Abstract
A large cohort of 220 HIV-1-infected children (median [range] age: 12 [4–17] years) was cared and followed up in the Central African Republic, including 198 in 1st-line and 22 in 2nd-line antiretroviral regimens. Patients were monitored clinically and biologically for HIV-1 RNA load and drug resistance mutations (DRMs) genotyping. A total of 87 (40%) study children were virological responders and 133 (60%) nonresponders. In children with detectable viral load, the majority (129; 97%) represented a virological failure. In children receiving 1st-line regimens in virological failure for whom genotypic resistance test was available, 45% displayed viruses harboring at least 1 DRM to NNRTI or NRTI, and 26% showed at least 1 major DRM to NNRTI or NRTI; more than half of children in 1st-line regimens were resistant to 1st-generation NNRTI and 24% of the children in 1st-line regimens had a major DRMs to PI. Virological failure and selection of DRMs were both associated with poor adherence. These observations demonstrate high rate of virological failure after 3 to 5 years of 1st-line or 2nd-line antiretroviral treatment, which is generally associated with DRMs and therapeutic failure. Overall, more than half (55%) of children receiving 1st-line antiretroviral treatment for a median of 3.4 years showed virological failure and antiretroviral-resistance and thus eligible to 2nd-line treatment. Furthermore, two-third (64%) of children under 2nd-line therapy were eligible to 3rd-line regimen. Taken together, these observations point the necessity to monitor antiretroviral-treated children by plasma HIV-1 RNA load to diagnose as early as possible the therapeutic failure and operate switch to a new therapeutic line.
Keywords: antiretroviral treatment, Central Africa, HIV-1 subtype, pediatrics, plasma HIV-1 RNA load, resistance mutations, virological failure, virological outcomes
1. Introduction
More than 90% of the 2.6 million children infected by HIV-1 live in sub-Saharan Africa. In 2015, around 220,000 children were newly infected, and 150,000 HIV-1-infected children under the age of 15 died because of AIDS.[1] Without antiretroviral treatment, about half of the children living with HIV die before the age of 2 years.[2,3]
Along with access to antiretroviral drugs in adults, antiretroviral treatment for children living in resource-constrained countries is available for about 15 years. During the last decade, the use of antiretroviral drugs was widespread in sub-Saharan Africa for preventing mother-to-child transmission, dramatically reversed the spread of HIV and significantly reduced the morbidity and mortality of this epidemic in the child population.[4–8] However, compared to adults, children living with HIV are less likely to receive antiretroviral treatment.[9] Furthermore, if the extension of access to antiretroviral drugs in African children has significantly reversed the infant mortality curve associated with AIDS, it has also facilitated emergence and spread of drug-resistant virus in sub-Saharan Africa.[10,11] Various factors are involved in the fact that HIV-infected children and adolescents are more vulnerable than adults to virological failure and drug resistance including the HIV resistance risk during prevention of mother-to-child transmission,[12] frequently high HIV-1 RNA plasma level in children,[13] limited number of available pediatric-formulated antiretroviral drugs for the different age classes, variable pharmacokinetics, rapid changes in body weight, frequently observed poor adherence, social environment, psychosocial factors, and frequent absence of biological monitoring.[8,14–25] Thus, recent studies in African children receiving 1st-line antiretroviral treatment according to the treatment guidelines of the World Health Organization (WHO) for resource-limited countries have reported generally high degrees of virological failure depending in part on treatment duration, ranging from 6% in Kwazulu-Natal (South Africa),[26,27] 15% in Cape Town (South Africa),[28] 17%[29] to 44%[30] in Ghana, 26% in Uganda,[31] 29% in Rwanda,[32] 34% in Kenya,[33] 35% in Ivory Coast,[16] 40% in the Central African Republic,[23] 53% in rural Cameroon,[34] 55% in Senegal,[24] 56% in Togo,[25] 58% in Tanzania[35,36] to 61% in Mali.[37] In addition, circulating virus resistant to at least 1 antiretroviral drug could be detected very frequently in 61%[33] to 98%[38] of children with a detectable viral load while receiving antiretroviral treatment. Moreover, the problem of perinatally infected adolescents has recently emerged. In 2013, it was estimated that the majority of adolescents living with HIV in Africa were never diagnosed, or were lost to follow-up or dropped out of treatment and care programs.[9] AIDS-related deaths are also increasing among adolescents.[9] Finally, several studies have reported on the outcome of antiretroviral treatment in children in Africa, but only a few reports are available on long-term outcomes and in adolescents.[19,23–25,35,37,39,40]
For over 10 years, the Ministry of Health of the Central African Republic has developed an operational, structured program aim to prevent the spread of HIV and to provide care for HIV-infected patients, with particular attention to HIV-infected children. The HIV-1 prevalence in children less than 18 months in the whole country may be estimated between 0.7% to 1.1% by taking into account HIV prevalence in 15 to 49-year old women and the rate of mother to child prevention.[41–43] To evaluate the management of pediatric AIDS, an observational cohort of HIV-infected children was followed-up since 2007 in the “Complexe Pédiatrique” of Bangui, the main health care clinic for HIV-infected children of the Central African Republic.[21,23] In 2009, Charpentier and colleagues[23] reported that one-third (34%) of children receiving 1st-line regimen (median of treatment = 18 months) was in virological failure with selection of drug resistance mutations (DRMs), and therefore eligible to 2nd-line treatment. In children under 2nd-line therapy, virological failure appeared more prevalent (47%), and the selection of at least 1 major DRM to nucleosidic reverse transcriptase inhibitor (NRTI) or non-nucleosidic reverse transcriptase inhibitor (NNRTI), and less frequently to protease inhibitor (PI).[23] These observations pointed the crucial need of the improvement in regards of pediatric antiretroviral drugs distribution in Central African Republic, to increase the adherence and to offer an adequate HIV monitoring to treated children.
Recent political events affecting the Central African Republic were associated with deterioration of health care support for HIV/AIDS in the country,[44] exacerbating HIV epidemic, considered as “out of control.”[45] These findings prompt us to process a reassessment of virologic failure, selection of resistant mutations to antiretroviral and failure rate to antiretroviral treatment in the cohort of HIV-infected children follow up at the “Complexe Pédiatrique” of Bangui and receiving antiretroviral regimen according to the 2013-revised WHO guidelines.[46]
2. Material and methods
2.1. Study population
All HIV-1-infected children attending the Complexe Pédiatrique of Bangui for their antiretroviral treatment follow up were prospectively included from January to March 2013. Children attending the pediatric complex are mainly born from HIV-infected mothers, and have in principle received HIV prevention of mother-to-child following the national guidelines. The newborn children infected by HIV despite prevention are followed-up according to the WHO-recommendations for resource-limited settings. In addition, a minority of HIV-infected children is suffering from sickle-cell disease. The active file comprised in 2013 around 1500 patients, whose 750 were treated by antiretroviral therapy according to the 2013-revised WHO recommendations.[46]
Inclusion criteria for this study were as follows: (i) Antiretroviral therapy since at least 6 months, consisting in 1st- or 2nd-line regimens as recommended by 2013-revised WHO recommendations[46]; (ii) availability of simple demographic data of children (age, gender), treatment history (duration of treatment; therapeutic line) and compliance; (iii) informed consent from children's biological parents or guardians.
2.2. Assessment of antiretroviral treatment adherence
Adherence was assessed as described previously,[21,23] using an empirical questionnaire addressed to the parent or the child, according to the child's age, including the following variables: (1) α: number of pill(s) forgotten during the period of the last week; (2) β: number of pills taken inappropriately during the period of the last week; and (3) ɣ: number of days without drug intake during the period of the last week. Quantitative estimation of adherence, “Ad,” was calculated as follows: Ad = (1 – [(α/14) + (β/7) + (ɣ/24)]/3) × 100). The variables α, β, and ɣ were rounded up to the nearest integer. Finally, the adherence was estimated as “very good” if Ad ≥ 90%, “good” if 80% < Ad < 90%, “middle” if 60% < Ad ≤ 80%, and “bad” if Ad ≤ 60%.
2.3. Plasma HIV-1 RNA load
Plasma HIV-1 RNA load were carried out at the Laboratoire National de Biologie Clinique et de Santé Publique of Bangui, using using the Amplix platform developed by Biosynex (Strasbourg, France), which integrates a fully automated station for nucleic acids extraction (RNA and/or DNA) and real-time PCR amplification station, using lyophilized Amplix HIV-1 RNA quantitative reagents (Biosynex). The assay detects HIV-1 groups M, O and several circulating recombinant forms (CRFs).[47] The Laboratoire National de Biologie Clinique et de Santé Publique participates in an external quality assurance testing program organized by the virology laboratory of the Hôpital Européen Georges Pompidou, Paris.
2.4. Classification of children
Children were classified into 2 groups of virological responses to treatment, as virological responders (V+), when their viral load at time of inclusion was undetectable (i.e., less than 20 copies/mL or 1.3 log copies/mL), and virological nonresponders (V–), when their viral load was detectable (i.e., >20 copies/mL). The threshold of virological failure was 1000 copies/mL as it is recommended by the WHO.[46]
2.5. Drug resistance mutations and estimation of the accumulation of resistance mutations
Aliquots of plasma were obtained from all infected children and were sent in dry ice to the virology unit of the Hôpital Européen Georges Pompidou, Paris, and then kept frozen at –80°C until their processing for DRMs genotyping.
The antiretroviral resistance genotype was performed randomly in half patients with detectable plasma HIV-1 RNA viral load.
HIV-1 protease and reverse transcriptase pol genes were sequenced by the ViroSeq HIV-1 genotyping system (Celera Diagnostics, Alameda, CA) with 1 mL of plasma sample. Resistance mutations were reported and interpreted as listed by the Agence Nationale de Recherches sur le SIDA et les hépatites virales (ANRS) algorithm (updated in September 2016; http://www.hivfrenchresistance.org). This algorithm distinguishes between the major drug resistance mutations and polymorphism of protease and reverse transcriptase genes of HIV-1 group M, as previously demonstrated,[48,49] and is frequently used to analyze and interpret resistance mutations of non-B HIV-1 variants from Africa.[23,24,50–53]
HIV-1 subtype was obtained by phylogenic analyses of pol gene sequences using reference sequences for HIV-1 genetic subtypes and circulating recombinant forms obtained from the Los Alamos Database (available at: http://hiv-web.lanl.gov).
The accumulation of DRMs was estimated through an empirical scoring system attributing a quantitative score regarding the number of major DRMs for the PI class, or the number of resistance to antiretroviral molecules for the NRTI/NNRTI classes. In brief, the empirical score for DRMs to PI (“PI resistance score”) was as follows: the score 0 corresponds to lack of DRM to PI, whereas the scores 1, 2, 3, 4, 5, and 6 correspond to the presence of DRMs to 1, 2, 3, 4, 5, or 6 PI, respectively. The empirical score for NRTI/NNRTI molecules (“NRTI/NNRTI resistance score”) was as follows: the score 0 corresponds to the lack of DRM, whereas the score 1 is attributed to resistance to EFV or NVP, resistance to ETR or RPV, resistance to TDF 1, resistance to 3TC or FTC, resistance to AZT, and resistance to ABC, d4T or ddI. The accumulation of drug resistance mutations or “Total resistance score” corresponded finally to the sum of “PI score” and “NRTI/NNRTI score.” The selected molecules interested by these resistances are the main antiretroviral drugs recommended by the WHO for resource-limited settings.[46]
For each child, was assigned an overall score of accumulated resistance or “Total resistance score,” corresponding to the sum of the “PI resistance score” and the “NRTI/NNRTI resistance score.”
Protease and RT sequences were submitted to European Nucleotide Archive with the following accession numbers: LT577626 to LT577673 and LT726745 to LT726792 (available at: http://www.ebi.ac.uk/ena/data/view/). HIV-1 subtype was evaluated by comparing the polymerase sequence to consensus sequences using the Los Alamos database (available at: http://hiv-web.lanl.gov).
