Abstract
Background:
The survival of advanced gastric cancer (GC) is dismal, and effects of antiangiogenic agents remain inconclusive. The purpose of this study is to assess combination of chemotherapy with antiangiogenic therapy versus traditional chemotherapy.
Methods:
To achieve the goal of scientific rigor, statistics from both referenced works and experiments were analyzed. We carefully searched for the referenced works by retrieving, as well as analyzing, literature databases for information on antiangiogenic therapy compared to other therapeutic approaches used to treat GC patients. Two groups were defined in the experiment: experimental and control groups. The experimental group was treated with antiangiogenic drug, and the control group was treated with standard chemotherapy or placebo.
Results:
The study included a total of 3240 participants. Overall, there was significant improvement in overall survival (hazard ratio [HR] = 0.78, 95% confidence interval [CI]: 0.67–0.91, P = 0.002), progression-free survival (HR 0.65, 95% CI: 0.52–0.81, P = 0.0002), objective response rate (risk ratio [RR] = 1.58, 95% CI: 1.33–1.88, P < 0.00001), and disease control rate (RR 2.44, 95% CI: 1.57–3.78, P < 0.0001) in the group with antiangiogenic drug versus the group with standard chemotherapy or placebo. Moreover, this new treatment approach showed tolerable toxicity.
Conclusion:
This study confirms the superior efficacy of combination therapy with antiangiogenic agents in comparison to traditional chemotherapy regimens for patients with GC. Moreover, this new treatment approach showed tolerable toxicity. This meta-analysis provides important information for clinicians who are interested in using antiangiogenic therapies to treat GC patients.
Keywords: antiangiogenic agents, gastric cancer, meta-analysis, systematic review, traditional chemotherapy
1. Introduction
Gastric cancer (GC) is a common digestive tract malignancy and one of the leading causes of cancer deaths. GC morbidity is also quite high worldwide, especially in Asia. GC is the eighth most common malignancy and sixth leading cause of cancer death worldwide, with approximately 900,000 new cases and 500,000 deaths/y. As the most frequent histological type, adenocarcinoma accounts for 90% of GCs. Although surgery is considered the only radical treatment modality for early disease, 80% of patients who present with locally advanced or metastatic GC receive limited benefit from gastrectomy. Therefore, chemotherapy plays an important role in the multidisciplinary approach to GC treatment.[1–6] Typically, traditional chemotherapy for GC includes platinum, taxanes, fluorouracil, and adriamycin.[7] However, the average survival time of 10 to 12 months demonstrates the limited effectiveness of current treatments.[8–10] In recent decades, chemotherapy has shown a poor curative effect and duration of effect, which suggests that GC treatment should involve combined therapy. At present, clinical research is focusing on whether to combine small-molecule targeted medicines with chemotherapeutics. Antiangiogenic compounds, as a type of small-molecule targeted therapy, have also been considered. Thus, new approaches are currently under investigation for GC treatment.[11–14]
Angiogenesis is associated with the processes of tumor proliferation, metastasis, and migration.[15,16] Tumor-related aberrant angiogenesis provides the nutrients necessary to grow carcinomas, thereby enhancing the antianoxia ability of bulky tumors.[17] Antiangiogenic treatment is a successful targeted approach for a variety of cancer types and has recently been added to traditional chemotherapy.[16,18–22]
Angiogenesis is the main pathway for the emergence and development of malignant tumors. Angiogenesis in the tumor environment provides the necessary nutrients and removes the metabolites produced as a result of tumor growth. In addition, cancer cells can migrate to other parts of the body via angiogenesis. Thus, the inhibition of angiogenesis in the tumor environment is vital for inhibiting tumor growth and metastasis.[23–25]
Drugs of angiogenesis function to block angiogenesis-mediated endothelial factors, thereby restraining cancer growth and metastasis. Because angiogenesis is the main mechanism responsible for tumor growth, antiangiogenic therapy has become a leading choice for the treatment of cancers.[26,27] Indeed, antiangiogenic treatment is also considered a promising therapy for GC.
Recently, several randomized controlled trials (RCTs) were conducted to evaluate the efficacy and toxicity of antiangiogenic agents in combination therapy for GC. Nevertheless, the results of these studies, in terms of survival and toxicities, are inconsistent.[28–32] Thus far, there is still no systematic review of prospective clinical trials investigating the pooled efficacy and safety of antiangiogenic agents. Therefore, in this study, a systematic review and a meta-analysis to assess the role of antiangiogenic agents in GC were conducted.
2. Methods
To achieve the goal of scientific rigor, statistics from both referenced works and experiments were analyzed. We carefully searched for the referenced works by retrieving, as well as analyzing, literature databases for information on antiangiogenic therapy compared to other therapeutic approaches used to treat GC patients. Two groups were defined in the experiment: the experimental and control groups. The experimental group was treated with antiangiogenic drug, and the control group was treated with standard chemotherapy or placebo.
