Abstract
Subsets of patients with a remote history of Kawasaki disease (KD) have coronary artery aneurysms with associated risks of late morbidity. In a pilot study we previously showed that computed tomography (CT) coronary artery calcium (CAC) scoring detects late CAC in patients with aneurysms and a remote history of KD. We performed CT calcium volume scoring in 166 subjects (median age 19.5 years) with a remote history of KD (median interval from KD to CT 15.1 years). Coronary arteries were classified as normal (n = 100), transiently dilated (n = 23), persistently dilated (n = 10), remodeled aneurysm (n = 9), or aneurysm (n = 24) based on echocardiography. All subjects with coronary arteries classified as normal, persistently dilated, or remodeled aneurysm had zero CAC. Of the 24 subjects with coronary aneurysms, all but five had CAC (median volume 542 mm3; range 17 mm3 to 8,218 mm3). For subjects imaged nine or more years after their acute KD (n=144), the presence of CAC had a sensitivity of 95% and a specificity of 100% for detecting coronary artery abnormalities (defined as coronary artery aneurysm and/or stenosis). In conclusion, coronary calcification was not observed in subjects with a history of KD who had normal coronary arteries by echocardiography during the acute phase. Coronary calcification, which may be severe, occurs late in patients with coronary aneurysms. Therefore, CAC scanning may be a useful tool to screen patients with a remote history of KD or suspected KD and unknown coronary artery status.
Keywords: Calcium Scoring, Kawasaki Disease, Aneurysm, Computed Tomography
Introduction
Computed tomography (CT) coronary artery calcium (CAC) scoring requires no contrast and relatively little radiation. This test is widely used to detect plaque due to atherosclerotic coronary artery disease.1 In a pilot study we previously showed that CAC scoring also detects calcium in patients with coronary artery aneurysms (CAA) and a remote history of Kawasaki disease (KD).2 Here we present the results of a larger follow-up study evaluating the utility of CAC scoring by CT to detect coronary artery abnormalities in patients with a history of KD.
Methods
We recruited subjects of age ≥ 10 years from 2 cohorts. Cohort 1 (n=90) consisted of patients with a history of KD diagnosed, treated, and followed by the KD Research Center at UCSD Medical Center and Rady Children’s Hospital San Diego. Cohort 2 (n=76) were self-referred, had a remote history of KD, and were originally diagnosed at another center. In 3 subjects in Cohort 2, the diagnosis of KD was based on a history of a KD-compatible illness and the presence of proximal coronary artery aneurysms diagnosed by CT or coronary angiography independently reviewed by two cardiologists. This study was approved by the Institutional Review Board of the University of California and all subjects provided informed consent or assent with parental consent as appropriate.
Coronary artery status was determined based upon initial echocardiograms performed during the acute and subacute phase of KD. Subjects with a coronary artery Z score (standard deviation from the mean for the internal diameter of the coronary artery normalized for body surface area) for the right coronary artery (RCA) or left anterior descending coronary artery (LAD)) <2.0 on serial echocardiograms during the acute and subacute illness were designated as normal. Subjects with a CA Z score of >2.0 and <3.0 that returned to normal within six weeks of fever onset were designated as transiently dilated. Subjects with a Z score ≥3.0 but <10.0 were designated as aneurysm and subjects with Z ≥10.0 or CA internal diameter ≥ 8mm were designated as giant aneurysm. For subjects with dilated or aneurysmal coronary artery abnormalities or unknown initial coronary artery status, the most recent CTA or invasive coronary angiogram was used when available. Subjects were classified into five categories by coronary status: 1) No history of coronary artery dilation (AHA Risk Level I), 2) transient (< 6 weeks) coronary artery dilation (AHA II), 3) persistent (≥ 6 weeks) coronary artery dilation, 4) remodeled aneurysm (AHA III/IV), 5) aneurysm (AHA III/IV). Subjects completed a standardized questionnaire documenting medications, cardiac history, and risk factors for atherosclerotic coronary artery disease (physician-diagnosed hypertension, diabetes mellitus, hyperlipidemia, family history of early atherosclerotic coronary artery disease defined as disease diagnosed at age < 55 years in a first degree relative, and current smoking). Coronary artery abnormality was defined as the presence of coronary artery aneurysm and/or stenosis on the most recent available imaging. Major adverse cardiac events (MACE) were defined as myocardial infarction, coronary artery bypass graft (CABG) surgery, or percutaneous coronary intervention.