3. Ethics statement
The study was formally approved by the Scientific Committee of Faculté des Sciences de la Santé (“FACSS”) de Bangui, (so-called Comité Scientifique de la Validation des Protocoles et des Résultats de Recherche en Santé”/ CSVPR) constituting the National Ethical Committee (agreement #2UB/FACSS/CSVPR/09). Informed written consent was obtained from mothers for themselves and on behalf of their respective child participating in the study. The collected data were anonymously. Finally, a return of laboratory results to clinicians was conducted to achieve a better management of the treated patient. Feedback was given to parents’children and their pediatricians on all tested parameters carried out during the study period, allowing changes of antiretroviral treatment, and improvement of medical care.
3.1. Statistical analyses
The raw data were entered into an Excel spreadsheet and analyzed using Epi Info version 3.5.1 (Center for Disease Control and Prevention, Atlanta, GA). HIV-1 viral load, resistance scores (Total, PI, and NRTI/NNRTI scores), virological response, virological failure, therapeutic regimen and sex were compared between adherence categories using χ2 test, with a significance level of 0.05. Multivariate logistical regression analysis was performed by taking the significant variables in the binary logistical regression model. The Odds ratio (OR) and 95% confidence interval were estimated for all variables. The strength of the statistical association was measured by adjusted OR and 95% confidence intervals.
4. Results
The major final study results and conclusions have been reported to the Ministry of Health and to the National Council for AIDS (Conseil National de Lutte contre le SIDA or “CNLS”), Bangui, Central African Republic.
4.1. Study children
A total of 220 HIV-1-infected children were included over a period of 4 months. The median age of the children was 12 years (range, 4–17 years). Girls were as prevalent as boys [118 (54%) vs 101 (46%)]. The majority of children (n = 198, 90%) were receiving 1st-line regimen according to the 2013-revised WHO recommendations,[46] for a mean duration in 1st-line of 4.7 years (range, 3.8–9.9), whereas the remaining (n = 22, 10%) received 2nd-line regimen, under generic tablet formulation, for a mean duration in 2nd-line of 3.8 years (range, 3.3–8.3) and for a mean duration in 1st- or 2nd-lines of 5.4 years (range, 3.8–13.3). Children taking 2nd-line regimens were treated in their line for lesser time than those in the 1st-line (3.8 vsvs 4.7 years; P<0.001).
The 1st-line regimens consisted of the following combinations: zidovudine (AZT) + lamivudine (3TC) + nevirapine (NVP) (n = 166, 83.8%), AZT + 3TC + efavirenz (EFV) (n = 18, 9.0%), stavudine (d4T) + 3TC + EFV (n = 6, 3.1%), d4T + 3TC + lopinavir (LPV) boosted by ritonavir (LPV/r) (n = 6, 3.1%), abacavir (ABC) + didanosine (ddI) + LPV/r (n = 1, 0.5%) and AZT + 3TC + indinavir (IDV) (n = 1, 0.5%). The 2nd-line regimens contained primarily LPV/r in 86.5% (AZT + 3TC + LPV/r [n = 11, 50.0%] and d4T + 3TC + LPV/r [n = 8, 36.5%]), the other combinations being d4T + ddI + EFV (n = 1, 4.5%), AZT + d4T + NVP (n = 1, 4.5%) and d4T + 3TC + EFV (n = 1, 4.5%).
4.2. Plasma HIV-1 RNA load monitoring
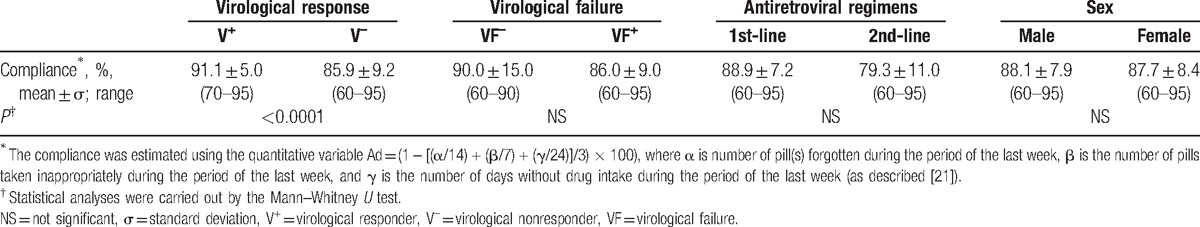
According to the detectability threshold of the assay used for plasma HIV-1 RNA load, 87 (40%) of study children were virological responders (V+) and 133 (60%) nonresponders (V–) (Table 1). Thus, only a minority of children showed undetectable HIV-1 RNA load. In children with detectable viral load, the majority were in virological failure (129, 97%), according to the 2013-revised WHO criteria[46] (Table 1). Interestingly, only 4 children (3%) were virological nonresponders (plasma viral load >1.3 log copies/mL), but not in virological failure (viral load <1000/mL); 2 were in 1st-line and 2 in 2nd-line regimens. These latter children with detectable but low HIV-1 RNA load showed shorter treatment duration than children in virological failure, but had yet accumulated similar levels of DRMs (Table 1). Virological responders showed less prolonged treatment duration (4.3 vs 5.0 years; P < 0.0001) and higher compliance (91.1% vs 85.9%; P < 0.0001) than nonresponders (Table 1). Nonresponders in virological failure showed higher treatment duration (5.0 years vs 3.9 years; P < 0.02) and higher HIV-1 RNA load (4.6 vs 2.2 log copies/mL; P < 0001) than those with detectable viral load below 1000 copies/mL (Table 1). Children in 1st-line regimen and those in 2nd-line were similar regarding their age, sex ratio, total treatment duration (2nd-line: 5.4 years vs 1st-line: 4.7 years), compliance and viral load (Table 1). A total of 119 (60.1%) and 14 (63.6%) of children under 1st-line and 2nd-line regimens, respectively, were virological nonresponders, and 117 (59.1%) and 12 (54.5%) of children under 1st-line and 2nd-line regimens, respectively, were in virological failure.
Table 1.
Characteristics of the 220 antiretroviral drugs-experienced children living in Bangui according to their virological responses at inclusion and classified as responders (e.g., viral load under the threshold of detection; V+) and nonresponders (detectable viral load; V–); of the 133 antiretroviral drugs-experienced children living in Bangui classified as nonresponders (detectable viral load; V–), according to the threshold of virological failure (VF) as defined by the 2013-revised WHO recommendations (e.g., viral load below the threshold of virological failure, 1000 copies/mL, VF–; viral load ≥ 1000 copies/mL, FV+)[46]; and of the 220 antiretroviral drugs-experienced children living in Bangui according to their therapeutic lines (1st-line or 2nd-line regimens) of antiretroviral treatment as defined by the 2013-revised WHO recommendations for resource-limited settings.[46]
4.3. Genotypic resistance tests result
Antiretroviral resistance genotypes in reverse transcriptase and protease pol genes were carried out in 66 plasma samples randomly selected in half of 133 children with detectable plasma HIV-1 RNA viral load; successful genotypes were obtained in 58 children, including 2 children with viral load below 1000 copies/mL and 56 children with HIV-1 RNA load above 1000 copies/mL in virological failure; and 50 children in 1st-line regimens and 8 children in 2nd-line regimens. Since plasmas for genotyping were randomly selected, the results in percentages shown in Table 1 are expected to be representative of the entire study population and sub-groups.
4.4. Genetic variability
Genetic analysis of the 58 HIV-1 pol sequences showed broad genetic diversity. Thus, most children were infected with the CRFs, CRF11_cpx (34.4%) and CRF01-AE (18.9%), or with HIV-1 subtype A (12.1%). Furthermore, a large variety of HIV-1 subtypes could be observed: 5.1% respectively for CFR02_AG, CRF13_cpx, H, D, 3.4%, respectively, for CRF15 and subtypes F1 and B. Finally, with the lowest proportion (1.7%) subtypes C and G.
4.5. Responders and nonresponders children
Among the 58 genotypes representative of the children with detectable viral load (V–) and/or in virological failure (V–, VF+), 54 (93.1%) harbored at least 1 DRM (DRM+). Only 4 (6.9%) children in 1st-line regimens with (V–, VF+) profile showed sensitive HIV-1 virus (DRM-) (Table 1). The distributions of DRM in virological nonresponders and in children in virological failure were similar: a minority showed DRMs to PI and around half of them DRMs to NRTI or NNRTI (Table 1).
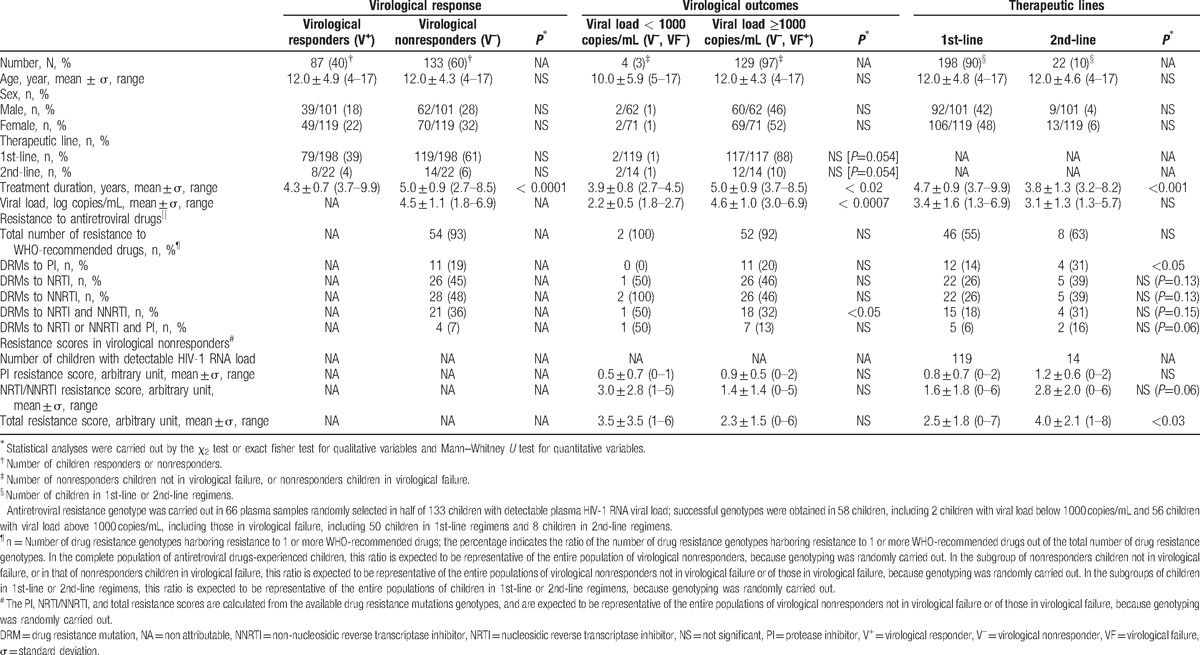
DRMs to PI corresponded mainly to natural polymorphism in protease pol gene as expected with HIV-1 non-B subtype protease sequences. The distribution of DRMs to PI is depicted in Fig. 1A. The DRMs V82A/F (n = 6; 10.3%), L33F (n = 2, 3.4%), I50L (n = 2, 3.4%), L76 V (n = 1, 1.7%) and I84 V (n = 1, 1.7%) were the major DRMs to PI found in the 58 study genotypes. A high frequency of natural polymorphisms was observed: the L63P/V mutation in 54 genotypes (93.1%) followed by M36I/L (53, 91.4%), L89I (53, 91.4%), H69H/K (52, 89.6%), G16E (32, 55.2%), D60E (30, 51.7%), L10F (27, 46.5%), I62 V (17, 29.3%), K20I/M (16, 27.6%), I15 V (11, 18.9%) and E35G (6, 10.3%). Among the 50 genotypic resistance tests performed in plasma samples from virological nonresponders receiving their 1st-line regimens, a total of 12 (24.0%) children in virological failure showed at least 1 major DRM to the PI drug class (Table 1). The percentages of DRMs to PI were higher in children in 2nd-line regimen than in those in 1st-line regimens (P < 0.05) (Table 1). Among the 8 genotypic resistance tests performed in plasma samples from virological nonresponders receiving their 2nd-line regimen, the DRM V82A/F was found in 2 (25.0%).
