Abstract
Objective
Laryngotracheal stenosis (LTS) is a fibrotic process that narrows the upper airway and has a significant impact on breathing and phonation. Iatrogenic injury from endotracheal and/or tracheostomy tubes is the most common etiology. This study investigates differences in LTS etiologies as they relate to tracheostomy dependence and dilation interval.
Study Design
Case series with chart review
Setting
Single-center tertiary care facility
Subjects and Methods
Review of adult patients with LTS was performed between 2004–2015. The association of patient demographics, comorbidities, disease etiology, and treatment modalities with patient outcomes was assessed. Multiple logistic regression analysis and Kaplan-Meier analysis was performed to determine factors associated with tracheostomy-dependence and time to second procedure respectively.
Results
262 patients met inclusion criteria. Iatrogenic patients presented with greater stenosis (p=0.023), greater length (p=0.004), and located further from the vocal folds (p<0.001) compared with other etiologies. Iatrogenic patients were more likely to be African-American and have obstructive sleep apnea, type II diabetes, hypertension, COPD, stroke, and use tobacco. Iatrogenic LTS (OR=3.1, CI= 1.2–8.2), Cotton-Myer Grade 3–4 (OR=2.6, CI=1.1–6.4), and lack of intraoperative steroids (OR=2.9, CI=1.2–6.9) were associated with tracheostomy-dependence. Non-smokers, patients without tracheostomy, and idiopathic LTS patients had a significantly longer time to second dilation procedure.
Conclusion
Iatrogenic LTS presents with a greater disease burden and higher risk of tracheostomy dependence compared to other etiologies of LTS. Co-morbid conditions promoting microvascular injury, including smoking, COPD, and diabetes, were prevalent in the iatrogenic cohort. Changes in hospital practice patterns to promote earlier tracheostomy in high risk patients could reduce the incidence of LTS.
Introduction
Laryngotracheal stenosis (LTS) is a fibrotic process narrowing the upper airway passages which has a significant impact on breathing and phonation.1,2 The stenosis usually occurs within the larynx or proximal trachea, though it can occur more distally in the trachea as well. Surgical decision-making incorporates the characteristics of the stenosis on flexible laryngoscopy and bronchoscopy, subjective complaints of dyspnea, and at times CT Scan and/or pulmonary function tests. Herrington et al. contrasted the chronic nature of endoscopic treatment of LTS and its limited morbidity with more definitive open procedures and associated higher rates of morbidity and mortality.3 Large studies are limited given the relatively uncommon nature of LTS, yet they have established key patient outcomes including tracheostomy and time to second procedure or more generally, the interval between dilations.4
The principal etiologies of LTS include iatrogenic, idiopathic, autoimmune, and traumatic.1,4 Studies examining etiology-specific LTS characterize specific variations and disparate responses to treatment.1,4,5 In a large multi-institutional study of idiopathic subglottic stenosis, Gelbard et al. demonstrated that idiopathic patients were usually able to avoid tracheostomy irrespective of whether an open or endoscopic surgical approach was used for treatment.6 In a another study with a high proportion of autoimmune patients, Hseu et al. demonstrated that surgical techniques, use of steroid injection, and mitomycin C application did not affect procedure interval between procedures.1 In a single-institution study, Gelbard et al demonstrated that tracheostomy dependence is higher in iatrogenic and autoimmune associated LTS than in traumatic or idiopathic LTS.4 Iatrogenic injury from endotracheal and/or tracheostomy tubes occurs more frequently than other etiologies.4,7 Studies show endotracheal tube sizes larger than 7.5 place patients at higher risk for tracheal stenosis and posterior glottic stenosis.8,9 Additionally, comorbidities adversely affecting wound healing, including diabetes, were found to be intrinsic risk factors for glottic stenosis and associated with iatrogenic LTS.9–11 Nevertheless further studies are necessary to improve our understanding of etiology-specific factors, patient comorbidities, and treatment modalities impact on patient outcomes.
This study investigates a large cohort of LTS patients with a focus on prevalent comorbidities stratified by LTS etiology that may influence tracheostomy-dependence and dilation interval. We aim to build on recent publications that show iatrogenic LTS patients to have higher-grade stenosis, higher association with tracheomalacia, and worse outcomes, which in part may be due to these patients’ comorbidities. Identification of intrinsic patient characteristics may impact the management of LTS patients and reduce its incidence. Furthermore, we anticipate the data presented in this study will inform clinician and patient expectations regarding treatment options and outcomes in LTS.
