Abstract
Gold nanoparticles have received much attention recently as carriers for anticancer drugs and therapeutic oligonucleotides, but little research has investigated their potential to act as stand-alone therapeutics. Previous studies interrogating their short- and long-term systemic toxicity have found that although gold nanoparticles accumulate within and clear slowly from the liver and spleen, they do not appear to exert toxic effects in these organs. Interestingly, gold nanoparticles innately exhibit the ability to modulate the tumor microenvironment specifically by interfering with crosstalk between tumor cells and stromal cells. In this issue of ACS Nano, Mukherjee and colleagues demonstrate that bare gold nanoparticles can disturb crosstalk between pancreatic stellate cells and pancreatic cancer cells by modulating the cellular secretome to reduce the growth of desmoplastic tissue and inhibit tumor growth. In this Perspective, we highlight opportunities for anticancer targeting within the tumor microenvironment and discuss gold nanoparticles as potential mediators of microenvironment-targeted therapy.
Graphical abstract
In 1889, Stephen Paget observed that tumor metastases appear to follow nonrandom patterns and proposed the hypothesis that tumor cells, or “seeds”, preferentially metastasize to organs that optimally support their growth, or the best “soil”.1 Therefore, metastatic events can only occur if viable tumor cells reach a supportive microenvironment. This hypothesis has since withstood more than a century of scientific testing and continues to direct and to illuminate modern cancer research, both in the context of metastasis and in the context of primary tumorigenesis. Several decades of research have demonstrated that malignant cancer cells are capable of transforming stromal cells to produce growth factors and cytokines that cooperate to support tumor cell proliferation, recruit vasculature, and enable immune evasion. Thus, it has been illustrated that the seed fertilizes the soil to promote tumor growth.2 Consistently, current frontline cancer treatment strategies often incorporate the use of agents to modulate the tumor microenvironment, such as anti-angiogenic therapeutics, in order to target both the seed and the soil. Accordingly, there has been a movement in the field of cancer nanomedicine to understand basic interactions between nanomaterials and both tumor cells and the tumor microenvironment to refine nanoparticle design criteria and maximize tumor eradication.3 In this Perspective, we briefly overview the current understanding of therapeutic targeting opportunities within the tumor microenvironment and describe recent advances and the future outlook of nanomedicine exploiting the tumor micro-environment.
In order to exploit therapeutic opportunities within the tumor microenvironment, it is imperative first to understand its biology. The tumor microenvironment is defined by several cellular and extracellular components that act in concert to promote tumor growth.4 The most prominently studied cellular components that characterize the tumor microenvironment include fibroblasts, endothelial cells, and immune cells. Fibroblasts within the tumor microenvironment, known as cancer-associated fibroblasts (CAFs), take on an “activated” phenotype distinct from normal fibroblasts and are characterized by alpha-smooth muscle actin (α-SMA) expression, increased proliferation, and increased soluble factor secretion. These CAFs promote tumor growth by secreting growth factors, pro-angiogenic factors, and matrix metalloproteinases (MMPs) to enable tumor cell invasion and ultimately metastasis. Additionally, endothelial cells are recruited to the tumor site largely by vascular endothelial growth factors (VEGFs) secreted from tumor cells, fibroblasts, and immune cells. Vascular endothelial growth factor-mediated angiogenesis produces leaky vasculature that promotes hypoxic conditions characteristic of the tumor microenvironment. Immune cells within the tumor microenvironment, including tumor-associated macrophages and neutrophils, upregulate hypoxic signaling and promote angiogenesis through the release of soluble factors. Importantly, these stromal cells cooperatively maintain the tumor microenvironment through multidirectional crosstalk with tumor cells.