Figure 1.
Drug resistance mutations profiles in HIV-1 strains detected among study children in virological failure. Drug resistance mutations (DRMs) (represented by decreasing order) expressed in percentage observed in 58 successful genotypes in reverse transcriptase and protease inhibitor pol genes obtained in a representative subpopulation randomly selected from 133 children with detectable plasma HIV-1 RNA viral load (nonresponders V–) followed in the Complexe Pédiatrique of Bangui: (A) DRMs to protease inhibitors (PI); (B) DRMs to nucleosidic reverse transcriptase inhibitors (NRTI); (C) DRMs to non-nucleosidic reverse transcriptase inhibitors (NNRTI). NNRTI = non-nucleosidic reverse transcriptase inhibitors, NRTI = nucleosidic reverse transcriptase inhibitors, PI = protease inhibitor.
Regarding the NRTI class, nearly half of nonresponders children (V–) displayed viruses that harbored at least 1 mutation associated with NRTI resistance (Table 1). The distribution of DRMs to NRTI is depicted in Fig. 1B. The most prevalent DRMs to NRTI were M184 V (n = 25, 43.1%), followed by T215Y (n = 9, 15.5%), K70E/R (n = 7, 12.1%), D67N (n = 6, 10.3%), M41L (n = 4, 6.8%), L210W (n = 4, 6.8%), K219Q/E (n = 3, 5.1%), and Q151 M (n = 2, 3.4%). Thymidine-associated mutations (TAMs) were present in 33 (56.9%) genotypes, and 7 (12.0%) viruses showed an extensive resistance profile (e.g., at least 3 TAMs). In median, the genotyped viruses exhibited 1 mutation associated with resistance to NRTI (range, 0–6). The percentages of DRMs to NRTI showed a trend to be higher in children in 2nd-line regimen than in those in 1st-line regimen but the difference was not statistically significant (Table 1). No viruses harbored the insertion at codon 69 of the reverse transcriptase pol gene.
Regarding the NNRTI class, around 60% of nonresponders children (V–) displayed viruses that harbored at least 1 mutation associated with NNRTI resistance (Table 1). The distribution of DRMs to NNRTI is depicted in Fig. 1C. The most prevalent DRMs to NRTI were K103H/N/S (18, 31.0%), K101E (7, 12.0%), G190A (7, 12.0%), and H221Y (6, 10.0%). The other mutations were E138A, (2, 3.4%), P225H (1, 1.7%) and V106I/A (1, 1.7%). The mutations Y181C/I/V and Y181C/H were absent (Fig. 1C). In median, the genotyped viruses exhibited 1 mutations associated with resistance to NNRTI (range, 0–3). The percentages of DRMs to NNRTI showed a trend to be higher in children in 2nd-line regimen than in those in 1st-line regimen but the difference was not statistically significant (Table 1).
Finally, in nonresponders children (V–) receiving their 1st-line or 2nd-line regimens, 45% and 48%, respectively, displayed viruses harboring at least 1 DRM to NRTI or NNRTI. If 1 excluded the M184 V mutation, a proportion of 26% and 39% of nonresponders children receiving their 1st-line or 2nd-line regimens, respectively, displayed viruses harboring at least 1 DRM to NRTI or NNRTI.
DRMs to PI were associated with mean PI resistance score below 1, whereas DRMs to NRTI or NNRTI were associated with mean NRTI/NNRTI resistance score above 1 (Table 1). The NRTI/NNRTI resistance scores were higher than the PI scores in virologically nonresponders children (P < 0.01), in children in virological failure (P < 0.01) and children under 1st-line regimen (P < 0.01) as well as in children under 2nd-line regimen (P < 0.01). These observations confirmed the predominance of the resistance to NNRTI, followed by the resistance to NRTI, and the minority of the resistance to PI. The total number of accumulated resistance to antiretroviral drugs as evaluated by the “Total resistance score” was higher in children under 2nd-line regimens than in children receiving their 1st-line regimens (P < 0.03) (Table 1).
4.6. Genotypic resistance tests interpretation and possible future therapeutic options
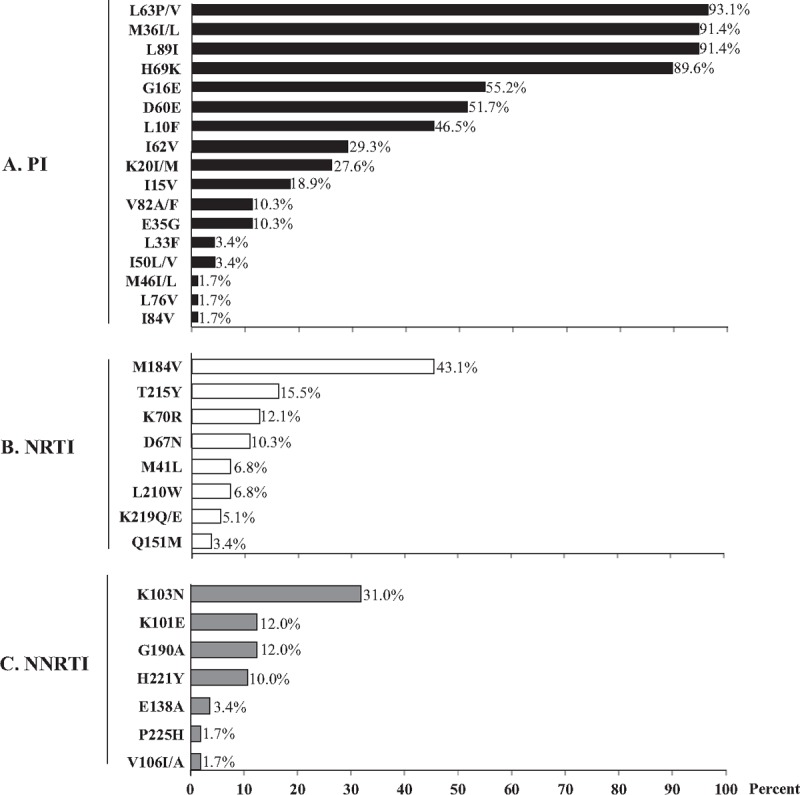
Possible antiretroviral drugs therapeutic options remaining in children under virological failure were further estimated according to their resistance genotypes interpreted using the ANRS algorithm for all the drugs proposed in 2nd-line regimen according to the 2013-revised WHO recommendations[46] (Figs. 2 and 3). Overall, the viruses of these latter patients remained susceptible to a median of 5 molecules of the 6 major available NRTI [AZT, d4T, 3TC/emtricitabine (FTC), ddI, ABC, tenofovir (TDF)], a median of 1 molecule of the 3 major NNRTIs [EFV, NVP, etravirine (ETR)], and a median of 7 molecules of the 7 major PIs [IDV, saquinavir (SQV), nelfinavir (NFV), fosamprenavir (FPV), LPV, atazanavir (ATV), darunavir (DRV)]. Thus, the use of 1st-generation NNRTI appeared largely compromised in the pediatric cohort of Bangui, as 53.5% and 55.3% of children with virological failure, in 1st-line or 2nd-line regimens were respectively resistant to EFV or NVP.
Figure 2.
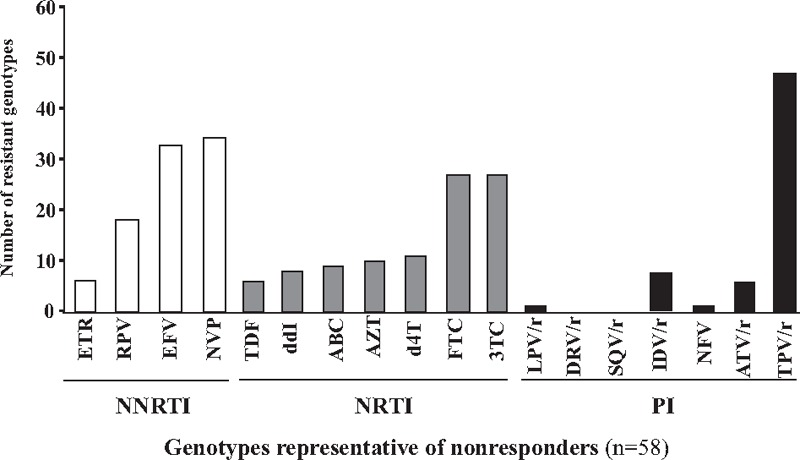
Resistance to major WHO antiretroviral drugs in HIV-1 from children in virological failure. The profiles of resistance to antiretroviral recommended by the WHO in 58 successful genotypes obtained in a representative sub-population randomly selected from 133 children with detectable plasma HIV-1 RNA viral load (nonresponders,V–) followed in the Complexe Pédiatrique Bangui. 3TC = lamivudine, ABC = abacavir, ATZ = atazanavir, AZT = zidovudine, d4T = stavudine, ddI = didanosine, DRV = darunavir, EFV = efavirenz, ETR = etravirine, FTC = emtricitabine, IDV = Indinavir, LPV = lopinavir, NFV = nelfinavir, NNRTI = non-nucleosidic reverse transcriptase inhibitor, NRTI = nucleosidic reverse transcriptase inhibitor, NVP = nevirapine, PI = protease inhibitor, r = ritonavir (which boosted other PIs), RPV = rilpivirine, SQV = saquinavir, TDF = tenofovir, TPV = tipravirine.
Figure 3.
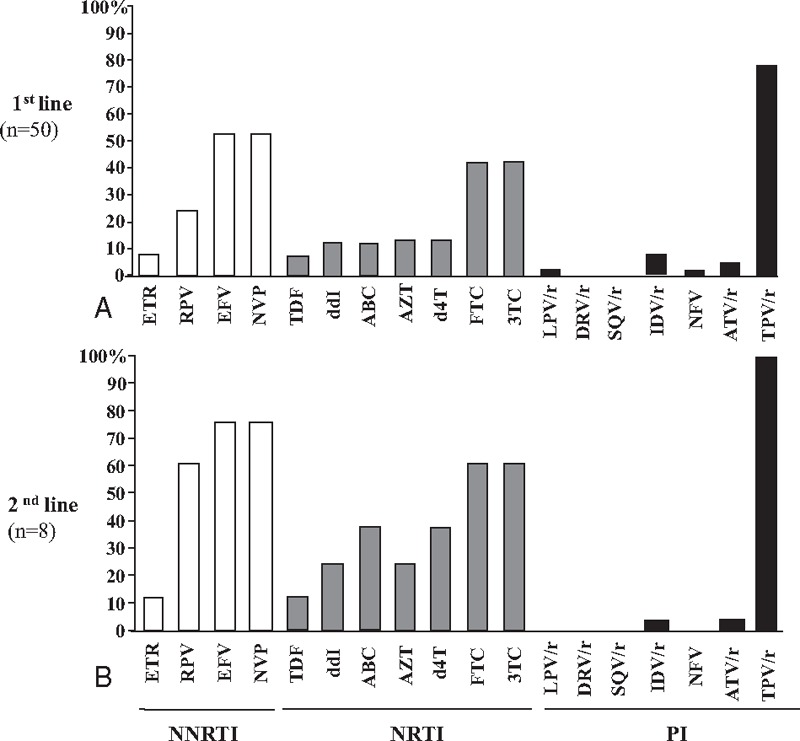
Resistance to antiretroviral drugs in HIV-1 from children in virological failure according to therapeutic regimens. (A) 1st-line regimens, (B) 2nd-line regimens. 3TC = lamivudine, ABC = abacavir, ATZ = atazanavir, AZT = zidovudine, d4T = stavudine, ddI = didanosine, DRV = darunavir, EFV = efavirenz, ETR = etravirine, FTC = emtricitabine, IDV = Indinavir, LPV = lopinavir, NFV = nelfinavir, NNRTI = non-nucleosidic reverse transcriptase inhibitor, NRTI = nucleosidic reverse transcriptase inhibitor, NVP = nevirapine, PI = protease inhibitor, r = ritonavir (which boosted other PIs), RPV = rilpivirine, SQV = saquinavir, TDF = tenofovir, TPV = tipravirine.