Methods
Patient Selection
IRB approval was obtained from the Johns Hopkins University Institutional Review Board (NA00081469) prior to beginning this study. Medical records were reviewed from 2004–2015 and all patients with LTS were reviewed. Patients included in the study were ≥ 18 years old and had documented stenosis on flexible laryngoscopy or CT imaging that was not the result of bilateral vocal fold paralysis or tracheomalacia alone. LTS etiology was determined by review of the medical record including classification within the Otolaryngology notes. Patients with isolated glottic or supraglottic stenosis were excluded from this study.
Recorded Variables
Patient demographics were recorded. Co-morbidities, smoking history, autoimmune blood markers, and tracheostomy status at first presentation were documented. Prior history of an open procedure was defined as open procedures of the trachea except tracheostomy. Intraoperative usage of steroids, mitomycin C, incision type, balloon or bougie dilation was also documented. Distance, length, and degree of compromise were recorded from flexible laryngoscopy exam or CT scan and Cotton-Myer grade was determined. Location of stenosis was recorded and patients who had stenosis at multiple sites were identified. Patients with subglottic stenosis with a component of glottic stenosis were analyzed separately from patients with multilevel stenosis that did not have any glottic involvement.
Patient Outcomes
The primary outcome in this study is tracheostomy status at last follow-up visit with the secondary outcome being time to second procedure. Operative interventions, defined as any procedure relating to managing the stenosis, and the total number of procedures were documented. The number of procedures per year was calculated by dividing the follow-up duration in years by the number of procedures that the patient underwent during this time.
Statistical Analysis
All statistical analysis was performed using STATA 12.0. (STATACorp, College Station, Texas). Parametric vs. non-parametric variables were identified graphically and with the Skewness-Kurtosis test. Chi-squared tests were used for normally distributed categorical variables. Student t-tests were used for continuous variables. Analysis of variance (ANOVA) tests were used to compare parametric continuous variables between etiology-specific LTS groups, and Kruskal-Wallis one-way analysis of variance was used for nonparametric variables. A Holm-Sidak post-hoc pairwise multiple comparison analysis was used to further identify statistically significant sub-groups for ANOVA tests and Dunn’s test of multiple comparison was used for post-hoc analysis of Kruskal-Wallis tests. Chi-squared post-hoc analyses were performed by splitting groups into two-by-two tables for analysis. For multiple logistic regression models, all outcome variables were initially tested with univariate analysis. Statistically significant associations were subsequently included in the multiple logistic regression model. Kaplan-Meier curves were used to model time to second procedure. P-values of less than 0.05 were considered statistically significant.
Results
Demographic characteristics and co-morbid conditions of study participants stratified by LTS etiology are shown in Table 1. 98.7% of idiopathic stenosis participants were female, as compared to 53.1% of iatrogenic and 63.3% of autoimmune participants (p<0.001). Twenty-nine percent of iatrogenic stenosis participants were African American, which was significantly (p<0.001) higher than the 8.5% of autoimmune and 4.1% idiopathic participants. Iatrogenic LTS participants had a significantly higher Charlson Co-morbidity Index (CCI) when compared to autoimmune or idiopathic stenosis patients(p<0.001). A comparison between specific comorbidities within the CCI is listed in Table 1. Stenosis characteristics were significantly (p<0.001) different between LTS etiologies with iatrogenic stenosis more likely to be further from the glottis when compared to autoimmune and idiopathic stenosis, and more likely to have a longer stenotic segment when compared to those with idiopathic stenosis. Additionally, the compromise percentage for iatrogenic stenosis was significantly higher than that for autoimmune stenosis (61.2 ± 21.3 vs. 49.9 ± 19.3, p<0.001). A majority of the multi-level stenosis patients in this study had glottic involvement (61.5%), but, interestingly, glottic involvement for multilevel stenosis was more commonly seen in autoimmune LTS than in the iatrogenic cohort. Figure 1 compares the total number of patients within each etiology stratified by Cotton-Myer grade. The average number of procedures per year was not significantly different for patients irrespective of stenosis etiology. However, idiopathic LTS patients had a significantly (p<0.001) longer time to second procedure than all patients with all other types of LTS. (Figure 2A) Patients that were tracheostomy-dependent at last follow-up had a shorter time to second procedure when compared to patients who were tracheostomy-free. (Figure 2B) Additionally, smokers also had a shorter time to second procedure than non-smokers. (Figure 2C)
Table 1.