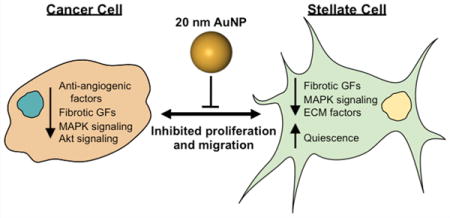
In epithelial cancers, the prevailing understanding of tumor–stroma interactions suggests that mutated, precancerous epithelial cells induce transformation in local fibroblasts through mechanisms that remain poorly understood, which trigger fibroblast production of several families of growth factors, most notably including transforming growth factor-β (TGF-β), fibroblast growth factor (FGF), epidermal growth factor (EGF), and insulin-like growth factor (IGF).5 These factors promote further epithelial transformation and proliferation to induce carcinogenesis. Therefore, disruption of tumor–stroma crosstalk by interfering with growth factor signaling may represent a promising strategy to halt tumor progression. In this issue of ACS Nano, Mukherjee and colleagues report that bare gold nanoparticles (AuNPs) can modulate the tumor microenvironment to inhibit the proliferation and migration of both tumor and stromal cells in pancreatic ductal adenocarcinoma (PDAC) (Figure 1).6 This work suggests that AuNPs exhibit innate properties that can be exploited to interrogate crosstalk mechanisms within the tumor microenvironment and disrupt these communications to produce a therapeutic outcome.
Figure 1.
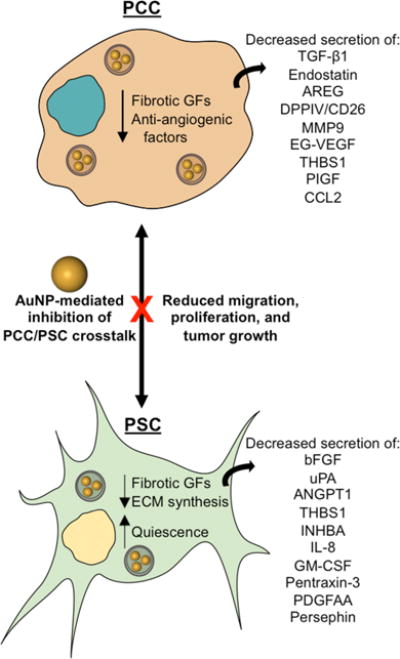
Schematic illustrating AuNP-mediated inhibition of pancreatic cancer cell (PCC) –pancreatic stellate cell (PSC) crosstalk. In the absence of treatment, PCCs and PSCs undergo bidirectional crosstalk by secreting growth factors and cytokines that promote disease progression. Gold nanoparticle treatment disrupts the crosstalk between PCCs and PSCs by altering the cellular secretome, leading to reduced migration, proliferation, and tumor growth.
Pancreatic ductal adenocarcinoma is characterized by its desmoplastic microenvironment, in which dense fibrous connective tissue envelops the tumor cells. Although research is only beginning to delineate the role of desmoplastic tissue in PDAC progression, increased desmoplasia has been linked to poorer prognosis and is potentially responsible for chemotherapy resistance;7 this underscores the significance of the microenvironment as a therapeutic target in PDAC.8 Opportunities to target the PDAC desmoplastic microenvironment largely arise from crosstalk between pancreatic stellate cells (PSCs) and pancreatic cancer cells (PCCs). Pancreatic stellate cells are fibroblasts that normally maintain the extracellular matrix (ECM) of the pancreas through the regulation of matrix proteins and MMPs. During PDAC tumorigenesis, PCCs recruit and transform PSCs to exhibit a myofibroblast-like phenotype. Activated PSCs then display increased proliferation, ECM production, soluble factor production, and migration. The effects of activated PSCs, in turn, induce PCC proliferation and apoptosis evasion. Further, activated PSCs may regulate epithelial-to-mesenchymal transition in PCCs, which increases their ability to migrate.9 Although crosstalk between PSCs and PCCs remains under investigation, several growth factors and cytokines have been implicated in their cooperative signaling, including TGF-β, FGF, connective tissue growth factor (CTGF), interleukin-1β, VEGF, and sonic hedgehog (Shh). Using AuNPs as a platform to investigate and to manipulate signaling between PSCs and PCCs, Mukherjee and colleagues report several interesting findings regarding the use of AuNPs as both stand-alone therapeutics and tools for crosstalk analysis.6