2.1. Ethical clearance
The study was approved by the institutional review board, the Second Affiliated Hospital of Kunming Medical University Ethics Committee for Clinical Investigation, and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
2.2. Literature review and retrieval strategy
Twelve articles on this topic were identified, including 9 prospective studies and 3 American Society of Clinical Oncology (ASCO) meeting abstracts. These resources were found in both the PubMed and ASCO databases and included a total of 3240 participants. A flowchart for inclusion/exclusion of individual studies (or articles) is presented in Fig. 1. The final literature retrieval was updated on August 20, 2015. Two investigators independently collected the data that met the inclusion criteria, and differences were resolved by discussion. The retrieval strategy included a combination of GC and other factors, including “anti-angiogenesis drugs” or “bevacizumab,” “sunitinib,” “apatinib,” “epirubicin,” “ramucirumab,” “trebananib,” or “TSU68.”
Figure 1.
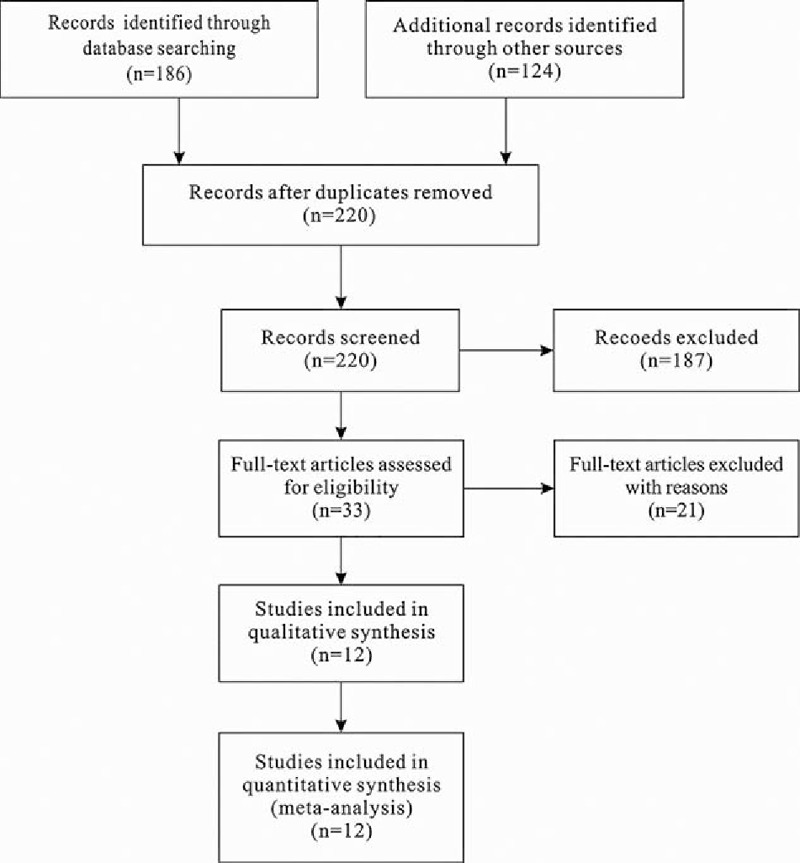
Select the relevant articles of process flowchart.
2.3. Criterion of acceptability and data extraction
The works included in the meta-analysis met the following criteria: evaluated eligible patients (≥18 years) who were histologically or cytologically diagnosed with GC and evaluated the effectiveness of antiangiogenic drug.
Information extracted from the studies included the following: the first author, publication year, total number of enrolled participants, hazard ratio (HR) of the median progression-free survival (PFS) and median overall survival (OS), risk ratio (RR) and 95% confidence interval (CI) for objective response rate (ORR) and disease control rate (DCR), common grade ≥3 adverse events, age (mean ± standard deviation or age range, years), gender, Eastern Cooperative Oncology Group (ECOG) performance status, stage, and prior surgery.
2.4. Statistical analysis and quality assessment
The main outcomes evaluated included OS and toxicity. Additional outcomes included PFS, ORR, and DCR, as well as a meta-analysis of the significance of antiangiogenic treatment for GC.
First, 2 authors evaluated the articles according to the inclusion criteria. After evaluating the articles, the 2 authors discussed and resolved any differences to reach a consensus.
The meta-analysis was strictly performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statements, as shown in the Checklist.