Considering the PI class, plasma viruses of 58 children with detectable HIV-1 RNA load with interpretable protease pol gene sequences were found to be susceptible to DRV, SQV in 100.0%, LPV and NFV in 98.3% (n = 57), FPV in 96.5% (n = 56), ATV in 89.6% (n = 52) and IDV in 87.9% (n = 51). In children under 1st-line regimen in virological failure, 4 (8.0%) showed genotype demonstrating resistance to IDV, 4 (8.0%) to ATV, 2 (4.0%) to FPV, 1 (2.0%) to LPV, and 1 (2.0%) resistance to IDV and NFV (Figs. 2 and 3). Viruses of children under 1st-line in virological failure were found resistant to IDV in 5 (10%) and to NFV in 1 (2.0%) genotypes, respectively. Viruses of children after 2nd-line therapeutic failure remained sensitive to ATV (n = 6, 75.0%), DRV (n = 8, 100.0%), FPV (n = 8, 100.0%), IDV (n = 6, 75.0%), LPV (n = 8, 100.0%), NFV (n = 8, 100.0%), SQV (n = 8, 100.0%) of the PI drug class. More than 88% (51/58) of children in virological failure (V–, VF+) showed remaining susceptibility to LPV and ATV, which constitute the major PI of the 2nd-line regimen in Africa, and DRV, which is recommended in the 3rd-line regimen.[46] Finally, the WHO-recommended drugs of the PI class remained mostly active in 76% and 50% of children in therapeutic failure in 1st-line or 2nd-line, respectively (P>0.05).
Considering the NRTI class, plasma viruses of the 58 nonresponders children (V–) with interpretable pol gene sequences were found to be susceptible to AZT and d4T in 82.7% (n = 48), 3TC/FTC in 55.1% (n = 32), ABC in 84.5% (n = 49), ddI in 86.2% (n = 50), and TDF in 89.6% (n = 52). Thus, the vast majority (84.5%; 49/58) of nonresponders children harbored resistant viruses remaining sensitive to AZT and TDF, the 2 main antiretroviral drugs included in the 2nd-line regimens in Africa.[46]
Regarding to the NNRTI class, children with detectable viral load and/or in virological failure were found to be susceptible to EFV in only 44.8% (n = 26) and to NVP in only 43.1% (n = 25).
Interestingly, DRM genotyping showed viruses presenting a resistance genotypic profile whose interpretation by the ANRS algorithm predicted resistance to antiretroviral drugs that they never received (Figs. 2 and 3). Thus, the majority of children displayed viruses remaining susceptible to the 2nd-generation NNRTI ETR. Only 5 (8.6%) had virus harboring predicted resistance and 2 (4.0%) had possible resistance to this drug due to the presence of the E138A mutation. Similarly, the majority of children displayed viruses remaining susceptible to the 2nd-generation NNRTI rilpivirine (RPV); 17 genotypes of 58 (29.3%) showed virus harboring predicted resistance to RPV. Regarding the PI drug class, 6 (10.3%) genotypes showed viruses resistant to ATV, 1 of the 2 major PI molecules recommended in 2nd-line antiretroviral treatment.[46] Finally, 47 (81.0%) genotypes showed viruses resistant to the PI tipravirine (TPV).
Taken together, among children receiving 1st-line therapy, the majority were virological nonresponders (60.1%, 119 of 198), and an estimated 92% of them (109 of 119) showed viruses harboring major DRMs to NNRTI, NRTI, or PI; finally, a proportion of 55.0% (109 of 198) of children in 1st-line regimens could be eligible for 2nd-line treatment (Table 1). Among children receiving 2nd-line therapy, the majority were virological nonresponders (63.6%, 14 of 22), and an estimated 100% of them (n = 14) showed viruses harboring major DRMs to NNRTI, NRTI, or PI; finally, a proportion of 63.6% (14 of 22) of children in 2nd-line regimens could be eligible for 3rd-line treatment (Table 1).
4.7. Adherence
Among study children, the adherence was “very good” in 80.0% (n = 176), “good” in 6.3% (n = 14), “middle” in 10.4% (n = 23) and “bad” in 3.2% (n = 7). HIV-1 RNA load was inversely correlated to the adherence among the whole study population (P < 0.002), the children receiving 1st-line (P < 0.004) or 2nd-line (P < 0.006) regimens, and the female children (P < 0.006) (Fig. 4). In males, the HIV-1 RNA load showed a trend to be inversely correlated to the adherence, but the correlation was not statistically significant (P = 0.10). Furthermore, the age was inversely correlated to the adherence (P < 0.03). In bivariate analysis, adherence significantly associated with virological response, virological failure, therapeutic regimen and sex (not shown). In multivariate logistical regression analysis using the variables shown as significant in bivariate analysis, the adherence remained significantly associated only with virological response, the categories “very good” and “good adherence” being strongly associated with effective virological response (e.g., undetectable HIV-1 viral load under treatment) (crude OR: 4.5; adjusted OR: 3.3, 95%CI[2.1–5.3]) (P < 0.0001) (Table 2).
Figure 4.
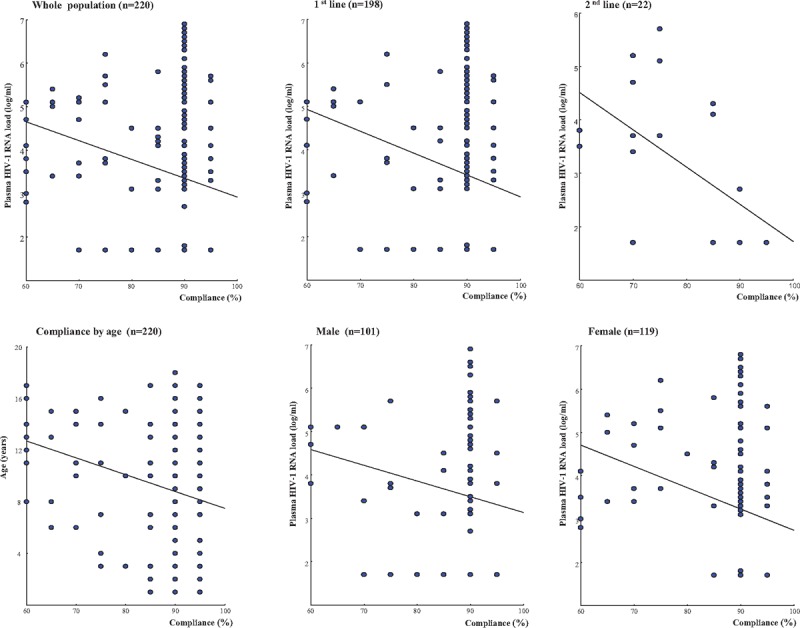
Adherence to antiretroviral treatment among the 220 study children. Adherence (percentage) according to HIV-1 RNA load (log copies/mL) or age (years) among the 220 antiretroviral drugs-experienced children living in Bangui, regarding their therapeutic lines (1st-line or 2nd-line regimens), and sex (male or female).
Table 2.
Compliance among the 220 antiretroviral drugs-experienced children living in Bangui according to their virological response to treatment at inclusion [(responders (e.g., viral load below the threshold of detection; V+) and nonresponders (detectable viral load; V–), the diagnosis of virological failure (e.g., viral load below the threshold of virological failure, 1000 copies/mL, FV–; viral load ≥ 1000 copies/mL, FV+), the antiretroviral treatment line regimens (1st- or 2nd- lines) and the sex.
5. Discussion
In this study, we reported on a large cohort of 220 HIV-1-infected children followed-up at the Complexe Pédiatrique of Bangui, Central African Republic, including 198 patients in 1st-line and 22 in 2nd-line antiretroviral regimens, as suggested by the 2013-revised WHO recommendations for resource-constrained countries.[46] The median age of the children was relatively elevated, with a high proportion (63%) of young adolescents. The measurement of plasma HIV-1 RNA load allowed to demonstrate high proportion (60%) of virological nonresponders (V–), in children under 1st-line regimens and in those under 2nd-line regimens. Nonresponder children were in their great majority (97%) in virological failure, that is, showed circulating HIV-1 RNA load above 1000 copies/mL. In children receiving 1st-line regimens in virological failure for whom genotypic resistance test was available, 45% displayed viruses with at least 1 DRM to NNRTI or NRTI, 26% showed at least 1 major DRM to NNRTI or NRTI following the exclusion of M184 V mutation; and more than half of children in 1st-line regimens were resistance to 1st-generation NNRTI. Furthermore, 24% children in 1st-line regimens showed viruses harboring major DRMs to PI. Finally, most children under 1st-line regimens (92%) and under 2nd-line regimens (100%) with detectable HIV-1 RNA load were resistant to at least 1 drug of the NNRTI, NRTI, or PI molecules as recommended by WHO. Interestingly, virological failure and thus selection of DRMs was associated with poor compliance. Taken together, these observations demonstrate the high rate of virological failure after 3 to 5 years of 1st-line or 2nd-line antiretroviral treatments, which generally is associated with DRMs and thus therapeutic failure, but also a minority of cases (7%) with viruses still sensitive to antiretroviral drugs. Overall, the percentage of children receiving 1st-line antiretroviral treatment for a median of 3.4 years in virological failure and displaying antiretroviral-resistant viruses, and thus eligible to 2nd-line treatment, may be estimated to more than half (55%) of children in 1st-line regimens. In children under 2nd-line therapy, the prevalence of the virological failures is equal to 1st-line therapy. As well, the selection of at least 1 major DRM to NNRTI, followed by NRTI and less commonly to PI; represents two-third (64%) of children which were eligible to 3rd-line treatment regimen. These findings complete and extent those reported from the same pediatric cohort in 2006 by Gody and colleagues[21] and in 2009 by Charpentier and colleagues,[23] and point the necessity to monitor antiretroviral drugs-treated children by plasma HIV-1 RNA load to diagnose early as possible situations of therapeutic failure and operate a shift to a new therapeutic line.
In this study, sensitive viruses were detected in 7% (n = 4) of resistance genotypes, corresponding to children under 1st-line antiretroviral treatment who were virological nonresponders (plasma viral load > 1.3 log copies/mL), but not in virological failure (viral load <1000/mL). In the previous series by Charpentier and colleagues,[23] sensitive viruses were detected in blood samples from 15% of children under 1st-line antiretroviral treatment with detectable HIV-1 RNA load (i.e., plasma viral load > 3.7 log copies/mL). These findings demonstrate that a minority of treated children with detectable HIV-1 RNA load possess sensitive viruses at time of sampling, a transient situation to the selection of full resistance if the virus continues to replicate. Virological failure with sensitive viruses seems to be due to poor adherence that must be urgently corrected. Indeed, the level of adherence in antiretroviral drug-treated children was associated inversely with plasma HIV-1 RNA at 6 months of antiretroviral treatment.[21] Virological monitoring seems to be of particular interest in HIV-infected children known to have difficulties to display a good adherence, in order to decrease the duration of HIV replication under antiretroviral drugs pressure. A contrario, the vast majority of resistance genotypes (93%) carried out in virological nonresponders children harbored genetic patterns compatible to resistance to WHO-recommended drugs.