While most investigations of AuNPs in anticancer strategies simply use them as bioinert carriers for therapeutic cargo (chemotherapy drugs, nucleic acids, etc.), the current research has identified exciting novel properties of AuNPs as stand-alone cancer therapeutics for PDAC.6 These results are particularly interesting because AuNPs were demonstrated to exert this therapeutic behavior specifically within populations of cancer cells but not nonmalignant cells. For example, AuNPs (20 nm diameter) were found to reduce proliferation in PSCs and PCCs significantly but not in normal human pancreatic ductal epithelial cells or nonmalignant NIH3T3 fibroblasts.6 These observations are in good agreement with previous findings that AuNPs do not modulate the proliferation of normal ovarian epithelial cells10,11 and do not induce systemic toxicity following 3–4 weeks of repeated injections.10,11 This reduction in proliferation was determined to result, at least in part, from suppression of mitogen-activated protein kinase (MAPK) signaling, which mediates growth-factor-induced proliferation. Further, AuNP-treated PSCs return to a more quiescent phenotype, indicated by decreased ECM protein production, reduced α-SMA expression, and restored lipid metabolism.6
Significantly, AuNPs were found to disrupt PCC–PSC crosstalk and ultimately to impair PCC-mediated PSC activation. These experiments were conducted by harvesting conditioned media (CM) from PCCs and PSCs treated with AuNPs. Pancreatic cancer cells and PSCs cultured in CM from AuNP-treated PSCs or PCCs, respectively, exhibited reduced proliferation and migration relative to cells cultured in CM from untreated PSCs/PCCs.6 Mechanistically, the authors reported that AuNP treatment reduced the expression of several key autocrine and paracrine signaling factors in both PCCs and PSCs. As a result of decreased soluble factor secretion, CM from AuNP-treated PCCs impaired PSC production of ECM proteins and reduced PSC α-SMA expression. Thus, AuNP-mediated crosstalk suppression may prevent the activation of PSCs and reduce their tumor-promoting behavior.
These findings may be surprising under current assumptions in the field that AuNPs are bioinert and do not induce systemic toxicity. However, several reports have indicated that nanoparticles adsorb a number of proteins under physiological conditions that may largely determine their interactions with cells (Figure 2).12,13 Consistent with this understanding, the authors of the current research have previously reported that one mechanism by which AuNPs interfere with cellular crosstalk is by adsorbing heparin-binding growth factors such as VEGF165, FGF-2, and PIGF, which, in turn, changes their conformations to suppress their signaling.10,14 In the present study, they identify key signaling molecules that mediate PSC/PCC crosstalk in PDAC. They found that AuNPs sequester anti-angiogenic, pro-fibrotic molecules secreted by both PSCs and PCCs, as evidenced by decreased levels of these factors present within CM from AuNP-treated PSCs and PCCs. However, mRNA encoding these specific signaling molecules was also reduced in both PSCs and PCCs, indicating that AuNPs interfere with cellular crosstalk by additional mechanisms beyond protein adsorption. Interestingly, AuNPs were also found to induce endoplasmic reticulum (ER) stress, which consequently activated Ire1-dependent decay of mRNAs (RIDD) to modulate the PSC/PCC secretome. Pancreatic stellate cells and PCCs treated with AuNPs for 48 h showed increased expression of proteins that mediate RIDD, whereas this behavior was not present in cells with impaired RIDD. These results were further corroborated by the use of a predictive computational model to elucidate the downstream consequences of suppressing PSC–PCC crosstalk in PDAC. This model revealed a number of “hub” proteins, which exhibited maximal regulation over other pathways, including Activin A, THBS1, PLAU, IL-8, SERPINE1, and PTX3. Overall, this model demonstrated that AuNP-mediated crosstalk depletion produces a complex series of molecular events defined by autocrine and paracrine signaling, which is, in turn, regulated by several hub proteins.6
Figure 2.
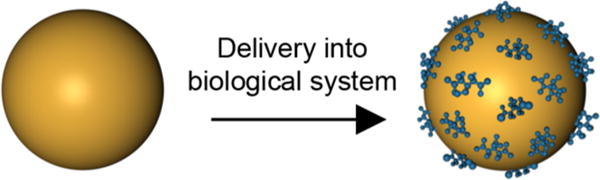
Gold nanoparticles delivered into biological systems are rapidly coated with proteins, which stabilize the AuNPs to prevent aggregation. AuNP binding also alters protein conformation to inhibit protein function.