2.5. Statistical analysis
The analysis of statistics was based on the results of the comparison of the experiment and control groups. All study groups were well balanced in terms of baseline characteristics in each study enrolled. The results found that more than half of the patients were men or ECOG 0 to 1. Heterogeneity was shown by the frost plot and P value. In addition, a fixed method, the Mantel–Haenszel method, was used to calculate the pooled HR for survival outcomes (PFS and OS). In addition, a random method, the DerSimonian–Laird method, was used to calculate the pooled RR for dichotomous data (ORR and DCR) and toxicity with a 95% CI.
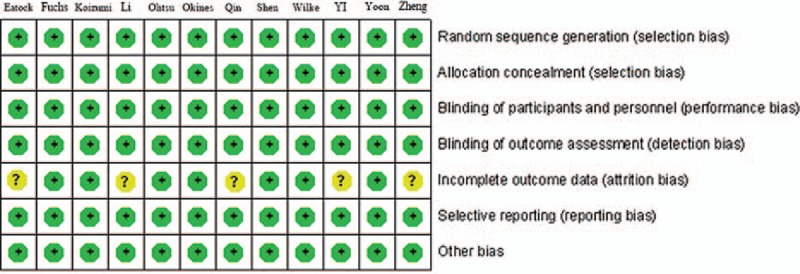
Other factors were also considered and limited in a reasonable range. All risk of bias calculations were assessed using Review Manager (version 5.2 for Windows; the Cochrane Collaboration, Oxford, UK). Publication bias was assessed using graphic funnel plots (P < 0.05 was considered statistically significant). Meanwhile, the Jadad scale was adopted to evaluate the studies in this meta-analysis and obtain a Jadad score. Figure 2 shows the results of this assessment.
Figure 2.
Evaluation of each study bias.
3. Results
3.1. Study characteristics
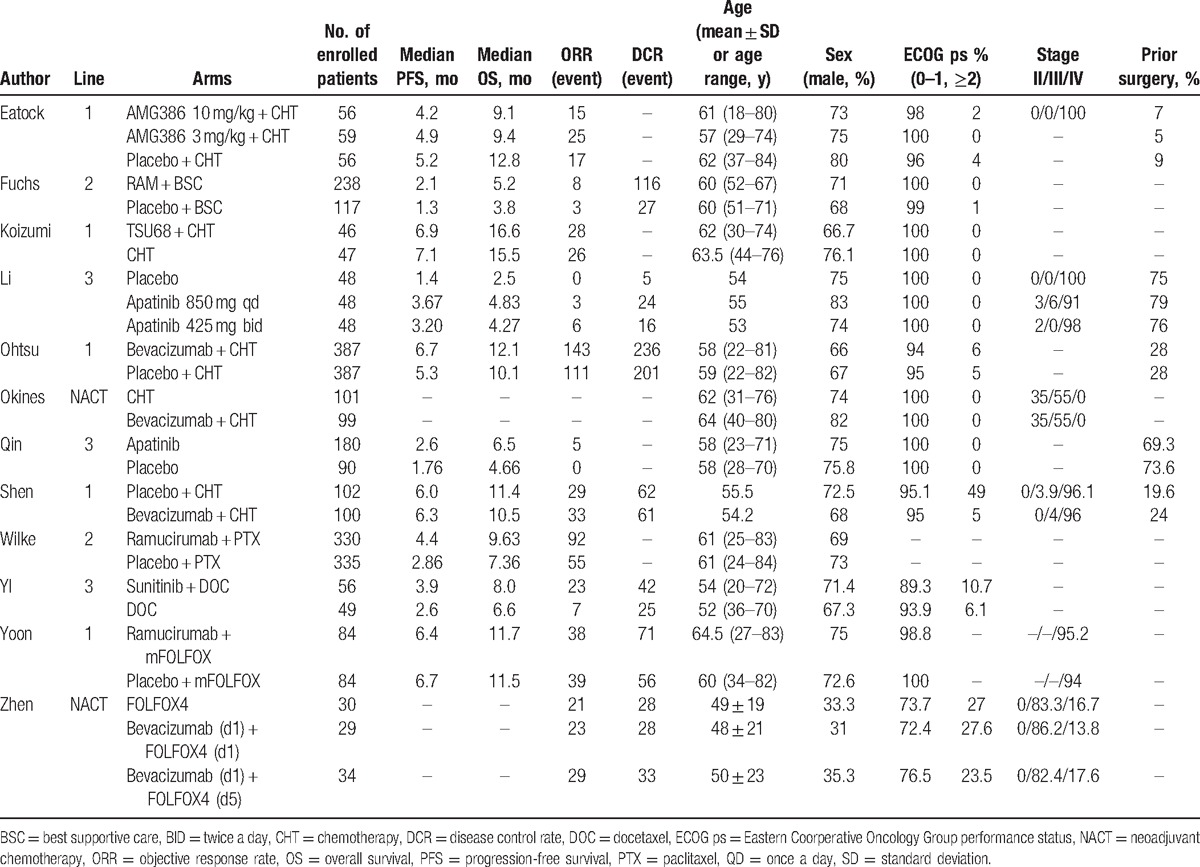
The work characteristics, patient demographic details, therapeutic methods, and curative effects for each study are presented in Table 1.