A minority of children (3%) were virologically nonresponders (plasma viral load >1.3 log copies/mL), but not in virological failure (viral load <1000/mL). These children with detectable but low HIV-1 RNA load showed shorter treatment duration than children in virological failure, but had yet accumulated similar levels of DRMs.
In a previous cohort of 52 children under 1st-line antiretroviral treatment since 6 months, conducted at the Complexe Pédiatrique in 2006,[21] detectable plasma HIV-1 RNA (e.g., >400 copies/mL) was observed in 27 (52%), and virological failure could be diagnosed in 12 children (23%) according to the 2010-revised WHO criteria (unpublished personal data).[54] In 2009, the percentage of therapeutic failure in 150 children under 1st-line regimens since 18 months was twice higher (40%), whereas the treatment duration increased 3-fold.[23] In 2013, the proportion of the 198 study children under 1st-line regimens since 4.7 years (median) in virological failure, a situation generally associated with selection of antiretroviral drug resistance, was as high as 59%, such 3-time higher than in 2006 and 1.5-time higher than in 2009. The current threshold (3.0 log copies/mL) proposed by the 2013-revised WHO recommendations for virological failure[46] was stronger than the threshold to HIV-1 viral load detection (e.g., >400 copies/mL) used in 2007 and likely more sensitive than the threshold of 3.7 log copies/mL (5000 copies/mL) recommended by WHO in 2010[54] to assess virological failure in treated children. These findings show that maintaining 1st-line antiretroviral treatment despite detectable viral load is associated over time with increased rate of virological failure.
The interaction between limited resources, living in conflict areas such as in the Central African Republic during the study period, and complexity of HIV treatment may have impacted negatively on adherence to antiretroviral therapy in some children.[55,56] Interruptions in antiretroviral treatment, promoted by political crisis or conflicts in sub-Saharan Africa, may lead to unplanned treatment interruptions due to medical supplies disruption, displacement of the population, unsafe travel, limited access to health care, and incapability to obtain medications, and may be associated with worsening HIV infection[57] as well as with virological failure.[58] Children are particularly vulnerable in crises and are at increased risk of abandonment, abduction, malnutrition, and communicable diseases.[56] Studies on the impact of the political and humanitarian crisis after the contested 2007 Kenyan presidential election showed small but statistically significant disruptions in clinical care and medication adherence among children on antiretroviral treatment despite a comprehensive health care response.[56,59,60] The Central African Republic was suffering during the study period from a major and out of control health and humanitarian crisis[44,61,62] which affected particularly the HIV epidemic.[45] The Central African Republic represents the largest HIV prevalence in French-speaking sub-Saharan Africa.[1] In the extremely difficult geo-political context of the country during the period 2009–2013, the failure of antiretroviral treatment has reached uncontainable proportions.[45] The degrees of virological failure among the 15,000 individuals receiving 1st-line antiretroviral treatment, including 1400 children, are estimated at 30% in adults and 50% in children, representing more than one-fifth (3100) patients in therapeutic failure with antiretroviral treatment-resistant HIV strains, necessitating the shift of the treatment to 2nd-line.[23,45,52] However, the capability of health system to manage antiretroviral treatment failure is insufficient, with lack of qualified human resources, no availability of 2nd-line treatment, and nascent biological monitoring, in a context of frequent and prolonged nationwide shortage in medical materials. The disastrous HIV epidemic in the Central African Republic requires immediate and specific advocacy and massive and adapted interventions. The national and/or international reactions have not been so far appropriate to resolve this crisis, and international contributors are in fact separating from the country's health provision.[61,62] More effective strategies are in truth needed to overcome the HIV epidemic in the Central African Republic as well as humanitarian disaster. Finally, understanding the consequences of conflict on HIV treatment in resource-limited settings, where prevalence of HIV is very high and political crises may occur more often, is imperative to avoid avoidable complications, particularly in children and adolescents.
Despite improvements in antiretroviral availability, adherence is still a main problem and antiretroviral treatment may be complicated for children in resource-limited countries.[63] Accurately gauging the adherence to antiretroviral therapy children is very important as the maintenance of high adherence is essential for a successful HIV treatment. In the present study, the adherence was assessed using an empirical questionnaire completed by the parent or the child, according to the child's age, as previously proposed by Gody and colleagues.[21] A high rate (80%) of study children showed “very good” (i.e., ≥90%) adherence, as yet previously reported as possible in conflict-affected areas, demonstrating that effective antiretroviral treatment may be feasibly and effectively provided despite conflict or post-conflict settings.[64,65] In study children, the adherence was higher in virological responders (≥90% in mean) than in nonresponders (<86% in mean), and was inversely correlated to HIV-1 RNA load among the whole study population, including the children receiving 1st-line or 2nd-line antiretroviral regimens. These observations validate the Gody's empiral scoring system to assess adherence in pediatric HIV-infected population, at least in Central African settings, by reference to an objective surrogate marker (HIV-1 viral load). Furthermore, they show that a “very good” adherence was predictive of virological response in treated children, in accordance to previous estimation stating that 90%- adherence or greater is recommended for optimal virologic suppression and minimizing failure rates.[66,67] A minority (13.6%) of study children showed “middle” or “bad” adherence. Poor antiretroviral treatment adherence was associated with viral resistance, opportunistic infections and ultimately failure of therapy.[68,69] However, in our hands, the adherence was not predictive of virological failure, whatever the therapeutic lines or the sex, and thus of the existence or absence of DRMs in viruses escaping to antiretroviral treatment. Adherence was inversely proportional to age in study children. The patterns of antiretroviral adherence in adolescents varies in different areas worldwide.[70] Adolescents in developing countries are shown to have poorer antiretroviral treatment adherence versus other age groups.[71–75] Numerous factors of poor adherence among adolescents have been identified, including specific behavioral, physiological and psychosocial complexities associated with the development in adolescence and the difficulty of acceptance of their health status,[76] most of them are supposed to act simultaneously.[70] In addition, the shift of responsibility for treatment from caregivers to adolescents themselves is likely implicated.[70] Poor adherence behavior among study adolescents as more generally in sub-Saharan Africa is a major concern, considering the limited available antiretroviral treatment options and the risk of drug resistance.[70] We found also that male gender was slightly associated with nonadherence, consistent with other studies on adults or adolescents with HIV.[77,78] Whatever their numerous possible causes, poor adherence or nonadherence may lead to development of resistant strains that may further increase the costs of management. Despite these limitations, the study has several important implications. First, the results suggest that child and family characteristics should be evaluated not only before initiation of antiretroviral treatment but also during the course of therapeutic management in HIV-infected children to identify those at higher risk of nonadherence. Such an evaluation will allow preventive or ongoing education and intervention efforts to be initiated early if risks to adherence are apparent. Second, the objective to reach an adherence more than 90% appears a priority, since “very good” adherence is as a predictor of good virological responses. Finally, the adherence is insufficient by itself to predict virological failure and/or accumulation of DRMs, and cannot be substituted to biological monitoring, that is, in practice circulating HIV-1 RNA load.
In sub-Saharan Africa, the rate of virological failure in antiretroviral drugs-treated children is diversely appreciated according to the study and its definition (quantitative threshold of plasma viral load), ranging from 6% to 61%.[16,20,21,23–25,27,28,30–37,79–92] Furthermore, the frequency of therapeutic failure seems quite higher when NNRTI-based regimens are used in 1st-line treatment,[82] ranging from 12% to 98%[16,20,21,24,33,37,38,79–82,84,87,88,92,93] than when PI-based regimen are used, ranging from 26% to 44%.[83,86,87,89,91]
The overall virological failure rates observed among perinatally HIV-1-infected children in the Central African Republic, ranging from 40.0% in 2009[23] to 59.1% in the present series enrolled in 2013, are generally higher than those observed in previous reports in African countries. However, in these previous studies the median time on antiretroviral treatment was often lower or fewer number of children were studied; for example 6% virological failure was observed after 3.3 years (range, 2.5–4.4) on antiretroviral treatment in Kwazulu-Natal (South Africa)[27]; 16.7% with a median of 16 months on antiretroviral treatment in Ghana[29]; 32, 53 and 55%, respectively, at 6, 12 and 24 months on antiretroviral treatment in Senegal[24]; 15% in Cape Town (South Africa) after a median time of 2.4 years on a 1st-line antiretroviral treatment protocol[28]; and 29% in Rwanda with a median duration of antiretroviral treatment of 3.4 years.[32] In Togo, virological failure rates ranged from 45.6% (12–24 months on antiretroviral treatment) to 55.7% (25–36 months on antiretroviral treatment) and did not change significantly with time on antiretroviral treatment or age.[25] Taken together, these reports indicate that virological failure depends on the impact of multiple factors such as CD4 T cell counts, HIV-1 RNA load, and stage of HIV disease at antiretroviral treatment initiation as well as adherence to treatment. Furthermore, all previously published studies as in the present series likely present inclusion bias because only individuals still retained on antiretroviral treatment are enrolled, suggesting that significant proportion of children who died or lost to follow-up are not taken into account.[14,94,95]
The pattern of DRMs found in the present series is consistent with the reports from several studies conducted in Africa,[16,19,20] as in the Complexe Pédiatrique of Bangui in 2009.[23] In this study cohort, prescribed mostly 1st-line antiretroviral treatment including NNRTI, virological failure was often associated with the selection of major NNRTI resistance mutations, while major PI resistance mutations could be observed on in the minority of children treated by 1st-line treatment including PI. The impact of DRMs acquired via prevention of mother-to-child transmission could not be evaluated in our study, but we previously reported a moderate (between 5% and 15%) prevalence of DRMs in the Central African HIV-infected pediatric population of Bangui.[96] These observations support the use of lopinavir-based 1st-line regimens in children in Africa as recommended by the WHO,[7,8,46,82,85,90,97] especially with the recent national recommendations to implement lifelong antiretroviral treatment for mothers. However, only 1 (12.5%) of the 8 children aged below 5 years was on a 1st-line PI-based antiretroviral treatment regimen in 2013 as recommended by the WHO, illustrating that these guidelines have not yet been implemented.
To our knowledge, only few studies reported on virological response in HIV-infected children receiving a 2nd-line regimen in resource-limited settings.[28,97–100] Although limited number of children under 2nd-line regimen in the present series, the rate of virological success appeared very low, with only 36.4% of children having undetectable plasma viral load. Explanations for failure on 2nd-line regimens include persistent poor adherence or adherence to suboptimal treatment regimens or dosing. Although the estimated rates of PI, NNRTI and NRTI (even after excluding the M184 V mutation) resistance mutations selection were similar in children under 1st-line or 2nd-line treatments, the “Total resistance score,” which takes into account the total number of DRMs to PI and the number of resistances to the principal WHO-recommended antiretroviral drugs, was higher in children under 2nd-line treatment than in those under 1st-line regimen, confirming that the risk to develop therapeutic failure in children in 2nd-line antiretroviral treatment is maintained, and was even slightly higher that in children in 1st-line treatment. These observations likely indicate that the unique therapeutic switching from 1st-line to 2nd-line antiretroviral treatment is not sufficient by it-self to avoid further therapeutic failure. The lack of genotypic resistance assessment at the beginning of the 2nd-line regimen, preventing to predict the antiviral activity of antiretroviral drugs comprised in the new regimen, may be an additional factor, especially in the minority of children in virological failure harboring viruses resistant to the NRTI recommended in the 2nd-line regimen in Africa. Finally, appropriate use of genotypic resistance sequencing in sub-Saharan African children failing 2nd-line antiretroviral treatment appears essential for rational and efficient use of limited treatment options.