Finally, the capability of AuNPs to disrupt crosstalk between PSCs and PCCs was tested in an orthotopic model of PDAC.6 Initial studies validated the significance of crosstalk in promoting PDAC tumor growth; tumors established by co-implantation of both PSCs and PCCs were larger than tumors established by implantation of PCCs alone. Interestingly, intraperitoneally injected AuNPs suppressed the growth of both PCC only and PSC + PCC tumors. Supporting these findings, AuNP-treated tumors exhibited decreased Ki67 staining and increased TUNEL staining, indicating that AuNPs also decrease proliferation of PSCs and PCCs in vivo and that this impaired crosstalk actually promotes apoptosis in malignant cells. This action was attributed to a specific therapeutic effect within the tumor rather than overall systemic toxicity because there was no significant change in animal weight across treatment/control groups nor evidence of toxicity in major organs by hematoxylin and eosin immunohistochemical staining. Further, the authors demonstrated that AuNPs’ ability to reverse the activated phenotype of PSCs in vitro does prevail in vivo, and this phenotype reversal was found to translate to decreased desmoplasia within the tumor. Tumors treated with AuNPs exhibited decreased α-SMA expression relative to controls as well as decreased staining for fibronectin and collagen. The observed decrease in desmoplastic indicators was accompanied by an increase in CD31-positive vessels in AuNP-treated PSC + PCC tumors. This effect was particularly impressive given the additional proteins and tissue micro-environments likely encountered by the AuNPs after intraperitoneal injection relative to the proteins present in controlled, in vitro experiments.6
Taken together, these results underscore the importance of PSC–PCC crosstalk in PDAC progression and reveal the therapeutic opportunities posed by the tumor microenvironment. This work also suggests that bare AuNPs may be a promising tool to investigate and to perturb cellular crosstalk in various disease models. It is remarkable that such a significant therapeutic effect was observed using such a simple technology. Much of the focus in the field of cancer nanomedicine has shifted to nanocomposites that interact with biological systems through some cooperative behavior across multiple components, for example, to release a payload or to undergo a therapeutic conformational change. However, this work suggests that there is still more to learn about the simple individual components of more complex systems. Similarly, there have been extensive efforts toward designing anticancer nanomaterials that target one signaling pathway with high specificity.15,16 While these materials have been successful in preclinical testing, few have advanced to clinical trials, and those that have been tested clinically have shown little benefit. One possible reason for this discrepancy between preclinical and clinical efficacy is that cancer is the result of complex interplay between many signaling pathways, as demonstrated by the computational model in the current research. Accordingly, therapeutic strategies that target just a single pathway are susceptible to inevitable resistance as cancer cells utilize alternative pathways to compensate for suppression of one pathway. The work by Mukherjee and colleagues suggests that suppressing multiple oncogenic signaling pathways may produce greater therapeutic outcomes and warrants further investigation.6
OUTLOOK AND FUTURE CHALLENGES
Until now, few studies have explored the potential for bare AuNPs to act as bioactive, stand-alone therapeutics. In this issue of ACS Nano, Mukherjee and colleagues provide evidence that AuNPs can disrupt crosstalk between PSCs and PCCs to halt the progression of PDAC tumors and reduce the growth of associated desmoplasia.6 This effect was attributed to growth factor and cytokine adsorption by AuNPs and AuNP-induced ER stress, which modulated the secretome of both PSCs and PCCs. This research opens up several interesting avenues of investigation for future studies of the use of nanomaterials to interrupt disease-promoting cellular crosstalk.