Patient Demographics and Stenosis Characteristics Stratified By Etiology
| Patient Characteristics | Total | Iatrogenic (N=128) | Autoimmune (N=49) | Idiopathic (N=74) | Othera (N=11) | P-value | |
|---|---|---|---|---|---|---|---|
| Age in years | (Mean ± SDb) | 46.7 ±16.4 | 48.8 ±18.2 | 40.7 ±14.7 | 47.9 ±13.0 | 39.5 ±16.7 | 0.477 m |
| Sex N (%) | Male Female |
85 (32.4) 177 (67.6) |
60 (46.9)* 68 (53.1)* |
18 (36.7)* 31 (63.3)* |
1 (1.4)Ψ 73 (98.7)Ψ |
6 (54.6)* 5 (45.5)* |
<0.001k |
| Race N (%) | Caucasian African American Asian Hispanic Other |
186 (72.1) 45 (17.4) 4 (1.6) 6 (2.3) 17 (6.6) |
66 (52.4)Ψ,* 37 (29.4)Ψ,* 3 (2.4)Ψ,* 5 (4.0)Ψ,* 15 (12.0)Ψ,* |
41 (85.4) 4 (8.5) 1 (2.1) 0 (0) 1 (2.1) |
70 (94.6) 3 (4.1) 0 (0) 1 (1.4) 0 |
9 (81.8) 1 (9.1) 0 (0) 0 (0) 1 (9.1) |
<0.001k |
| BMIc | 30.1 ± 9.5 | 30.2 ± 9.6 | 28.1 ± 9.2 | 31.3 ± 9.8 | 29.8 ± 6.8 | 0.643 m | |
| Co-morbidities | |||||||
| Charlson Co-morbidity Index | Mean ± SD | 0.82 ±1.1 | 1.1 ±1.3 Ψ | 0.40 ± 0.8 | 0.66 ±0.9 | 0.73 ± 1.0 | 0.003 m |
| GERDd | N (%) | 57 (22.3) | 21 (16.7) * | 8 (16.7) | 24 (33.8) | 4 (40.0) | 0.014 k |
| OSAe | N (%) | 20 (7.8) | 17 (13.5) * | 2 (4.1) | 1 (1.4) | 0 (0) | 0.009 k |
| DMIIf | N (%) | 45 (17.7) | 33 (26.4) * | 4 (8.3) | 8 (11.3) | 0 (0) | 0.007 k |
| HTNg | N (%) | 79 (30.9) | 51 (40.5) * | 10 (20.4) | 15 (21.1) | 3 (30.0) | 0.011 k |
| COPDh | N (%) | 26 (10.2) | 24 (19.1)Ψ,* | 1 (2.0) | 1 (1.4) | 0 (0) | <0.001 k |
| CVAi | N (%) | 5 (2.0) | 5 (4.0) | 0 (0) | 0 (0) | 0 (0) | 0.154 k |
| MIj | N (%) | 2 (0.80) | 2 (1.6) | 0 (0) | 0 (0) | 0 (0) | 0.556 k |
| Tobacco Use | N (%) | 69 (27.8) | 54 (44.6)Ψ,* | 7 (14.9) | 6 (8.5) | 2 (22.2) | <0.001 k |
| Stenosis Characteristics | |||||||
| Distance from Glottis | Mean (cm) ± SD | 2.2 ± 1.4 | 2.9 ± 1.6 Ψ,* | 1.1 ± 0.7 | 1.8 ± 0.8 | 1.5 ± 0.5 | <0.001n |
| Length of Stenosis | Mean (cm) ± SD | 1.9 ± 1.2 | 2.2 ± 1.5 * | 1.9 ± 0.84 | 1.5 ± 0.8 | 2.1 ± 0.5 | 0.01 n |
| Compromise % | Mean (%) ± SD | 57.4 ± 19.4 | 61.2 ± 21.3Ψ | 49.9 ± 19.3 | 56.0 ±15.3 | 63.5 ± 21.1 | 0.022 m |
| Location N (%) |
Subglottic Tracheal MLS o with GI p MLS o without GI p |
161 (61.7) 75 (28.6) 10 (3.8) 16 (16.1) |
51 (39.8) Ψ,* 65 (50.8) Ψ,* 3 (2.3) Ψ,* 7 (5.5) Ψ,* |
39 (81.3) 1 (2.1) 6 (12.2) 4 (8.2) |
66 (89.2) 6 (8.1) 1 (1.4) 1 (1.6) |
5 (45.5) 2 (18.2) 0 4 (36.4) |
<0.001k |
| Procedural Outcomes | |||||||
| Average Number of Procedures per Year | Mean ± SD | 1.3 ± 1.9 | 1.0 ± 2.2 | 1.4 ± 1.7 | 1.3 ± 1.1 | 2.2 ± 2.9 | 0.206 m |
Bolded indicates statistical difference between a group when compared to ‘Iatrogenic’ group determined through post-hoc analysis.
Asterisk (*) indicates statistical difference between a group when compared to ‘Idiopathic’ group determined through post-hoc analysis.