In children displaying detectable HIV-1 RNA load under antiretroviral drugs pressure, a high frequency (93%) of DRMs was observed, as previously described in HIV-infected African children treated by antiretroviral drugs, 71% in Ivory Coast,[16] 73% in Mali,[37] and 77% to 86% in the Central African Republic.[21,23] In this study, the most common resistance profiles were associated with the wide use of 3TC and 1st-generation NNRTIs, as recommended by the WHO in 1st-line antiretroviral treatment for resource-limited settings.[46,59] Our findings highlight the emergency of virological monitoring upon the usage of 1st-generation of NNRTIs, particularly in the children having NNRTI resistance mutations due to perinatal prophylaxis drugs.[12,101–103] Indeed, the lack of treatment monitoring by HIV-1 RNA load seems to delay the virological failure diagnosis, resulting in an increase in the duration of persisting viral replication under antiretroviral drugs pressure, and consequently the risk of accumulation of NNRTI mutations.
In this study, in the children under virological failure, the virus remained susceptible to a median of 5 molecules of the 6 major available NRTIs (AZT, d4T, 3TC/FTC, ddI, ABC and TDF), a median of 1 molecule of the 3 major NNRTIs (EFV, NVP and ETR), and a median of 7 molecules of the 7 major PIs (IDV, SQV, NFV, FPV, LPV, ATV and DRV). Thus, the use of 1st-generation NNRTIs (EFV and NVP) appeared largely compromised in more than half children with virological failure, in 1st-line or 2nd-line regimens. However, the antiviral activity of NRTIs and PIs comprised in the 2nd-line regimen proposed by the 2013-revised WHO recommendations in case of virological failure was not largely impacted in the present series, as it was yet the case for the children of the Complexe Pédiatrique of Bangui followed up in 2009.[23] Indeed, AZT and TDF, the 2 main antiretroviral which are included in the 2nd-line regimens in Africa,[46] remained mostly sensitive in patients with resistant viruses. Furthermore, the majority (88%) of children in virological failure presented residual susceptibility to LPV and ATV, which establish the major PI of the 2nd-line regimen in Africa, and DRV, which is recommended in the 3rd-line regimen.[46] However, the selection of PI-resistant viruses occurring during 1st-line or 2nd-line regimens could compromise the future therapeutic options since drugs of PI class could not be active in 24% and 50% of children taking 1st-line or 2nd-line regimens in therapeutic failure, respectively. Indeed, children with viruses harboring PI resistance, although initially effective, the long-term durability of PI-based treatment regimen can be compromised by the accumulation of resistance mutations.[82,104] Furthermore, as clearly demonstrated by our observations, a second-line NNRTI regimen is often not durable in these children, as previously pointed.[82] Children who are switched to an NNRTI regimen at the time of PI failure are likely to have an increased risk of failure and resistance, due to the low genetic barrier of the regimen, previous exposure to NVP for prevention of mother-to-child transmission, and probable sub-optimal adherence. Furthermore, those failing a PI regimen, but with NRTI resistance (such as TAMs) are unlikely to achieve full virological suppression on a second-line NNRTI regimen, and thus rapidly acquire NNRTI resistance. There is therefore a need for a durable 3rd-line combination which should be at best guided by therapy history and genotypic resistance testing.[104] The best choice could be a salvage potent PI with high genetic barrier to resistance, such as DRV/r.[82,104,105] Recent data has shown that integrase inhibitors such as raltegravir and elvitegravir are also valuable in pediatric treatment.[104–106] Finally, successful 3rd-line therapy of pediatric patients is hindered by the lack of pediatric formulations and high costs, with dosing especially problematic for children younger than 6 years, largely a result of the low priority that is given globally to the development of pediatric formulations and regimens.[107]
According to the genotypic resistance results as interpreted by the ANRS algorithm, the ETR activity, a new 2nd-generation NNRTI, appeared yet compromised in around 12% of circulating HIV-1 strains, despite the fact that the drug has been never introduced in the Central African Republic. Such prevalence of predicted ETR resistance appears much higher than the rates previously reported in Western countries (2.4% and 3.8%).[108,109] In this study, primary resistance to ETR may reflect possible genetic specificity of non-B subtypes,[110] in addition to long duration of viral replication under 1st-generation NNRTI drugs pressure in Africa, as previously hypothesized.[23] Similarly, the activity of RPV, another new 2nd-generation NNRTI, appeared yet compromised according to the ANRS algorithm in around 29% of circulating HIV-1 strains, despite the fact that the drug has been never introduced in the Central African Republic. High rate (59%) of genotypic resistance to RPV was previously reported in France,[111] and is thought to be associated with natural resistance to this drug of non-B subtypes of HIV-1.[111] Finally, a very high rate (81%) of study children showed viruses resistant to TPV, a new PI approved for treatment-experienced pediatric and adolescent HIV-infected patients, according to the ANRS resistance interpretation algorithm which classifies non-B subtypes HIV-1 as naturally resistant to this drug.[112] Natural resistance to TPV is attributed to the high natural polymorphism of the protease gene of non-B subtypes,[113] in association in some children with the accumulation of DRMs to PI, as previously described.[114]
In conclusion, despite certain limitations such as absence of information on how many children died of HIV or were lost to follow-up, our study provided important information on virological outcomes of lifelong antiretroviral treatment in perinatally infected children and adolescents. Access to routine plasma HIV-1 RNA load monitoring is crucial and necessary,[30,107,115–119] although difficult in resource-constrained countries,[120] in addition to the use of potent PI-based regimens and adapted formulations for the different age classes.
Acknowledgments
The authors are particularly grateful to Dr Alexis Naissem and Mr Dionke Fofana from Expertise France. They thank Miss Rosine Feissona for excellent technical assistance. The authors also thank Dr Pierre Roques, Commissariat à l’Energie Atomique, Division of Immuno-Virologie, Institute of Emerging Diseases and Innovative Therapies, Fontenay-aux-Roses, France, for HIV-1 pol sequences analyses and European Nucleotide Archive submission. Dr M.A. Jenabian is the holder of the tier 2 Canada research chair in immuno-virology.
Footnotes
Abbreviations: 3TC = lamivudine, ABC = abacavir, Ad = adherence, AIDS = acquired immunodeficiency syndrome, ANRS = Agence Nationale de Recherches sur le SIDA et les hépatites virales, ATV = atazanavir, AZT = zidovudine, CRF = circulating recombinant form, d4T = stavudine, ddI = didanosine, DRM = drug resistance mutation, DRV = darunavir, EFV = efavirenz, ETR = etravirine, FPV = fosamprenavir, FTC = emtricitabine, IDV = indinavir, LPV = lopinavir, NNRTI = non-nucleosidic reverse transcriptase inhibitor, NRTI = nucleosidic reverse transcriptase inhibitor, NVP = nevirapine, PCR = polymerase chain reaction, PI = protease inhibitor, r = ritonavir (which boosted other PIs), RPV = rilpivirine, SQV = saquinavir, TAM = thymidine-associated mutations, TDF = tenofovir, TPV = tipravirine, V– = virological nonresponder, V+ = virological responder, VF+ = presence of virological failure, VF– = lack of virological failure, VL = viral load, WHO = World Health Organization.
Preliminary results of the study were presented at Second International African Society for Laboratory Medicine (ASLM), 30 November-4 December 2014, Cape Town, South Africa, at 7° Conférence Francophone VIH/Hépatites (AFRAVIH 2014), 27–30 avril 2014, Montpellier, France, and at 8° Conférence Francophone VIH/Hépatites (AFRAVIH 2016), 20–23 avril 2016, Bruxelles, Belgique.
Funding: Partial funding of the study was received by Ministry of Health and Population, Central African Republic, for routine analyses and by the GIP-ESTHER (“Ensemble pour une Solidarité Thérapeutique en Réseau” which is currently named as Expertise France Santé), Paris, France, for HIV genotyping. We thank Dr Thomas Lamy, Biosynex, Strasbourg, France, for providing kits for HIV-1 RNA load measurements of the study.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Joint United Nations Programme on HIV/AIDS (UNAIDS). Global AIDS response progress reporting 2015 (Updated December 2015). Available at: http://www.unaids.org/en/resources/documents/2015/GARPR_2015_guidelines. Accessed March 01, 2017. [Google Scholar]
- [2].Newell ML, Coovadia H, Cortina-Borja M, et al. Ghent International AIDS Society (IAS) Working Group on HIV Infection in Women and Children. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004;364:1236–43. [DOI] [PubMed] [Google Scholar]
- [3].Brahmbhatt H, Kigozi G, Wabwire-Mangen F, et al. Mortality in HIV-infected and uninfected children of HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr 2006;41:504–8. [DOI] [PubMed] [Google Scholar]
- [4].Manosuthi W, Chottanapand S, Thongyen S, et al. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr 2006;43:42–6. [DOI] [PubMed] [Google Scholar]
- [5].Puthanakit T, Aurpibul L, Oberdorfer P, et al. Hospitalization and mortality among HIV-infected children after receiving highly active antiretroviral therapy. Clin Infect Dis 2007;44:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Patel K, Hernán MA, Williams PL, et al. Pediatric AIDS Clinical Trials Group 219/219C Study Team. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin Infect Dis 2008;46:1751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Violari A, Lindsey JC, Hughes MD, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med 2012;366:2380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jenabian MA, Costiniuk CT, Mboumba Bouassa RS, et al. Tackling virological failure in HIV-infected children living in Africa. Expert Rev Anti Infect Ther 2015;13:1213–23. [DOI] [PubMed] [Google Scholar]
- [9].Joint United Nations Programme on HIV/AIDS (UNAIDS). ‘15 by 15’ a global target achieved. Available at: http://www.unaids.org/en/resources/documents/2015/15_by_15_a_global_target_achieved. Accessed March 01, 2017. [PubMed] [Google Scholar]
- [10].Johnson JA, Li JF, Morris L, et al. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose Nevirapine is substantially underestimated. J Infect Dis 2005;192:16–23. [DOI] [PubMed] [Google Scholar]
- [11].Sutcliffe CG, van Dijk JH, Bolton C, et al. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis 2008;8:477–89. [DOI] [PubMed] [Google Scholar]
- [12].Kébé K, Bélec L, Diop Ndiaye H, et al. The case for addressing primary resistance mutations to non-nucleoside reverse transcriptase inhibitors to treat children born from mothers living with HIV in sub-Saharan Africa. J Int AIDS Soc 2014;17:18526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rouet F, Sakarovitch C, Msellati P, et al. Abidjan ANRS 049 Ditrame Study Group. Pediatric viral human immunodeficiency virus type 1 RNA levels, timing of infection, and disease progression in African HIV-1-infected children. Pediatrics 2003;112:e289. [DOI] [PubMed] [Google Scholar]
- [14].Abrams EJ, Weedon J, Steketee RW, et al. Association of human immunodeficiency virus (HIV) load early in life with disease progression among HIV-infected infants. New York City Perinatal HIV Transmission Collaborative Study Group. J Infect Dis 1998;178:101–8. [DOI] [PubMed] [Google Scholar]
- [15].van Rossum AM, Fraaij PL, de Groot R. Efficacy of highly active antiretroviral therapy in HIV-1 infected children. Lancet Infect Dis 2002;2:93–102. [DOI] [PubMed] [Google Scholar]