One of the most striking results of this research is that while AuNPs can sequester signaling molecules to disrupt PSC/PCC crosstalk in vitro, this effect is maintained in vivo following intraperitoneal injection of AuNPs. This result was consistent with the authors’ previous findings investigating the antiangiogenic properties of AuNPs, which also demonstrated that modifying the AuNP surface with charged ligands prevents AuNP-mediated heparin-binding growth factor inhibition.10,14 Additional work investigating NP–protein interactions has demonstrated that NPs exposed to biomolecules develop both a “hard” corona and a “soft” corona, where the hard corona consists of biomolecules with high affinity to the NP surface.12 Importantly, the hard corona is quite stable, so even after the NP is introduced to a new environment containing different biomolecules, the NP hard corona largely remains the same and will continue to dictate NP interactions with subsequently encountered environments.12 In this context, we can question: how does the route of AuNP administration affect their ability to modulate the signaling efficacy of heparin-binding growth factors? In the current research, AuNPs were delivered intraperitoneally, so they may have quickly reached the tumor site to modulate PSC/PCC crosstalk.6 It is reasonable to hypothesize that intratumoral delivery strategies, if possible, may achieve the highest degree of crosstalk suppression. However, much research aims to deliver NPs intravenously, so NPs are exposed to serum proteins before reaching their target site. One study revealed that AuNPs incubated in undiluted human serum adsorb 71 ± 22 distinct serum proteins based on the AuNP diameter and surface chemistry, and the composition of the resulting protein corona defined the cellular interaction of the AuNPs.13 Therefore, researchers may be able to tune AuNP cellular interactions by altering their route of administration.
These results lead to another interesting avenue of future investigation: how do the physicochemical properties of AuNPs, such as size, shape, and surface chemistry, influence their ability to disrupt the tumor microenvironment? The authors’ previous work investigated a range of AuNP sizes (5–100 nm) and found that 20 nm AuNPs maximally disrupted heparin-binding growth-factor-mediated signaling.10 One potential reason for this could be that this particular size efficiently balances the increased radius of curvature offered by smaller NPs, which maximizes biomolecule loading density, with maintaining sufficient surface area to facilitate ligand binding. This increased surface biomolecule density may, in turn, promote cellular uptake to mediate ER stress-related secretome modulation. Similarly, much research has been dedicated to comparing cellular interactions among various NP shapes, including spheres, rods, cylinders, cubes, and prisms. This work has revealed that NP size and shape are coupled in their effects on cellular interactions,17 suggesting that AuNPs larger than 20 nm of different shapes may exert similar or greater abilities to suppress cellular crosstalk. Finally, surface chemistry is known to impact NP–cellular interactions significantly and may play a role in regulating cellular crosstalk. One of the most common NP surface modifications is the addition of polyethylene glycol (PEG) to prevent protein adsorption, to increase blood circulation time, and to enhance NP biocompatibility. However, this behavior is highly dependent on PEG molecular weight and grafting density.18 Because PEGylation is often required to prevent intravenously injected NPs from immediate phagocytic clearance,17 the ability of AuNPs modified with variable PEG coatings to regulate cellular crosstalk poses an interesting question for future work in this area. As researchers investigate how the physicochemical properties of AuNPs influence their ability to regulate the tumor microenvironment, we will learn the answer to two important questions. First, are the parameters that maximize crosstalk inhibition consistent across tumor types? Second, how do cancer cells versus normal cells perceive AuNPs to enable manipulation of the tumor microenvironment without perturbing normal tissues?
Finally, much exciting research has investigated the potential for NP systems to potentiate the toxicity of additional therapies to produce a synergistic anticancer effect. Because desmoplasia acts as a significant barrier to drug delivery in PDAC,19 mitigating the desmoplastic reaction may improve the transport of chemotherapeutics to tumor cells and improve overall tumor reduction. Therefore, we encourage researchers to investigate whether changes in the tumor microenvironment afforded by AuNPs, such as reduced desmoplasia and increased angiogenesis, can be exploited for enhanced drug delivery and tumor regression. Others have shown that increasing tumor permeability by targeting the vasculature or disrupting the tumor ECM can enhance intratumoral drug delivery. For example, photothermal therapy can increase both vascular and cellular permeability to enhance the delivery of additional therapeutics.20–22 These studies suggest that AuNP-mediated reprogramming of the tumor microenvironment will have similar effects. Moreover, it is possible that combining AuNP-mediated crosstalk suppression to target the tumor micro-environment with traditional chemotherapies to target tumor cells directly may further expand the ability to inhibit tumor growth, as it will enable manipulation of multiple oncogenic signaling pathways simultaneously, as well as inhibition of multiple tumor-associated cells.