Cross (Ψ) indicates statistical difference between group and ‘Autoimmune’ group determined through post-hoc analysis.
Italicized indicates statistical difference between group and ‘Other’ group determined through post-hoc analysis.
“Other” etiology includes radiation, external trauma, infection, and congenital causes
SD: Standard Deviation;
BMI: Body Mass Index;
GERD: Gastroesophageal reflux disease;
OSA: Obstructive sleep apnea;
DMII: Type II Diabetes Mellitus;
HTN: Hypertension;
COPD: Chronic Obstructive Pulmonary Disease;
CVA: Cerebrovascular Accident;
MI: Myocardial Infarction;
Chi squared test used;
Student’s t-test used;
ANOVA test used;
Kruskal-Wallis test used;
MLS: Multilevel Stenosis;
GI: Glottic involvement
Figure 1. Cotton-Myer Stenosis Grade Frequency By Etiology.
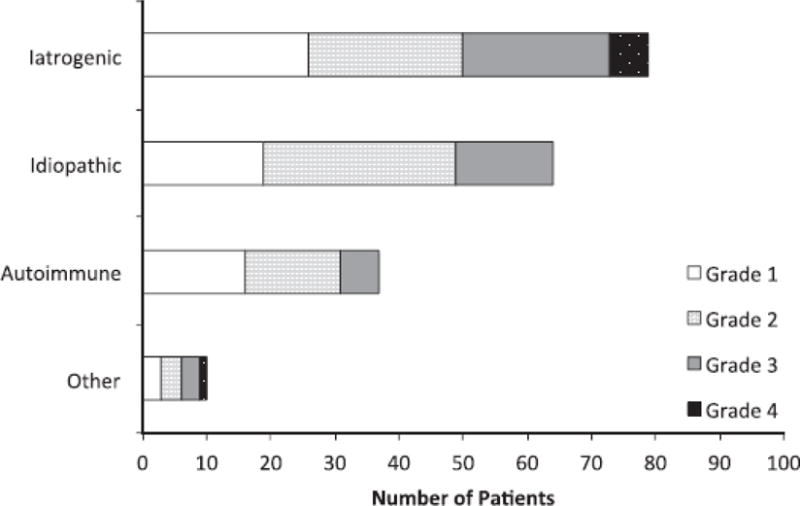
Demonstrates iatrogenic group has greater percentage of Grade 3/Grade 4 stenosis than other sub-categories. Grade 1 (<50% compromise), Grade 2 (50–70%), Grade 3 (70–99%), Grade 4 (100%)
Figure 2. Kaplan-Meier Time to Second Procedure Curves.
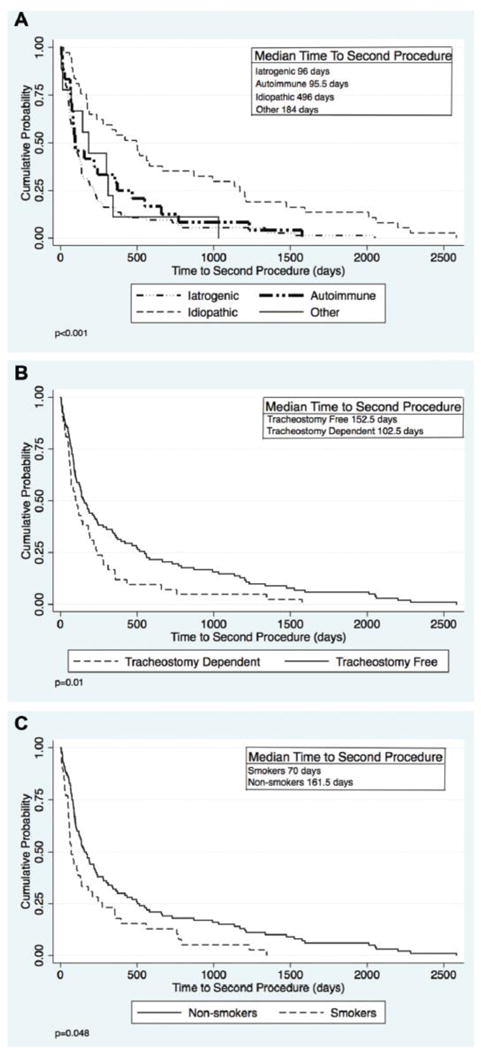
Time to second procedure stratified by (A) etiology (B) tracheostomy status (C) smoking status. Only patients who had a second procedure were included (N=144).