Table 1.
Characteristics of included studies and agents.
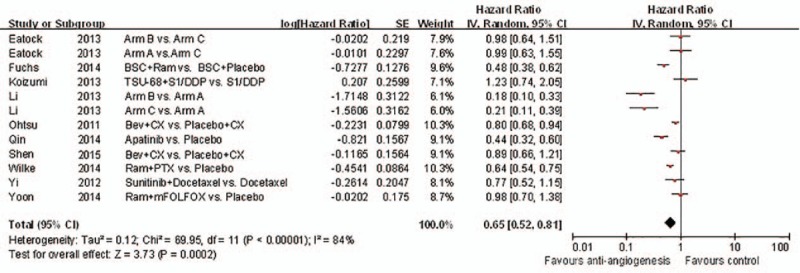
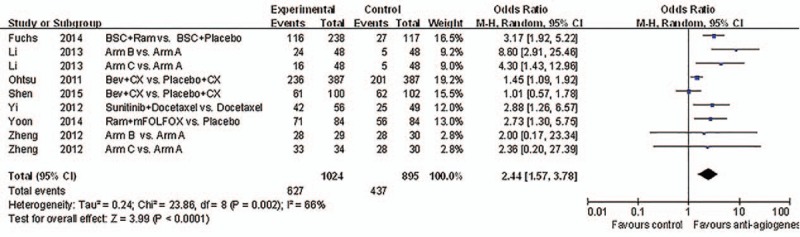
The included RCTs evaluated a total of 3240 patients. In the RCTs, 1 study provided no statistics on PFS, OS, ORR, and DCR, and 3 studies lacked statistics on the DCR. However, on the whole, the results showed improvements in PFS, OS, ORR, and DCR following antiangiogenic treatment, as shown in Figs. 3–6, respectively.
Figure 3.
Forest plot and pooled HR & 95%CI for progression-free survival: Anti-angiogeneis agents of experimental versus control group.
Figure 6.
Forest plot and pooled HR & 95%CI for disease control rate: Anti-angiogeneis agents of experimental versus control group.
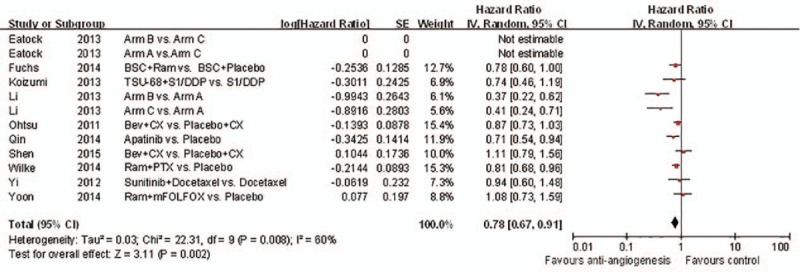
3.2. Primary outcome: OS and toxicity
Ten studies met the inclusion criteria and were ultimately included for the OS analysis (Fig. 4). The pooled HR for OS was 0.78 (95% CI: 0.67–0.91, P = 0.002). In summary, for patients with GC, joint antiangiogenic therapy increased the likelihood of survival and reduced the risk of death compared to standard chemotherapy (HR for OS 0.78, 95% CI: 0.67–0.91, P = 0.002). As a result, patients with GC benefitted from antiangiogenic agents.
Figure 4.
Forest plot and pooled HR & 95%CI for Overall survival: Anti-angiogeneis agents of experimental versus control group.
In spite of 1 study showing the heterogeneity of the included RCTs, there was no OS benefit. Similarly, other individual studies and works revealed no substantial influence on the overall result.
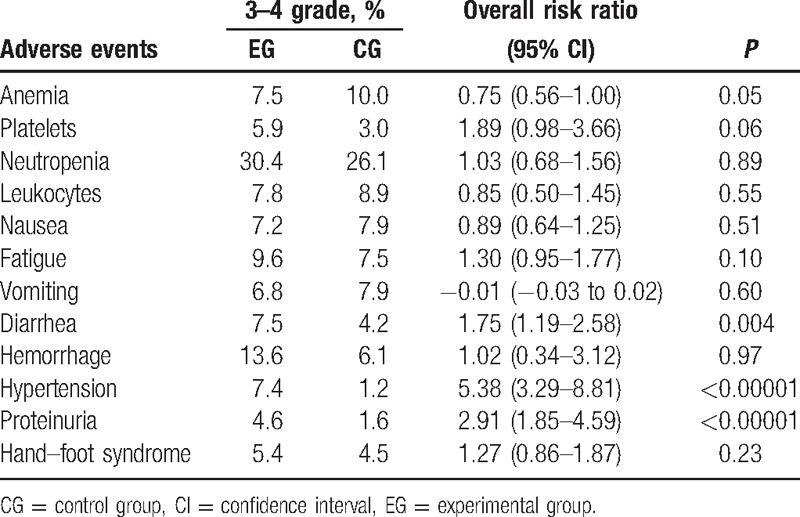
Regarding adverse events, antiangiogenic treatment resulted in a slight increase in the unit throughout the period studied. Nevertheless, the differences were not statistically significant. Table 2 lists the most common grade ≥3 toxicities.