Few strategies have been developed to overcome therapeutic challenges posed by the tumor microenvironment in PDAC. The exciting work reported by Mukherjee and colleagues6 demonstrates the merit of a simple NP system in suppressing multiple signaling pathways required to support the progression of a devastating disease. Further, this work underscores the importance of carefully characterizing the biological consequences that are contributed by individual components of more complex nanomedicine systems. In this context, we have proposed a number of questions to further elucidate the capability of AuNPs to suppress crosstalk and to impair the tumor microenvironment. Answering these questions will reveal the optimal design parameters for such systems, aid the rational design of synergistic combination treatment strategies, and ultimately improve patient outcomes.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under Award Number R35GM119659 and by Grant No. IRG 14-251-07-IRG from the American Cancer Society. J.R.M. received support from the Department of Defense through a National Defense Science and Engineering Graduate Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of these agencies.
ABBREVIATIONS
- CAFs
cancer-associated fibroblasts
- α-SMA
alpha-smooth muscle actin
- MMP
matrix metalloproteinases
- VEGF
vascular endothelial growth factor
- TGF-β
transforming growth factor beta
- FGF
fibroblast growth factor
- EGF
epidermal growth factor
- IGF
insulin-like growth factor
- AuNPs
gold nanoparticles
- PDAC
pancreatic ductal adenocarcinoma
- PSC
pancreatic stellate cell
- PCC
pancreatic cancer cell
- ECM
extracellular matrix
- CTGF
connective tissue growth fact
- Shh
sonic hedgehog
- MAPK
mitogen activated protein kinase
- CM
conditioned media
- PIGF
placental growth factor
- mRNA
messenger ribonucleic acid
- ER
endoplasmic reticulum
- RIDD
Ire1-dependent decay of mRNAs
- THBS1
Thrombospondin 1
- PLAU
plasminogen activator, urokinase
- IL-8
interleukin-8
- SERPINE1
plasminogen activator inhibitor-1
- PTX 3
Pentraxin 3
- TUNEL
terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling
- PEG
polyethylene glycol
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Langley RR, Fidler IJ. The Seed and Soil Hypothesis Revisited-The Role of Tumor-Stroma Interactions in Metastasis to Different Organs. Int J Cancer. 2011;128:2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuzet SE, Gaggioli C. Fibroblast Activation in Cancer: When Seed Fertilizes Soil. Cell Tissue Res. 2016;365:607–619. doi: 10.1007/s00441-016-2467-x. [DOI] [PubMed] [Google Scholar]
- 3.Kanapathipillai M, Brock A, Ingber DE. Nanoparticle Targeting of Anti-Cancer Drugs That Alter Intracellular Signaling or Influence the Tumor Microenvironment. Adv Drug Delivery Rev. 2014;79:107–118. doi: 10.1016/j.addr.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill FR, Capasso M, Hagemann T. The Tumor Microenvironment at a Glance. J Cell Sci. 2012;125:5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 5.Bhowmick Na, Neilson EG, Moses HL. Stromal Fibroblasts in Cancer Initiation and Progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saha S, Xiong X, Chakraborty PK, Shameer K, Arvizo RR, Kudgus RA, Dwivedi SKD, Hossen MN, Gillies EM, Robertson JD, Dudley JT, Urrutia RA, Postier RG, Bhattacharya R, Mukherjee P. Gold Nanoparticle Reprograms Pancreatic Tumor Microenvironment and Inhibits Tumor Growth. ACS Nano. 2016 doi: 10.1021/acsnano.6b02231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, Friess H. The Role of Stroma in Pancreatic Cancer: Diagnostic and Therapeutic Implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 8.Whatcott C, Han H, Posner RG, Von Hoff DD. Tumor-Stromal Interactions in Pancreatic Cancer. Crit Rev Oncog. 2013;18:135–151. doi: 10.1615/critrevoncog.v18.i1-2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apte MV, Wilson JS. Dangerous Liaisons: Pancreatic Stellate Cells and Pancreatic Cancer Cells. J Gastroenterol Hepatol. 2012;27:69–74. doi: 10.1111/j.1440-1746.2011.07000.x. [DOI] [PubMed] [Google Scholar]