Tracheostomy status was assessed with univariate and multi-variate analysis. Table 2 stratifies LTS patients by tracheostomy status at last follow-up shows tracheostomy dependence was associated with older age, female gender, iatrogenic etiology, ethnicity, higher CCI, type II diabetes, COPD, smoking, and Cotton-Myer grade 3 or grade 4 stenosis. Multiple logistic regression analysis in Table 3 shows that iatrogenic LTS (OR=3.1, CI= 1.2–8.2), Cotton-Myer Grade 3–4 (OR=2.6, CI=1.1–6.4), and lack of intraoperative steroids (OR=2.9, CI=1.2–6.9) were associated with tracheostomy-dependence.
Table 2.
Comparison of Factors Associated with Tracheostomy Free Survival at Last Follow-Up
| Patient Characteristics | Tracheostomy-Free (N=192) | Tracheostomy-Dependent (N=69) | P-value a | |
|---|---|---|---|---|
| Age in years | Mean ± SDb | 45.2 ±15.9 | 50.8 ±17.5 | 0.02d |
|
| ||||
| Sex N (%) | Male Female |
53 (63.1) 139 (78.5) |
31 (36.9) 38 (21.5) |
0.008e |
|
| ||||
|
Race N (%) |
Caucasian African American Asian Hispanic Other |
147 (79.0) 22 (48.9) 3 (75.0) 4 (66.7) 13 (76.5) |
39 (21.0) 23 (51.1) 1 (25.0) 2 (33.3) 4 (23.5) |
0.002e |
|
| ||||
| BMIc | Mean ± SD | 30.0 ± 9.4 | 30.2 ± 10.0 | 0.947d |
|
| ||||
| Charlson Co-morbidity Index | Mean ± SD | 0.70 ± 0.97 | 1.1 ± 1.4 | 0.006d |
|
| ||||
| COPDf (N%) | 14 (53.9) | 12 (46.2) | 0.023e | |
|
| ||||
| DMIIg (N%) | 27 (58.7) | 19 (41.3) | 0.019e | |
|
| ||||
| Etiology N(%) |
Iatrogenic Autoimmune Idiopathic Other |
71 (55.5) 42 (87.5) 71 (96.0) 8 (72.7) |
57 (44.5) 6 (12.5) 3 (4.1) 3 (27.3) |
<0.001e |
|
| ||||
| Location N (%) |
Subglottic |
124 (77.0) |
37 (23.0) |
0.197e |
|
|
||||
| Tracheal | 23 (30.7) | 52 (69.3) | ||
|
|
||||
| MLSh with GIi | 5 (31.3) | 11 (68.8) | ||
|
|
||||
| MLSh without GIi | 5 (50.0) | 5 (50.0) | ||
|
| ||||
| Tobacco Use N (%) | Smoker Non-smoker |
41 (59.4) 142 (79.3) |
28 (40.6) 37 (20.7) |
0.001e |
|
| ||||
| Cotton Myer Grade N (%) |
Grade 1 or 2 Grade 3 or 4 |
114 (84.4) 31 (57.4) |
22 (15.6) 23 (42.6) |
<0.001e |
|
| ||||
| Previous Open Tracheal Procedure | N (%) | 29 (64.4) | 16 (35.6) | 0.141e |
p value <0.05 considered significant;
SD: Standard Deviation;
BMI: Body Mass Index;
t-test was used to obtain p value;
Chi-squared test used to obtain p-value;
COPD: Chronic Obstructive Pulmonary Disease;
Type 2 Diabetes Mellitus;
Multilevel stenosis;
Glottic involvement; Table shows row percentages
Table 3.
Multiple Logistic Regression Model of Tracheostomy Free Survival At Last Follow-Up
| Tracheostomy-Free Survival Multiple Logistic Regression Model | |||
|---|---|---|---|
| Variable | Regression Coefficient (95% CI) | Odds Ratio (95% CI) | P-value |
| Non-Iatrogenic Etiology | 1.14 (0.18, 2.1) | 3.1 (1.2, 8.2) | 0.020 |
| Previous Open Tracheal Procedure | −0.35 (−1.4, 0.75) | 0.71 (0.24, 2.1) | 0.534 |
| Intraoperative Steroid Use | 1.04 (0.15, 1.9) | 2.8 (1.2, 6.9) | 0.022 |
| Tobacco Use | −0.55 (−1.5, 0.46) | 0.58 (0.21, 1.6) | 0.287 |
| Cotton-Myer Grade 1 or 2 Stenosis* | 0.95 (0.04, 1.9) | 2.6 (1.1, 6.4) | 0.041 |
Adjusted for age, gender, race, Charlson Co-morbidity Index score
Cotton-Myer Grade 3 or 4 stenosis used as reference
The association of treatment modalities on tracheostomy free survival when stratified by iatrogenic etiology was included in a separate multiple logistic regression model shown in Table 4. Overall, these analyses showed no significant differences in overall odds of remaining tracheostomy free for any treatment modality other than steroid use. Steroid use remained more likely to be associated with tracheostomy free survival (OR=3.5, 95% CI 1.6–7.7) in the overall population. Stratifying by iatrogenic etiology status made this association disappear.