Table 2.
Most common grade ≥3 adverse events analyzed in the meta-analysis.
3.3. Secondary outcomes: PFS, ORR, and DCR
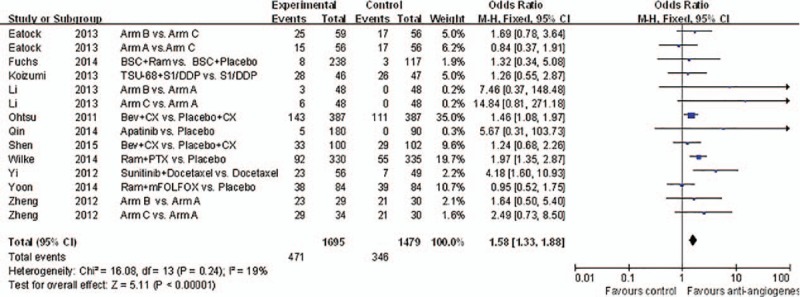
Almost all of the studies analyzed the PFS, ORR, and DCR. The experimental group showed slightly more improvement than the control group in terms of PFS (HR 0.65, 95% CI: 0.52–0.81, P = 0.0002). Figure 3 shows the comparison between the 2 groups. As shown in Fig. 5, antiangiogenic treatment improved the ORR (RR 1.58, 95% CI: 1.33–1.88, P < 0.00001).
Figure 5.
Forest plot and pooled HR & 95%CI for objective response rate: Anti-angiogeneis agents of experimental versus control group.
Patients treated with antiangiogenic drugs showed significantly better DCRs (RR 2.44, 95% CI: 1.57–3.78, P < 0.0001), as presented in Fig. 6.
Despite the pooled OS, PFS, ORR, and DCR, the above analysis showed that antiangiogenic treatment was beneficial for GC patients. As demonstrated by the statistics, the response rate to antiangiogenic therapy was higher than to control treatment, which helped to prolong the patient's life span.
3.4. Toxicity analyses
Table 2 shows detailed information for each study about the common adverse reactions that were equal to or greater than grade 3. In the analysis of hematological toxicity, nausea, and vomiting, we observed no differences between the experimental and control groups. With respect to the incidence of hemorrhage, proteinuria, and hypertension, the antiangiogenic group showed a higher incidence of these adverse reactions than the control group. However, the prevalence of these reactions was in the tolerable range.
3.5. Risk of bias and publication bias
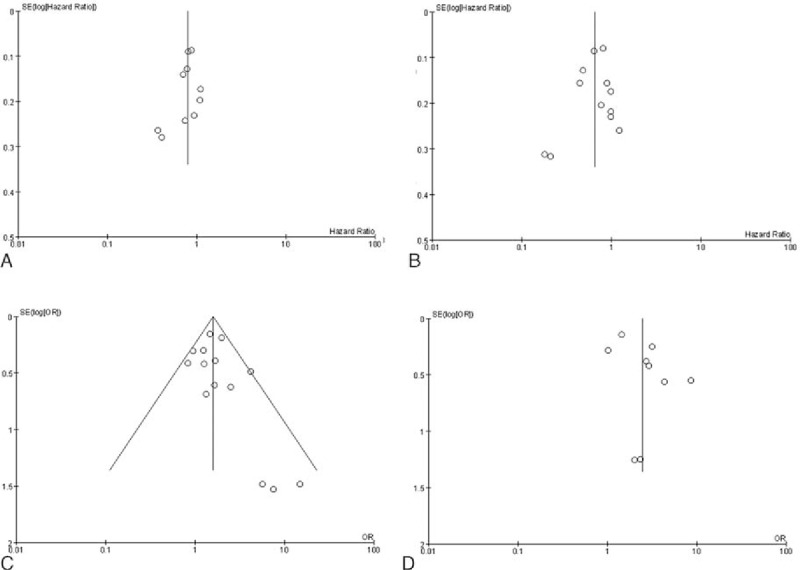
In summary, there was a low risk of bias for all analyses, including the meta-analysis. This low risk of bias was due to random sequence generation, allocation concealment, blinding of outcome assessments, incomplete outcome data, selective reporting, and other bias. In the 12 articles included in this meta-analysis, there was no high-risk bias. In 4 of the studies, the DCR risk of bias was unknown due to the lack of relative statistics data, as listed in Table 2. Figure 7 shows the statistical analysis for the publication bias, which was present in the OS analysis. However, there was no apparent publication bias in the other results, including for the PFS, ORR, and DCR.