- 10.Arvizo RR, Rana S, Miranda OR, Bhattacharya R, Rotello VM, Mukherjee P. Mechanism of Anti-Angiogenic Property of Gold Nanoparticles: Role of Nanoparticle Size and Surface Charge. Nanomedicine. 2011;7:580–587. doi: 10.1016/j.nano.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai Y-Y, Huang Y-H, Chao Y-L, Hu K-Y, Chin L-T, Chou S-H, Hour A-L, Yao Y-D, Tu C-S, Liang YJ, Tsai C-Y, Wu H-Y, Tan S-W, Chen H-M. Identification of the Nanogold Particle-Induced Endoplasmic Reticulum Stress by Omic Techniques and Systems Biology Analysis. ACS Nano. 2011;5:9354–9369. doi: 10.1021/nn2027775. [DOI] [PubMed] [Google Scholar]
- 12.Monopoli MP, Salvati A, Åberg C, Dawson KA. Biomolecular Coronas Provide the Biological Identity of Nanosized Materials. Nat Nanotechnol. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 13.Walkey CD, Olsen JB, Song F, Liu R, Guo H, Olsen DWH, Cohen Y, Emili A, Chan WC. Protein Corona Fingerprinting Predicts the Cellular Interaction of Gold and Silver Nanoparticles. ACS Nano. 2014;8:2439–2455. doi: 10.1021/nn406018q. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee P, Bhattacharya R, Wang P, Wang L, Basu S, Nagy JA, Atala A, Mukhopadhyay D, Soker S. Antiangiogenic Properties of Gold Nanoparticles. Clin Cancer Res. 2005;11:3530–3534. doi: 10.1158/1078-0432.CCR-04-2482. [DOI] [PubMed] [Google Scholar]
- 15.Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, Merkel TJ, Luthi AJ, Patel PC, Cutler JI, Daniel WL, Scott AW, Rotz MW, Meade TJ, Giljohann DA, Mirkin CA, Stegh AH. Spherical Nucleic Acid Nanoparticle Conjugates as an RNAi-Based Therapy for Glioblastoma. Sci Transl Med. 2013;5:209ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng ZJ, Morton SW, Ben-Akiva E, Dreaden EC, Shopsowitz KE, Hammond PT. Layer-by-Layer Nanoparticles for Systemic Codelivery of an Anticancer Drug and siRNA for Potential Triple-Negative Breast Cancer Treatment. ACS Nano. 2013;7:9571–9584. doi: 10.1021/nn4047925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albanese A, Tang PS, Chan WCW. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 18.Pelaz B, del Pino P, Maffre P, Hartmann R, Gallego M, Rivera-Fernández S, de la Fuente JM, Nienhaus GU, Parak WJ. Surface Functionalization of Nanoparticles with Polyethylene Glycol: Effects on Protein Adsorption and Cellular Uptake. ACS Nano. 2015;9:6996–7008. doi: 10.1021/acsnano.5b01326. [DOI] [PubMed] [Google Scholar]
- 19.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The Pancreas Cancer Microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Maltzahn G, Park J-H, Lin KY, Singh N, Schwöppe C, Mesters R, Berdel WE, Ruoslahti E, Sailor MJ, Bhatia SN. Nanoparticles That Communicate in Vivo to Amplify Tumour Targeting. Nat Mater. 2011;10:545–552. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fay BL, Melamed JR, Day ES. Nanoshell-Mediated Photothermal Therapy Can Enhance Chemotherapy in Inflammatory Breast Cancer Cells. Int J Nanomed. 2015;10:6931–6941. doi: 10.2147/IJN.S93031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raeesi V, Chan WCW. Improving Nanoparticle Diffusion through Tumor Collagen Matrix by Photo-Thermal Gold Nanorods. Nanoscale. 2016;8:12524–12530. doi: 10.1039/c5nr08463f. [DOI] [PubMed] [Google Scholar]