Table 4.
Multiple Logistic Regression Model of Tracheostomy Free Survival and Treatment Modalities
| Treatment | All LTS Patients | Iatrogenic | Non-Iatrogenic | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | P value | OR | 95% CI | P value | ||
| Steroids | 3.5 | 1.6 – 7.7 | 0.002 | 2.5 | 0.91 – 7.1 | 0.076 | 7.0 | 1.0 – 48.4 | 0.050 | |
| Incision Type | Cold Laser |
0.71 | 0.28 – 1.8 | 0.465 | 0.92 | 0.29 – 3.0 | 0.112 | 0.27 | 0.03–2.8 | 0.780 |
| 0.78 | 0.21 – 2.9 | 0.713 | 0.24 | 0.04 – 1.4 | 0.107 | * | * | * | ||
| Dilation Type | Bougie Balloon |
1.6 | 0.52 – 4.7 | 0.801 | 1.3 | 0.35 – 4.9 | 0.679 | 4.8 | 0.18–130.8 | 0.350 |
| 1.7 | 0.58 – 5.1 | 0.333 | 1.8 | 0.49 – 6.9 | 0.363 | 9.4 | 0.37 – 239.8 | 0.174 | ||
| Mitomycin C | 3.4 | 0.81–14.0 | 0.095 | 2.9 | 0.45 – 18.8 | 0.263 | * | * | * | |
| Open Procedure | 0.67 | 0.28 – 1.6 | 0.374 | 0.64 | 0.22 – 1.9 | 0.421 | 1.8 | 0.11 – 29.9 | 0.677 | |
Utilized multiple logistic regression; all adjusted for age, sex, race;
OR – odds ratio, CI – confidence interval
Mitomycin C application (N=12) and laser incision cases (N=20) were all tracheostomy free at last follow-up and could therefore not be included in the model as they predicted the outcome perfectly for non-iatrogenic stenosis alone.
Discussion
This study showed that primary outcome of tracheostomy dependence was associated with iatrogenic LTS, Cotton-Myer Grade 3–4, and lack of intraoperative local steroid injection. Tracheostomy dependence and tobacco use were associated with a decrease in time to second procedure, whereas idiopathic etiology was associated with a longer time to second procedure. Iatrogenic LTS demonstrated some unique features, located further from the vocal folds than idiopathic and autommune LTS, with a greater length of stenosis than idiopathic LTS, and greater narrowing than autommine LTS. Furthermore, iatrogenic LTS had prevalent comorbidities including a significantly higher rate of GERD, OSA, DM II, HTN, and tobacco use when compared with idiopathic LTS.
The disparate behavior of LTS etiology is evident when comparing iatrogenic LTS directly with idiopathic subglottic stenosis. The idiopathic cohort in this study demonstrated a predominant Cotton-Myer Grade 1–2 classification (76.6%), female gender (98.7%), Caucasian ethnicity (94.6%), and a low 4.1% incidence of tracheostomy-dependence, unique patient characteristics that correspond with other publications.1,4,12,13 A favorable treatment response was also seen in our cohort with a significant increased time interval to second procedure for idiopathic subglottic stenosis patients when compared to all other etiologies. While, this outcome may be related to the lower disease burden in patients with idiopathic subglottic stenosis, it may also be due to the specific pathophysiology causing this subset of LTS.1,4,12,13
This study demonstrated that patients who received a steroid injection were more likely to be tracheostomy free. As the tracheostomy dependent patients in this study had a shorter time to second procedure, these patients appeared to be treated more aggressively, likely to exhaust all options for decannulation. The underlying mechanism for this finding may be due to steroids reduction of inflammation slowing the recurrence of fibrosis. Evaluating treatment modalities beyond steroid use demonstrated that variations in technique were not associated with remaining tracheostomy-dependent or time to second procedure. Similarly, Hseu et al. showed similar conclusions that variations in dilation type (balloon vs. bougie), mitomycin C application, incision type (cold vs. CO2) did not affect procedural interval.1 Isolating treatment variations for iatrogenic LTS patients showed no differences, implying that surgeon preference may be used to guide instrument choice for excision and dilation.
