance was longer than the AWGS recommendation. However, a recent systematic review with 48 studies indicated that the “distance walked during the gait speed test did not influence the recorded gait speed.”[36] Fifth, 280 participants (31.6%) were excluded due to missing data for gait speed, handgrip strength, and/or sleep duration. The exclusion of participants may induce a selection bias in the results. Sixth, although we asked the participants the time they went to sleep at night and the time they woke up to make the data of sleep duration more reliable. We only recorded the categories of sleep duration in our database. This might cause statistical information loss. Finally, although we adjusted the analyses for many potential confounding factors, the possibility of residual confounding cannot be eliminated.
5. Conclusion
A U-shape relationship between self-reported sleep duration and sarcopenia were identified in a population of Chinese community-dwelling older adults, especially in women. This finding implies that there may be a potential relationship between sleep disorders and sarcopenia.
Acknowledgments
The authors thank the staff and participants of the Comprehensive Geriatric Assessment and Health Care Service System in Chinese Elderly Project. They also thank Dr. Joseph Flaherty from St. Louis University for English editing.
Footnotes
Abbreviations: ASM = appendicular skeletal muscle mass, AWGS = Asia Working Group for Sarcopenia, BMI = body mass index, BIA = bioelectrical impedance analysis, CI = confidence intervals, DXA = dual X-ray absorptiometry, GDS-30 = 30-item Geriatric Depression Scale, GH = growth hormone, GS = gait speed, HS = handgrip strength, ICC = intraclass correlation coefficient, IGF-1 = insulin-like growth factor-1, MMSE = Mini-Mental Status Examination, MNA-SF = Mini Nutritional Assessment Short-Form, OR = odd ratios, SEE = standard error of estimate, SMI = muscle mass index.
XH and JJ contributed equally to this work.
Funding: This work was supported by the National Department Public Benefit Research Foundation by the Chinese National Ministry of Health (No. 201002011) and the Sichuan Provincial Science and Technology Department (No. 2015GZ0344). The sponsors had no role in the design, performance, data analysis and preparation of this study.
The authors have no conflicts of interest to disclose.
References
- [1].Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult—a mini-review. Gerontology 2010;56:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sayer AA. Sarcopenia the new geriatric giant: time to translate research findings into clinical practice. Age Ageing 2014;43:736–7. [DOI] [PubMed] [Google Scholar]
- [3].Cruz-Jentoft AJ. Sarcopenia: a clinical review. Rev Clin Gerontol 2013;23:267–74. [Google Scholar]
- [4].Cederholm T, Morley JE. Sarcopenia: the new definitions. Curr Opin Clin Nutrit Metab Care 2015;18:1–4. [DOI] [PubMed] [Google Scholar]
- [5].Fex A, Barbat-Artigas S, Dupontgand S, et al. Relationship between long sleep duration and functional capacities in postmenopausal women. J Clin Sleep Med 2012;8:309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Loprinzi PD, Loenneke JP. Engagement in muscular strengthening activities is associated with better sleep. Prev Med Rep 2015;2:927–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang M, Jiang J, Hao Q, et al. Dynapenic obesity and lower extremity function in elderly adults. J Am Med Dir Assoc 2015;16:31–6. [DOI] [PubMed] [Google Scholar]
- [8].Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- [9].Wen X, Wang M, Jiang CM, et al. Anthropometric equation for estimation of appendicular skeletal muscle mass in Chinese adults. Asia Pac J Clin Nutr 2011;20:551–6. [PubMed] [Google Scholar]
- [10].Alexandre T, da S, Duarte YA, et al. Sarcopenia according to the European Working Group on Sarcopenia in Older People (EWGSOP) versus dynapenia as a risk factor for mortality in the elderly. J Nutrit Health Aging 2014;18:751–6. [DOI] [PubMed] [Google Scholar]