- [16].Chaix ML, Rouet F, Kouakoussui KA, et al. Genotypic human immunodeficiency virus type 1 drug resistance in highly active antiretroviral therapy-treated children in Abidjan, Côte d’Ivoire. Pediatr Infect Dis J 2005;24:1072–6. [DOI] [PubMed] [Google Scholar]
- [17].Averbuch D, Schapiro JM, Lanier ER, et al. Diminished selection for thymidine-analog mutations associated with the presence of M184 V in Ethiopian children infected with HIV subtype C receiving lamivudine-containing therapy. Pediatr Infect Dis J 2006;25:1049–56. [DOI] [PubMed] [Google Scholar]
- [18].Menson EN, Walker AS, Sharland M, et al. Collaborative HIV Paediatric Study Steering Committee. Underdosing of antiretrovirals in UK and Irish children with HIV as an example of problems in prescribing medicines to children, 1997–2005: cohort study. BMJ 2006;332:1183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lwembe R, Ochieng W, Panikulam A, et al. Anti-retroviral drug resistance-associated mutations among non-subtype B HIV-1-infected Kenyan children with treatment failure. J Med Virol 2007;79:865–72. [DOI] [PubMed] [Google Scholar]
- [20].Adjé-Touré C, Hanson DL, Talla-Nzussouo N, et al. Virologic and immunologic response to antiretroviral therapy and predictors of HIV type 1 drug resistance in children receiving treatment in Abidjan, Côte d’Ivoire. AIDS Res Hum Retroviruses 2008;24:911–7. [DOI] [PubMed] [Google Scholar]
- [21].Gody JC, Charpentier C, Mbitikon O, et al. High prevalence of antiretroviral drug resistance mutations in HIV-1 non-B subtype strains from African children receiving antiretroviral therapyregimen according to the 2006 revised WHO recommendations. J Acquir Immune Defic Syndr 2008;49:566–9. [DOI] [PubMed] [Google Scholar]
- [22].Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS 2008;22:1463–73. [DOI] [PubMed] [Google Scholar]
- [23].Charpentier C, Gody JC, Mbitikon O, et al. Virological response and resistance profiles after 18 to 30 months of first- or second-/third-line antiretroviral treatment: a cross-sectional evaluation in HIV type 1-infected children living in the Central African Republic. AIDS Res Hum Retroviruses 2012;28:87–94. [DOI] [PubMed] [Google Scholar]
- [24].Kebe K, Thiam M, Diagne Gueye NR, et al. High rate of antiretroviral drug resistance mutations in HIV Type 1-infected Senegalese children in virological failure on first-line treatment according to the World Health Organization Guidelines. AIDS Res Hum Retroviruses 2013;29:242–9. [DOI] [PubMed] [Google Scholar]
- [25].Salou M, Dagnra AY, Butel C, et al. High rates of virological failure and drug resistance in perinatally HIV-1-infected children and adolescents receiving lifelong antiretroviral therapy in routine clinics in Togo. J Int AIDS Soc 2016;19:20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Reddi A, Leeper SC, Grobler AC, et al. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC Pediatr 2007;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pillay S, Bland MR, Lessells RJ, et al. Drug resistance in children at virological failure in a rural KwaZulu-Natal, South Africa, cohort. AIDS Res Ther 2014;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Orrell C, Levison J, Ciaranello A, et al. Resistance in pediatric patients experiencing virologic failure with first and second line antiretroviral therapy. Pediatr Infect Dis J 2013;32:644–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barry O, Powell J, Renner L, et al. Effectiveness of first-line antiretroviral therapy and correlates of longitudinal changes in CD4 and viral load among HIV-infected children in Ghana. BMC Infect Dis 2013;13:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kukoyi O, Renner L, Powell J, et al. Viral load monitoring and antiretroviral treatment outcomes in a pediatric HIV cohort in Ghana. BMC Infect Dis 2016;16:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ruel TD, Kakuru A, Ikilezi G, et al. Virologic and immunologic outcomes of HIV-infected Ugandan children randomized to lopinavir/ritonavir or nonnucleoside reverse transcriptase inhibitor therapy. J Acquir Immune Defic Syndr 2014;65:535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mutwa PR, Boer KR, Rusine J, et al. Long-term effectiveness of combination antiretroviral therapy and prevalence of HIV drug resistance in HIV-1 infected children in Rwanda. Pediatr Infect Dis J 2014;33:63–9. [DOI] [PubMed] [Google Scholar]
- [33].Wamalwa DC, Lehman DA, Benki-Nugent S, et al. Long-term virologic response and genotypic resistance mutations in HIV-1 infected Kenyan children on combination antiretroviral therapy. J Acquir Immune Defic Syndr 2013;62:267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zoufaly A, Fillekes Q, Hammerl R, et al. Prevalence and determinants of virological failure in HIV-infected children on antiretroviral therapy in rural Cameroon: a cross-sectional study. Antivir Ther 2013;18:681–90. [DOI] [PubMed] [Google Scholar]
- [35].Bratholm C, Johannessen A, Naman E, et al. Drug resistance is widespread among children who receive long-term antiretroviral treatment at a rural Tanzanian hospital. J Antimicrob Chemother 2010;65:1996–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gamell A, Muri L, Ntamatungiro A, et al. A case series of acquired drug resistance-associated mutations in human immunodeficiency virus-infected children: an emerging public health concern in rural Africa. Open Forum Infect Dis 2015;3:ofv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Germanaud D, Derache A, Traore M, et al. Level of viral load and antiretroviral resistance after 6 months of non nucleoside reverse transcriptase inhibitor first-line treatment in HIV-1-infected children in Mali. J Antimicrob Chemother 2010;65:118–24. [DOI] [PubMed] [Google Scholar]
- [38].Sigaloff KC, Kayiwa J, Musiime V, et al. Short communication: high rates of thymidine analogue mutations and dual-class resistance among HIV-infected Ugandan children failing first-line antiretroviral therapy. AIDS Res Hum Retroviruses 2013;29:925–30. [DOI] [PubMed] [Google Scholar]
- [39].Vaz P, Chaix ML, Jani I, et al. Risk of extended viral resistance in human immunodeficiency virus-1-infected Mozambican children after first-line treatment failure. Pediatr Infect Dis J 2009;28:e283–7. [DOI] [PubMed] [Google Scholar]
- [40].Ruel TD, Kamya MR, Li P, et al. Early failure and the development of antiretroviral drug resistance mutations in HIV-infected Ugandan children. J Acquir Immune Defic Syndr 2011;56:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ministère du Plan et de l’Economie de la République Centrafricaine. République Centrafricaine Prévalence de l’Infection VIH et Facteurs Associés en République Centrafricaine en 2010. Institut Centrafricain des Statistiques et des Etudes Economiques et Sociales. 2012. Available at: https://www.dhsprogram.com/pubs/pdf/FR263/FR263.pdf. Accessed March 01, 2017. [Google Scholar]
- [42].Guifara GA, Experience of the prevention from mother to child transmission of HIV in Central African Republic (2001–2005), 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention, Abstract no CDB034, 2014. [Google Scholar]
- [43].Newman Owiredu M, Newman L, Nzomo T, et al. Elimination of mother-to-child transmission of HIV and syphilis: A dual approach in the African Region to improve quality of antenatal care and integrated disease control. Int J Gynaecol Obstet 2015;130(Suppl 1):S27–31. [DOI] [PubMed] [Google Scholar]
- [44].Green A. The Central African Republic's silent health crisis. Lancet 2012;380:964–5. [DOI] [PubMed] [Google Scholar]
- [45].Bélec L, Mbopi-Kéou FX. HIV epidemic out of control in Central African Republic. Lancet 2012;380:1993–4. [DOI] [PubMed] [Google Scholar]
- [46].World Health Organization (WHO) recommendations. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. June 2013. Available at: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/. Accessed March 01, 2017. [PubMed] [Google Scholar]
- [47].Mossoro-Kpinde CD, Mboumba Bouassa RS, Jenabian MA, et al. Analytical performances of human immunodeficiency virus type 1 RNA-based Amplix® real-time PCR platform for HIV-1 RNA auantification. AIDS Res Treat 2016;2016:7954810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Champenois K, Bocket L, Deuffic-Burban S, et al. Expected response to protease inhibitors of HIV-1 non-B subtype viruses according to resistance algorithms. AIDS 2008;22:1087–9. [DOI] [PubMed] [Google Scholar]
- [49].Liu L, May S, Richman DD, et al. Comparison of algorithms that interpret genotypic HIV-1 drug resistance to determine the prevalence of transmitted drug resistance. AIDS 2008;22:835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Anquetil D, Deshpande A, Zongo D, et al. Susceptibility to etravirine of HIV type 1 subtype C isolates from nevirapine/efavirenz-experienced patients: comparative interpretation of ANRS and STANFORD algorithms. AIDS Res Hum Retroviruses 2012;28:1793–7. [DOI] [PubMed] [Google Scholar]
- [51].Elmi Abar A, Jlizi A, Darar HY, et al. HIV-1 drug resistance genotyping from antiretroviral therapy (ART) naïve and first-line treatment failures in Djiboutian patients. Diagn Pathol 2012;7:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Péré H, Charpentier C, Mbelesso P, et al. Virological response and resistance profiles after 24 months of first-line antiretroviral treatment in adults living in Bangui, Central African Republic. AIDS Res Hum Retroviruses 2012;28:315–23. [DOI] [PubMed] [Google Scholar]
- [53].Loubet P, Charpentier C, Visseaux B, et al. Prevalence of HIV-1 drug resistance among patients failing first-line ART in Monrovia, Liberia: a cross-sectional study. J Antimicrob Chemother 2015;70:1881–4. [DOI] [PubMed] [Google Scholar]
- [54].World Health Organization (WHO) 2010 recommendations. Antiretroviral therapy for HIV infection in infants and children: Toward universal access. Available at: http://www.who.int/publications/2010/9789241599801_eng.pdf. Accessed March 01, 2017. [PubMed] [Google Scholar]
- [55].Garang PG, Odoi RA, Kalyango JN. Adherence to antiretroviral therapy in conflict areas: a study among patients receiving treatment from Lacor Hospital, Uganda. AIDS Patient Care STDS 2009;23:743–7. [DOI] [PubMed] [Google Scholar]
- [56].Yoder RB, Nyandiko WM, Vreeman RC, et al. Long-term impact of the Kenya postelection crisis on clinic attendance and medication adherence for HIV-infected children in western Kenya. J Acquir Immune Defic Syndr 2012;59:199–206. [DOI] [PubMed] [Google Scholar]
- [57].Bennett BW, Marshall BD, Gjelsvik A, et al. HIV incidence prior to, during, and after violent conflict in 36 sub-Saharan African nations, 1990–2012: an ecological study. PLoS One 2015;10:e0142343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mann M, Diero L, Kemboi E, et al. Antiretroviral treatment interruptions induced by the Kenyan postelection crisis are associated with virological failure. J Acquir Immune Defic Syndr 2013;64:220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vreeman RC, Nyandiko WM, Ayaya SO, et al. Factors sustaining pediatric adherence to antiretroviral therapy in western Kenya. Qual Health Res 2009;19:1716–29. [DOI] [PubMed] [Google Scholar]
- [60].Vreeman RC, Nyandiko WM, Sang E, et al. Impact of the Kenya post-election crisis on clinic attendance and medication adherence for HIV-infected children in western Kenya. Confl Health 2009;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Médecins Sans Frontières. November 2011. Central African Republic: A state of silent crisis. Available at: http://www.msf.org/shadomx/apps/fms/fmsdownload.cfm?file_uuid=59E8398E-B0FF-40EA-94F9–177AF5D108B4&siteName=msf. Accessed March 01, 2017. [Google Scholar]
- [62].Moszynski P. Excessive mortality in Central African Republic is out of control, warns charity. BMJ 2011;343:d8198. [DOI] [PubMed] [Google Scholar]
- [63].Fetzer BC, Mupenda B, Lusiama J, et al. Barriers to and facilitators of adherence to pediatric antiretroviral therapy in a sub-Saharan setting: insights from a qualitative study. AIDS Patient Care STDS 2011;25:611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mills EJ, Ford N, Singh S, et al. Providing antiretroviral care in conflict settings. Curr HIV/AIDS Rep 2009;6:201–9. [DOI] [PubMed] [Google Scholar]