Intrinsic comorbidities such as diabetes, chronic smoking, COPD, and higher CCI likely impact the dysregulated mucosal wound healing following intubation. Diabetes has previously been shown to increase the likelihood of tracheal injury and long-term tracheostomy dependence, presumably through microvascular injury resulting in impaired wound healing.4,9,11 It is possible that tobacco exposure may prime the large airway mucosa for injury and lead to worse outcomes, particularly in iatrogenic stenosis where direct damage to the airway through instrumentation is occurring. As in COPD, smoking-induced damage to large airway mucosa lessens the vascular supply, rendering the area more susceptible to mechanical injury, thereby promoting pathologic wound repair of the epithelium and lamina propria.14,15–17 This population would be interesting to characterize in future studies where the role of tobacco, a modifiable risk factor, on the large airways may be more thoroughly analyzed. Through a similar mechanism, the impact of a high CCI as a predisposing factor for iatrogenic LTS may be indicative of a number of ischemic conditions, including heart disease, diabetes, and peripheral vascular disease, that interfere with normal wound healing in intubated patients predisposing them to a scar phenotype. Changes in hospital practice patterns to promote earlier tracheostomy in high risk patients and reducing the use of large endotracheal tubes could have an outsized effect on reducing the incidence of LTS.
This study has several limitations. We included only stenosis patients and their treatment courses at this study institution. Previous treatment(s) at outside hospitals were not included, due to lack of available records. This confounder may bias our study towards fewer procedures in some patients. Additionally, we relied upon chart review to identify the stenosis etiology. It is possible that some non-iatrogenic cases of LTS were classified as iatrogenic because they were uncovered after an intubation. However, the large number of clinically homogenous patients in this cohort suggests that this was not a common problem. Furthermore, tracheomalacia has been shown to highly associated with iatrogenic LTS and to be a risk factor for tracheostomy, but was not analyzed in this study due to lack of regular reporting of this associated phenomenon.4 This study did not differentiate between current smokers and former smokers, which may impact the development of LTS and impact laryngotracheal injury healing. However, both current and former smokers have been shown to sustain long-term damage to airway mucosa.18–20 Finally, this is a retrospective study and is inherently limited by its design. It would therefore be inappropriate to draw any causal relationships from this study. However, the large number of LTS patients involved in this study, in combination with the extensive description of associations within LTS etiology specific sub-groups, provides valuable information to clinicians regarding patient outcomes and treatment management and may be included in a meta-analysis to better analyze this rare disease.
Conclusion
Iatrogenic LTS, Cotton-Myer Grade 3–4 stenosis, and not using local steroids were associated with tracheostomy-dependence in this large LTS cohort. Iatrogenic LTS presents further from the vocal folds, has a longer length of stenosis, and greater narrowing when compared with other etiologies. Co-morbid conditions promoting microvascular injury, including smoking, COPD, and diabetes, as well as obstructive sleep apnea and hypertension were more prevalent in iatrogenic LTS. Iatrogenic LTS is often difficult to treat and the results of this study will be useful for practicing airway surgeons as well as for other specialties that treat intubated patients. With raised awareness of factors associated with iatrogenic LTS, changes in hospital practice patterns to promote earlier tracheostomy in high-risk patients and reduce the use of large endotracheal tubes could have an outsized effect on reducing the incidence of LTS.
Acknowledgments
There were no contributors to this work who did not qualify for authorship.
Funding Source: This study was funded with grant number 1K23DC014082. In addition, research reported in this publication was supported by the Triological Society and American College of Surgeons. Support for Kevin Motz’s effort on this publication is through an NIH T32 training grant
Sponsor’s Role
Funding Source: Funding for this study had no direct impact on study design/conduct of the study/data collection, management/analysis/interpretation of the data, and preparation/review/or approval of the manuscript.