- [11].Delmonico MJ, Harris TB, Lee JS, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 2007;55:769–74. [DOI] [PubMed] [Google Scholar]
- [12].Newman AB, Kupelian V, Visser M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003;51:1602–9. [DOI] [PubMed] [Google Scholar]
- [13].Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2007;62:844–50. [DOI] [PubMed] [Google Scholar]
- [14].Liu HC, Lin KN, Teng EL, et al. Prevalence and subtypes of dementia in Taiwan: a community survey of 5297 individuals. J Am Geriatr Soc 1995;43:144–9. [DOI] [PubMed] [Google Scholar]
- [15].Cui GH, Yao YH, Xu RF, et al. Cognitive impairment using education-based cutoff points for CMMSE scores in elderly Chinese people of agricultural and rural Shanghai China. Acta Neurol Scand 2011;124:361–7. [DOI] [PubMed] [Google Scholar]
- [16].Chan AC. Clinical validation of the Geriatric Depression Scale (GDS): Chinese version. J Aging Health 1996;8:238–53. [DOI] [PubMed] [Google Scholar]
- [17].Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging 2009;13:782–8. [DOI] [PubMed] [Google Scholar]
- [18].Chien MY, Wang LY, Chen HC. The relationship of sleep duration with obesity and sarcopenia in community-dwelling older adults. Gerontology 2015;61:399–406. [DOI] [PubMed] [Google Scholar]
- [19].Morley JE, Malmstrom TK. Can sarcopenia be diagnosed without measurements? Eur Geriatr Med 2014;5:291–3. [Google Scholar]
- [20].Maurovich-Horvat E, Pollmacher TZ, Sonka K. The effects of sleep and sleep deprivation on metabolic, endocrine and immune parameters. Prague Med Rep 2008;109:275–85. [PubMed] [Google Scholar]
- [21].Kumari M, Badrick E, Ferrie J, et al. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab 2009;94:4801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Piovezan RD, Abucham J, Dos Santos RV, et al. The impact of sleep on age-related sarcopenia: possible connections and clinical implications. Ageing Res Rev 2015;23(Pt B):210–20. [DOI] [PubMed] [Google Scholar]
- [23].Spiegel K, Knutson K, Leproult R, et al. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol 2005;99:2008–19. [DOI] [PubMed] [Google Scholar]
- [24].Abbatecola AM, Paolisso G, Fattoretti P, et al. Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. J Nutr Health Aging 2011;15:890–5. [DOI] [PubMed] [Google Scholar]
- [25].Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol 2007;5:93–102. [DOI] [PubMed] [Google Scholar]
- [26].Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care 2012;15:12–22. [DOI] [PubMed] [Google Scholar]
- [27].Andersson DC, Betzenhauser MJ, Reiken S, et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 2011;14:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Umanskaya A, Santulli G, Xie W, et al. Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc Natl Acad Sci U S A 2014;111:15250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cappuccio FP, D’Elia L, Strazzullo P, et al. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep 2010;33:585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhan Y, Chen R, Yu J. Sleep duration and abnormal serum lipids: the China Health and Nutrition Survey. Sleep Med 2014;15:833–9. [DOI] [PubMed] [Google Scholar]
- [31].Baek SJ, Nam GE, Han KD, et al. Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: the 2008–2010 Korea National Health and Nutrition Examination Survey. J Endocrinol Invest 2014;37:247–60. [DOI] [PubMed] [Google Scholar]
- [32].Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options—a mini-review. Gerontology 2014;60:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Van Den Berg JF, Van Rooij FJ, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res 2008;17:295–302. [DOI] [PubMed] [Google Scholar]
- [34].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zeng P, Wu S, Han Y, et al. Differences in body composition and physical functions associated with sarcopenia in Chinese elderly: reference values and prevalence. Arch Gerontol Geriatr 2015;60:118–23. [DOI] [PubMed] [Google Scholar]
- [36].Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci 2013;68:39–46. [DOI] [PubMed] [Google Scholar]