- [65].O’Brien DP, Venis S, Greig J, et al. Provision of antiretroviral treatment in conflict settings: the experience of Médecins Sans Frontières. Confl Health 2010;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. February 23, 2009; pp 1–139. Available at: https://aidsinfo.nih.gov/contentfiles/pediatricguidelines001391.pdf. Accessed March 01, 2017. [Google Scholar]
- [67].Mullen J, Leech S, O'Shea S, et al. Antiretroviral drug resistance among HIV-1 infected children failing treatment. J Med Virol 2002;3:299–304. [DOI] [PubMed] [Google Scholar]
- [68].Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS 2000;14:357–66. [DOI] [PubMed] [Google Scholar]
- [69].Maggiolo F, Airoldi M, Kleinloog HD, et al. Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clin Trials 2007;8:282–92. [DOI] [PubMed] [Google Scholar]
- [70].Adejumo OA, Malee KM, Ryscavage P, et al. Contemporary issues on the epidemiology and antiretroviral adherence of HIV-infected adolescents in sub-Saharan Africa: a narrative review. J Int AIDS Soc 2015;18:20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Williams PL, Storm D, Montepiedra G, et al. PACTG 219C Team. Predictors of adherence to antiretroviral medications in children and adolescents with HIV infection. Pediatrics 2006;118:e1745–1757. [DOI] [PubMed] [Google Scholar]
- [72].Rudy BJ, Murphy DA, Harris DR, et al. Adolescent Trials Network for HIV/AIDS Interventions. Patient-related risks for nonadherence to antiretroviral therapy among HIV-infected youth in the United States: a study of prevalence and interactions. AIDS Patient Care STDs 2009;23:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ryscavage PA, Anderson EJ, Sutton SH, et al. Clinical outcomes of adolescents and young adults in adult HIV care. J Acquir Immune Defic Syndr 2011;58:193–7. [DOI] [PubMed] [Google Scholar]
- [74].Bygrave H, Mtangirwa J, Ncube K, et al. Antiretroviral therapy outcomes among adolescents and youth in rural Zimbabwe. PLoS One 2012;7:e52856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Agwu AL, Fairlie L. Antiretroviral treatment, management challenges and outcomes in perinatally HIV-infected adolescents. J Int AIDS Soc 2013;16:18579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hawkins A, Evangeli M, Sturgeon K, et al. AALPHI Steering Committee. Episodic medication adherence in adolescents and young adults with perinatally acquired HIV: a within-participants approach. AIDS Care 2016;(suppl):68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med 1999;131:81–7. [DOI] [PubMed] [Google Scholar]
- [78].Murphy DA, Wilson CM, Durako SJ, et al. Antiretroviral medication adherence among the REACH HIV-infected adolescent cohort. AIDS Care 2001;13:27–40. [DOI] [PubMed] [Google Scholar]
- [79].Song R, Jelagat J, Dzombo D, et al. Efficacy of highly active antiretroviral therapy in HIV-1 infected children in Kenya. Pediatrics 2007;120:e856–861. [DOI] [PubMed] [Google Scholar]
- [80].Pillay V, Pillay C, Kantor R, et al. HIV type 1 subtype C drug resistance among pediatric and adult South African patients failing antiretroviral therapy. AIDS Res Hum Retroviruses 2008;24:1449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].van Griensven J, De Naeyer L, Uwera J, et al. Success with antiretroviral treatment for children in Kigali, Rwanda: experience with health center/nurse-based care. BMC Pediatr 2008;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gupta RK, Gibb DM, Pillay D. Management of paediatric HIV-1 resistance. Curr Opin Infect Dis 2009;22:256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Müller AD, Myer L, Jaspan H. Virological suppression achieved with suboptimal adherence levels among South African children receiving boosted protease inhibitor-based antiretroviral therapy. Clin Infect Dis 2009;48:e3–5. [DOI] [PubMed] [Google Scholar]
- [84].Musoke PM, Barlow-Mosha L, Bagenda D, et al. Response to antiretroviral therapy in HIV-infected Ugandan children exposed and not exposed to single-dose nevirapine at birth. J Acquir Immune Defic Syndr 2009;52:560–8. [DOI] [PubMed] [Google Scholar]
- [85].Ramos J. Boosted protease inhibitors as a therapeutic option in the treatment of HIV-infected children. HIV Med 2009;10:536–47. [DOI] [PubMed] [Google Scholar]
- [86].van Zyl GU, van der Merwe L, Claassen M, et al. Protease inhibitor resistance in South African children with virologic failure. Pediatr Infect Dis J 2009;28:1125–7. [DOI] [PubMed] [Google Scholar]
- [87].Wallis CL, Erasmus L, Varughese S, et al. Emergence of drug resistance in HIV-1 subtype C infected children failing the South African national antiretroviral roll-out program. Pediatr Infect Dis J 2009;28:1123–35. [DOI] [PubMed] [Google Scholar]
- [88].Gupta RK, Ford D, Mulenga V, et al. Drug resistance in human immunodeficiency virus type-1 infected Zambian children using adult fixed dose combination stavudine, lamivudine, and nevirapine. Pediatr Infect Dis J 2010;29:e57–62. [DOI] [PubMed] [Google Scholar]
- [89].Reitz C, Coovadia A, Ko S, et al. Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of cotreatment for tuberculosis. J Infect Dis 2010;201:1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Barth RE, Tempelman HA, Smelt E, et al. Long-term outcome of children receiving antiretroviral treatment in rural South Africa: substantial virologic failure on first-line treatment. Pediatr Infect Dis J 2011;30:52–6. [DOI] [PubMed] [Google Scholar]
- [91].Taylor BS, Hunt G, Abrams EJ, et al. Rapid development of antiretroviral drug resistance mutations in HIV-infected children less than two years of age initiating protease inhibitor-based therapy in South Africa. AIDS Res Hum Retroviruses 2011;27:945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Tolle M, Howard L, Kirk B, et al. Reverse transcriptase genotypes in pediatric patients failing initial antiretroviral therapy in Gaborone, Botswana. J Int Assoc Physicians AIDS Care (Chic) 2012;11:260–8. [DOI] [PubMed] [Google Scholar]
- [93].Vergne L, Kane CT, Laurent C, et al. Low rate of genotypic HIV-1 drug-resistant strains in Senegalese government initiative of access to antiretroviral therapy. AIDS 2003;17:S31–38. [DOI] [PubMed] [Google Scholar]
- [94].Kamya MR, Mayanja-Kizza H, Kambugu A, et al. Academic Alliance for AIDS Care and Prevention in Africa. Predictors of long-term viral failure among Ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr 2007;46:187–93. [DOI] [PubMed] [Google Scholar]
- [95].World Health Organization (2014). Health for the world's adolescents: a second chance in the second decade. Geneva: World Health Organization; Available at: http://apps.who.int/adolescent/second-decade/files/1612_MNCAH_HWA_Executive_Summary.pdf. Accessed March 01, 2017. [Google Scholar]
- [96].Charpentier C, Gody JC, Tisserand P, et al. Surveillance of antiretroviral drug resistance mutations in untreated young children living in the Central African Republic. Antivir Ther 2011;16:1347–50. [DOI] [PubMed] [Google Scholar]
- [97].Schoffelen AF, Wensing AM, Tempelman HA, et al. Sustained virological response on second-line antiretroviral therapy following virological failure in HIV-infected patients in rural South Africa. PLoS One 2013;8: e58526.i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Barth RE, van der Meer JT, Hoepelman AI, et al. Effectiveness of highly active antiretroviral therapy administered by general practitioners in rural South Africa. Eur J Clin Microbiol Infect Dis 2008;27:977–84. [DOI] [PubMed] [Google Scholar]
- [99].Musiime V, Kaudha E, Kayiwa J, et al. Antiretroviral drug resistance profiles and response to second-line therapy among HIV type 1-infected Ugandan children. AIDS Res Hum Retroviruses 2013;29:449–55. [DOI] [PubMed] [Google Scholar]
- [100].Dow DE, Shayo AM, Cunningham CK, et al. Durability of antiretroviral therapy and predictors of virologic failure among perinatally HIV-infected children in Tanzania: a four-year follow-up. BMC Infect Dis 2014;14:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Eshleman SH, Hoover DR, Chen S, et al. Resistance after single-dose nevirapine prophylaxis emerges in a high proportion of Malawian newborns. AIDS 2005;19:2167–9. [DOI] [PubMed] [Google Scholar]
- [102].Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med 2007;356:135–47. [DOI] [PubMed] [Google Scholar]
- [103].Micek MA, Blanco AJ, Beck IA, et al. Nevirapine resistance by timing of HIV type 1 infection in infants treated with single-dose nevirapine. Clin Infect Dis 2010;50:1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].van Zyl GU, Rabie H, Nuttall JJ, et al. It is time to consider third-line options in antiretroviral-experienced paediatric patients? J Int AIDS Soc 2011;14:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Eley BS, Meyers T. Antiretroviral therapy for children in resource-limited settings: current regimens and the role of newer agents. Paediatr Drugs 2011;13:303–16. [DOI] [PubMed] [Google Scholar]
- [106].Lee JS, Calmy A, Andrieux-Meyer I, et al. Review of the safety, efficacy, and pharmacokinetics of elvitegravir with an emphasis on resource-limited settings. HIV AIDS (Auckl) 2012;4:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Calmy AL, Ford N. Improving treatment outcome for children with HIV. Lancet 2011;377:1546–8. [DOI] [PubMed] [Google Scholar]
- [108].Cotte L, Trabaud MA, Tardy JC, et al. Prediction of the virological response to etravirine in clinical practice: comparison of three genotype algorithms. J Med Virol 2009;81:672–7. [DOI] [PubMed] [Google Scholar]
- [109].Scherrer AU, Hasse B, von Wyl V, et al. Prevalence of etravirine mutations and impact on response to treatment in routine clinical care: the Swiss HIV Cohort Study (SHCS). HIV Med 2009;10:647–56. [DOI] [PubMed] [Google Scholar]
- [110].Maïga AI, Descamps D, Morand-Joubert L, et al. Resistance-associated mutations to etravirine (TMC-125) in antiretroviral-naïve patients infected with non-B HIV-1 subtypes. Antimicrob Agents Chemother 2010;54:728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lambert-Niclot S, Charpentier C, Storto A, et al. Rilpivirine, emtricitabine and tenofovir resistance in HIV-1-infected rilpivirine-naive patients failing antiretroviral therapy. J Antimicrob Chemother 2014;69:1086–9. [DOI] [PubMed] [Google Scholar]
- [112].Marcelin AG, Masquelier B, Descamps D, et al. Tipranavir-ritonavir genotypic resistance score in protease inhibitor-experienced patients. Antimicrob Agents Chemother 2008;52:3237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Champenois K, Bocket L, Deuffic-Burban S, et al. Expected response to protease inhibitors of HIV-1 non-B subtype viruses according to resistance algorithms. AIDS 2008;22:1087–9. [DOI] [PubMed] [Google Scholar]
- [114].Hsieh SM, Chang SY, Hung CC, et al. Impact of first-line protease inhibitors on predicted resistance to tipranavir in HIV-1-infected patients with virological failure. BMC Infect Dis 2009;9:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Orrell C, Harling G, Lawn SD, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther 2007;12:83–8. [PubMed] [Google Scholar]
- [116].Calmy A, Ford N, Hirschel B, et al. HIV viral load monitoring in resource-limited regions: optional or necessary? Clin Infect Dis 2007;44:128–34. [DOI] [PubMed] [Google Scholar]
- [117].Sawe FK, McIntyre JA. Monitoring HIV antiretroviral therapy in resource-limited settings: time to avoid costly outcomes. Clin Infect Dis 2009;49:463–5. [DOI] [PubMed] [Google Scholar]
- [118].Cole SR, Napravnik S, Mugavero MJ, et al. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol 2010;171:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].World Health Organization (WHO) recommendations. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. September 2015. Available at: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf. Accessed March 01, 2017. [PubMed] [Google Scholar]
- [120].Bélec L, Bonn JP. Challenges in implementing HIV laboratory monitoring in resource-constrained settings: how to do more with less. Future Microbiol 2011;6:1251–60. [DOI] [PubMed] [Google Scholar]