References
- 1.Hseu AF, Benninger MS, Haffey TM, Lorenz R. Subglottic stenosis: a ten-year review of treatment outcomes. The Laryngoscope. 2014;124(3):736–741. doi: 10.1002/lary.24410. [DOI] [PubMed] [Google Scholar]
- 2.Hillel AT, Karatayli-Ozgursoy S, Benke JR, et al. Voice quality in laryngotracheal stenosis: impact of dilation and level of stenosis. Ann Otol Rhinol Laryngol. 2015;124(5):413–418. doi: 10.1177/0003489414564249. [DOI] [PubMed] [Google Scholar]
- 3.Herrington HC, Weber SM, Andersen PE. Modern management of laryngotracheal stenosis. The Laryngoscope. 2006;116(9):1553–1557. doi: 10.1097/01.mlg.0000228006.21941.12. [DOI] [PubMed] [Google Scholar]
- 4.Gelbard A, Francis DO, Sandulache VC, Simmons JC, Donovan DT, Ongkasuwan J. Causes and consequences of adult laryngotracheal stenosis. The Laryngoscope. 2015;125(5):1137–1143. doi: 10.1002/lary.24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman GS, Thomas-Golbanov CK, Chan J, Akst LM, Eliachar I. Treatment of subglottic stenosis, due to Wegener’s granulomatosis, with intralesional corticosteroids and dilation. J Rheumatol. 2003;30(5):1017–1021. [PubMed] [Google Scholar]
- 6.Gelbard A, Donovan DT, Ongkasuwan J, et al. Disease homogeneity and treatment heterogeneity in idiopathic subglottic stenosis. The Laryngoscope. 2015 Nov; doi: 10.1002/lary.25708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadkaree SK, Schwartz D, Gerold K, Kim Y. Use of Bronchoscopy in Percutaneous Dilational Tracheostomy. JAMA Otolaryngol–Head Neck Surg. 2016;142(2):143–149. doi: 10.1001/jamaoto.2015.3123. [DOI] [PubMed] [Google Scholar]
- 8.Halum SL, Ting JY, Plowman EK, et al. A multi-institutional analysis of tracheotomy complications. The Laryngoscope. 2012;122(1):38–45. doi: 10.1002/lary.22364. [DOI] [PubMed] [Google Scholar]
- 9.Hillel AT, Karatayli-Ozgursoy S, Samad I, et al. Predictors of Posterior Glottic Stenosis: A Multi-Institutional Case-Control Study. Ann Otol Rhinol Laryngol. 2015 Oct; doi: 10.1177/0003489415608867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelbard A, Francis DO, Sandulache VC, Simmons JC, Donovan DT, Ongkasuwan J. Causes and consequences of adult laryngotracheal stenosis. The Laryngoscope. 2014 Oct; doi: 10.1002/lary.24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Su Z-Z, Hu L-J, et al. Analysis of the risk factors causing tracheal stenosis after tracheotomy for mechanical ventilation in 560 patients. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;42(11):839–842. [PubMed] [Google Scholar]
- 12.Pagliuca G, Rosato C, Martellucci S, et al. Cytologic and functional alterations of nasal mucosa in smokers: temporary or permanent damage? Otolaryngol–Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2015;152(4):740–745. doi: 10.1177/0194599814566598. [DOI] [PubMed] [Google Scholar]
- 13.Saetta M, Turato G, Facchini FM, et al. Inflammatory cells in the bronchial glands of smokers with chronic bronchitis. Am J Respir Crit Care Med. 1997;156(5):1633–1639. doi: 10.1164/ajrccm.156.5.9701081. [DOI] [PubMed] [Google Scholar]
- 14.Jeffery PK, Li D. Airway mucosa: secretory cells, mucus and mucin genes. Eur Respir J. 1997;10(7):1655–1662. doi: 10.1183/09031936.97.10071655. [DOI] [PubMed] [Google Scholar]
- 15.Rees PJ, Ayres JG, Chowienczyk PJ, Clark TJ. Irritant effects of cigarette and cigar smoke. Lancet Lond Engl. 1982;2(8306):1015–1017. doi: 10.1016/s0140-6736(82)90051-4. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado F, Loiselle A, Depew ZS, et al. Idiopathic subglottic stenosis: an evolving therapeutic algorithm. The Laryngoscope. 2014;124(2):498–503. doi: 10.1002/lary.24287. [DOI] [PubMed] [Google Scholar]
- 17.Grillo HC, Mark EJ, Mathisen DJ, Wain JC. Idiopathic laryngotracheal stenosis and its management. Ann Thorac Surg. 1993;56(1):80–87. doi: 10.1016/0003-4975(93)90406-8. [DOI] [PubMed] [Google Scholar]
- 18.Lee JJ, Liu D, Lee JS, et al. Long-term impact of smoking on lung epithelial proliferation in current and former smokers. J Natl Cancer Inst. 2001;93(14):1081–1088. doi: 10.1093/jnci/93.14.1081. [DOI] [PubMed] [Google Scholar]
- 19.van Oijen MG, Gilsing MM, Rijksen G, Hordijk GJ, Slootweg PJ. Increased number of proliferating cells in oral epithelium from smokers and ex-smokers. Oral Oncol. 1998;34(4):297–303. doi: 10.1016/s1368-8375(98)00007-4. [DOI] [PubMed] [Google Scholar]
- 20.Wistuba II, Lam S, Behrens C, et al. Molecular damage in the bronchial epithelium of current and former smokers. J Natl Cancer Inst. 1997;89(18):1366–1373. doi: 10.1093/jnci/89.18.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]