Figure 7.
Publication bias qualitative analysis: funnel plot includes the meta-analysis of all studies outcome. (A) Overall survival, (B) progression-free survival; (C) objective response rate; (D) disease control rate.
4. Discussion
Chemotherapy plays a vital role in GC treatment, and traditional chemotherapy with platinum-based regimens, taxane-based regimens, and fluorouracil is widely used for GC patients.[33] However, the clinical outcomes of GC patients remain unsatisfactory, especially for those with extensive disease. At present, research on cytotoxic drugs for GC has stagnated. However, due to advances in molecular biology research, GC treatments such as antiangiogenic therapy have been extended to the molecular level. The goal of GC treatment is targeted therapy with minimum toxicity.[34–37] In several studies, antiangiogenic treatment has been shown to improve the survival of patients with GC. However, inconsistent response rates, survival rates, and toxicities have been reported.[20,36,37] Therefore, this meta-analysis was conducted to determine the role of antiangiogenic agents in treating GC. Based on our analysis, the combination of antiangiogenic agents with chemotherapy may be beneficial for patients with GC in terms of OS, although potential publication bias should be considered when interpreting these results.
For patients with GC, the optimal aims are to relieve cancer-related symptoms, minimize treatment-related toxicity, prolong the survival time, and improve the quality of life. Therefore, toxicity is particularly relevant. In other words, efforts should be made to simultaneously prolong the survival time and decrease the toxicity, thus improving the patient's quality of life. According to reports in the literature, the antiangiogenic treatments applied to the experimental group led to better outcomes in comparison to the control group, with fewer digestive tract reactions and hematologic toxicities. Our results demonstrated that there was a higher incidence of grade ≥3 hemorrhage, hypertension, proteinuria, and diarrhea in patients administered antiangiogenic combination regimens. In addition, the risk of fatigue and hand–foot syndrome was equivalent in the 2 groups. Therefore, combination therapy with antiangiogenic agents may be associated with tolerable toxicity and better efficacy than traditional regimens.
Not all data were shown in the original studies. Baseline characteristics (age, sex, pathologic stage, previous surgery, ECOG score, etc.) were balanced across all patients. Further analysis found that age, gender, and staging had little effect on antiangiogenic effects. The clinical efficacy of antiangiogenic therapies was difficult to predict.[38] Furthermore, it remains unclear whether antiangiogenic agents dosage should be adjusted according to the patient's age, the progress of the disease throughout the process of treatment, or whether the original treatment should be changed or terminated according to the patient's toxicity tolerance.[39] There was no treatment difference in OS or PFS in subgroup analysis.[40] The percentage of patients with an ECOG performance status of 0 in the apatinib arm was relatively higher than that in the control arm (27.3% vs 16.5%), but there was no statistically significant difference between the 2 arms (P = 0.0674).[41] According to previous studies, a greater benefit with antiangiogenic strategies can be expected in Caucasian patients’ as compared with Asian patients’ GC.[42]
Improvements in the comprehensive diagnosis and treatment of GC hold significant promise for advancing GC prognosis and improving patient quality of life. With the continuous development of science and technology, diagnosis and treatment for GC are constantly being improved. In the near future, cancer morbidity will likely be better controlled worldwide, and the recovery rate will be greatly improved.
Due to small sample sizes and other factors related to individual studies, it can be difficult to draw reliable conclusions. However, with the integration and analysis of results from multiple studies, meta-analysis has become an important method for the analysis of a given treatment modality because this approach provides more stable estimates of the effect. However, several limitations should be considered when interpreting the results of this analysis. First and foremost, this analysis involved various patient groups in the early and advanced stages of GC, which increased the clinical heterogeneity of our meta-analysis and complicated the interpretation of our results. Moreover, a variety of combined regimens were included in our study, which made it difficult to determine which treatment was most effective or contributed most to the observed heterogeneity. Therefore, larger scale, high-quality studies should be performed to identify patients who would most likely benefit from antiangiogenic treatment regimens. Last, our analysis was based on assembled data from different studies instead of original individual patient data, making the treatment benefit more uncertain.
In conclusion, our results confirm the superior efficacy of combination therapy with antiangiogenic agents in comparison to traditional chemotherapy regimens for GC patients; moreover, this new treatment approach showed tolerable toxicity. This meta-analysis provides important information for clinicians who are interested in using antiangiogenic therapies to treat GC patients.[43–45]
Footnotes
Abbreviations: ASCO = American Society of Clinical Oncology, CI = confidence interval, DCR = disease control rate, ECOG = Eastern Cooperation Oncology Group, GC = gastric cancer, HR = hazard ratio, ORR = objective response rate, OS = overall survival, PFS = progression-free survival, RCT = randomized controlled trial, RR = risk ratio.
XL, FW, and YK have contributed equally to the article.
Funding/support: The work was supported by the National Natural Science Foundation of China (nos. 81360360 and 81660399), the Yunnan Provincial Engineering Research Center of Major Surgical Diseases (2014), the Innovation Team Project of Yunnan Institutions of Higher Education (2014), the Innovation Team Project of Yunnan Province (no. 2015HC033), the Yunnan Provincial Academician Workstation of Xiaoping Chen (2016), the Breeding Program for Major Scientific and Technological Research Achievements of Kunming Medical University (no. CGPY201607), and the Medical Leading Talent Project of Yunnan Province (2017) to LW.
The authors have no conflicts of interest to disclose.
References
- [1].Li XF, Tan YN, Cao Y, et al. A case report of gastrointestinal hemorrhage and perforation during apatinib treatment of gastric cancer. Medicine (Baltimore) 2015;94:e1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhu B, Wu JR, Zhou XP. Retrospective comparison of trastuzumab plus cisplatin and trastuzumab plus capecitabine in elderly HER2-positive advanced gastric cancer patients. Medicine (Baltimore) 2015;94:e1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tey J, Choo BA, Leong CN, et al. Clinical outcome of palliative radiotherapy for locally advanced symptomatic gastric cancer in the modern era. Medicine (Baltimore) 2014;93:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim KH, Kim SH, Kim MC. How much progress has been made in minimally invasive surgery for gastric cancer in Korea?: a viewpoint from Korean prospective clinical trials. Medicine (Baltimore) 2014;93:e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ma J, Yao S, Li XS, et al. Neoadjuvant therapy of DOF regimen plus bevacizumab can increase surgical resection rate in locally advanced gastric cancer: a randomized, controlled study. Medicine (Baltimore) 2015;94:e1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Orditura M, Galizia G, Sforza V, et al. Treatment of gastric cancer. World J Gastroenterol 2014;20:1635–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nakajima T, Fujii M. What make differences in the outcome of adjuvant treatments for resected gastric cancer? World J Gastroenterol 2014;20:11567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jacome AA, Wohnrath DR, Scapulatempo Neto C, et al. Prognostic value of epidermal growth factor receptors in gastric cancer: a survival analysis by Weibull model incorporating long-term survivors. Gastric Cancer 2014;17:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Luo D, Lu ML, Zhao GF, et al. Reduced Popdc3 expression correlates with high risk and poor survival in patients with gastric cancer. World J Gastroenterol 2012;18:2423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lim SM. Targeted therapy in gastric cancer: personalizing cancer treatment based on patient genome. World J Gastroenterol 2014;20:e2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu H, Chen X, Sun J, et al. The efficacy and toxicity of paclitaxel plus S-1 compared with paclitaxel plus 5-FU for advanced gastric cancer: a PRISMA systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2014;93:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aoyagi K, Kouhuji K, Kizaki J, et al. Molecular targeting to treat gastric cancer. World J Gastroenterol 2014;20:13741–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hua T, Qinsheng W, Xuxia W, et al. Nuclear factor-kappa B1 is associated with gastric cancer in a Chinese population. Medicine (Baltimore) 2014;93:e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang K, Zhang WH, Chen XZ, et al. Survival benefit and safety of no. 10 lymphadenectomy for gastric cancer patients with total gastrectomy. Medicine (Baltimore) 2014;93:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Suo WH, Zhang N, Wu PP, et al. Anti-tumour effects of small interfering RNA targeting anion exchanger 1 in experimental gastric cancer. Br J Pharmacol 2012;165:135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wong H, Yau T. Molecular targeted therapies in advanced gastric cancer: does tumor histology matter? Therap Adv Gastroenterol 2013;6:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hwang SW, Lee DH. Is endoscopic ultrasonography still the modality of choice in preoperative staging of gastric cancer? World J Gastroenterol 2014;20:13775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].De Vita F, Di Martino N, Fabozzi A, et al. Clinical management of advanced gastric cancer: the role of new molecular drugs. World J Gastroenterol 2014;20:14537–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Singh SR. Gastric cancer stem cells: a novel therapeutic target. Cancer Lett 2013;338:110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sousa JF, Ham AJ, Whitwell C, et al. Proteomic profiling of paraffin-embedded samples identifies metaplasia-specific and early-stage gastric cancer biomarkers. Am J Pathol 2012;181:1560–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wong H, Yau T. Targeted therapy in the management of advanced gastric cancer: are we making progress in the era of personalized medicine? Oncologist 2012;17:346–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Morishita A, Gong J, Masaki T. Targeting receptor tyrosine kinases in gastric cancer. World J Gastroenterol 2014;20:4536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fontana E, Sclafani F, Cunningham D. Anti-angiogenic therapies for advanced esophago-gastric cancer. Indian J Med Paediatr Oncol 2014;35:253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Boyle M, Chun C, Strojny C, et al. Chronic inflammation and angiogenic signaling axis impairs differentiation of dental-pulp stem cells. PLoS One 2014;9:e113419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Scribner E, Saut O, Province P, et al. Effects of anti-angiogenesis on glioblastoma growth and migration: model to clinical predictions. PLoS One 2014;9:e115018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee HJ, Song IC, Yun HJ, et al. CXC chemokines and chemokine receptors in gastric cancer: from basic findings towards therapeutic targeting. World J Gastroenterol 2014;20:1681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Asaka M, Mabe K. Strategies for eliminating death from gastric cancer in Japan. Proc Jpn Acad Ser B Phys Biol Sci 2014;90:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ciliberto D, Staropoli N, Caglioti F, et al. A systematic review and meta-analysis of randomized trials on the role of targeted therapy in the management of advanced gastric cancer: evidence does not translate? Cancer Biol Ther 2015;16:1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Iacovelli R, Pietrantonio F, Farcomeni A, et al. Chemotherapy or targeted therapy as second-line treatment of advanced gastric cancer. A systematic review and meta-analysis of published studies. PLoS One 2014;9:e108940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Luo HQ, Han L, Jiang Y. Meta-analysis of six randomized control trials of chemotherapy plus anti-HER monoclonal antibody for advanced gastric and gastroesophageal cancer. Asian Pac J Cancer Prev 2014;15:5343–8. [DOI] [PubMed] [Google Scholar]
- [31].Qi WX, Shen Z, Tang LN, et al. The role of anti-VEGF agents in the treatment of advanced gastric cancer: a meta-analysis of randomized controlled trials. Tumour Biol 2014;35:7675–83. [DOI] [PubMed] [Google Scholar]
- [32].Gurzu S, Sugimura H, Orlowska J, et al. New insights in histogenetic pathways of gastric cancer. Medicine (Baltimore) 2015;94:e1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Foo M, Leong T. Adjuvant therapy for gastric cancer: current and future directions. World J Gastroenterol 2014;20:13718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang Q, Chen ZY, Chen CD, et al. Training in early gastric cancer diagnosis improves the detection rate of early gastric cancer: an observational study in China. Medicine (Baltimore) 2015;94:e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Giordano A, Cito L. Advances in gastric cancer prevention. World J Clin Oncol 2012;3:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hamashima C. Current issues and future perspectives of gastric cancer screening. World J Gastroenterol 2014;20:13767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bilici A. Treatment options in patients with metastatic gastric cancer: current status and future perspectives. World J Gastroenterol 2014;20:3905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Triana-Baltzer G, Pavlicek A, Goulart A, et al. Predictive markers of efficacy for an angiopoietin-2 targeting therapeutic in xenograft models. PLoS One 2013;8:e80132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Weller M, Stupp R, Hegi M, et al. Individualized targeted therapy for glioblastoma: fact or fiction? Cancer J 2012;18:40–4. [DOI] [PubMed] [Google Scholar]
- [40].Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—a randomized, phase III study. J Clin Oncol 2014;32:2039–49. [DOI] [PubMed] [Google Scholar]
- [41].Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–54. [DOI] [PubMed] [Google Scholar]
- [42].Javle M, Smyth EC, Chau I. Ramucirumab: successfully targeting angiogenesis in gastric cancer. Clin Cancer Res 2014;20:5875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Xu M, Qiang F, Gao Y, et al. Evaluation of a novel functional single-nucleotide polymorphism (rs35010275 G>C) in MIR196A2 promoter region as a risk factor of gastric cancer in a Chinese population. Medicine (Baltimore) 2014;93:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xu MD, Qi P, Weng WW, et al. Long non-coding RNA LSINCT5 predicts negative prognosis and exhibits oncogenic activity in gastric cancer. Medicine (Baltimore) 2014;93:e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li Q, Wang JX, He YQ, et al. MicroRNA-185 regulates chemotherapeutic sensitivity in gastric cancer by targeting apoptosis repressor with caspase recruitment domain. Cell Death Dis 2014;5:e1197. [DOI] [PMC free article] [PubMed] [Google Scholar]