The CT scanning protocol has been previously described.2 In brief, subjects were scanned during a single breath-hold on a 64-slice Discovery CT750 HD scanner (GE Healthcare, Milwaukee, WI) in gated axial mode with 2.5 mm slice thickness. For three of the subjects the tube energy was set to 120 kVp and for the remainder the tube energy was set to100 kVp (instead of the tube energy of 120 kVp traditionally used for calcium scoring) in order to minimize the radiation dose and the tube current was chosen based on subjects’ body mass index. The median radiation dose-length product (DLP) was 43 mGy-cm, corresponding to a median effective radiation dose of 0.61 mSv. If clinically indicated, CT angiography was performed in selected patients using the scanner described above with prospective gating.
Images for CAC scoring were reconstructed using a 512 × 512 matrix and analyzed offline using a Ziostation workstation (QI Imaging, Redwood City, CA). Coronary calcium volume was measured using a minimum threshold of 130 Hounsfield units for the 3 patients imaged using 120 kVp and 147 Hounsfield units for the remainder imaged using 100 kVp, following the work of Nakazatu et al. to adjust for the lower tube energy, and a minimum volume of 2.5 mm3.3 Regions of calcification of the coronary arteries were manually identified on the workstation and for each subject the total volume of coronary calcification was measured.
Serum high sensitivity C-reactive protein (hsCRP) was measured by ELISA (GenWay Biotech, Inc) in a subset of subjects (n=106) on whom blood samples were collected as part of their participation in the San Diego Adult KD Collaborative Study. Concentration of hsCRP was also determined for 80 age-matched control subjects with a median value of 1.58 mg/L, IQR 0.39–1.11 mg/L.
We calculated medians and interquartile ranges (IQR) for all continuous parameters studied. The two-tailed Mann–Whitney U test was used to assess differences between continuous variables and the chi-squared test was used to compare proportions for discrete variables. Correlation between continuous variables was assessed using Pearson’s correlation coefficient. For all tests a two-sided p <0.05 was considered statistically significant.
Results
Demographic data are summarized in Table 1. The median subject age was 19.5 years (IQR 15.6 – 25.5 years, range 10.0 – 59.8 years) and the median interval from KD to CT was 15.1 years (IQR 11.1–20.6 years, range 1.5 – 53.3 years).
Table 1.
Patient Characteristics
| Cohort | |||
|---|---|---|---|
| Variable | 1 (N=90) |
2 (N=76) |
Total (N=166) |
| Males (%) | 60 (67) | 44 (58) | 104 (63) |
| Age at onset of KD (years): | 2.4 (1.4–5.2) | 4.0 (2.1–7.1) | 3.5 (1.5–5.9) |
| Age at time of calcium score, (years): | 16.7 (13.6–19.6) | 24.9 (20.1–31.1) | 19.7 (15.8–25.4) |
| Interval from onset of KD to calcium score (years): |
13.2 (10.6–15.8) | 20.0 (13.6–27.1) | 15.1 (11.1–20.6) |
| Body mass index (kg/m2): | 22 (19–24) | 23 (20–27) | 22 (20–25) |
| Coronary artery status | |||
| Normal: | 51 (57%) | 49 (64%) | 100 (60%) |
| Transiently dilated: | 22 (24%) | 1 (1%) | 23 (14%) |
| Persistently dilated: | 6 (7%) | 4 (5%) | 10 (6%) |
| Remodeled aneurysm: | 9 (10%) | 0 (0%) | 9 (5%) |
| Aneurysm: | 2 (2%) | 22 (29%) | 24 (14%) |
| Atherosclerosis risk factors | |||
| Hypertension: | 1 (1%) | 13 (17%) | 14 (8%) |
| Diabetes mellitus: | 1 (1%) | 3 (4%) | 4 (2%) |
| Tobacco use, ever: | 8 (9%)) | 14 (18%)) | 22 (13%)) |
| Hyperlipidemia: | 5 (5%)) | 18 (24%)) | 23 (14%)) |
| Family history of early CAD (< 55 years): | 18 (19%)) | 17 (22%)) | 35 (21%)) |
| Medications | |||
| Statin: | 1 (1%)) | 9 (12%)) | 10 (6%)) |
| ACE-I or ARB: | 2 (2%)) | 5 (7%)) | 7 (4%)) |
| Beta blocker: | 0 | 10 (13%)) | 10 (6%)) |
| Calcium channel blocker: | 0 | 3 (4%)) | 3 (2%)) |
| Warfarin or NOAC: | 1 (1%)) | 11 (14%)) | 12 (7%)) |
| Aspirin: | 3 (3%)) | 26 (34%)) | 29 (17%)) |
| Clopidogrel: | 0 | 5 (7%)) | 5 (3%)) |
| MACE | 1 (1%)) | 12 (16%)) | 13 (8%)) |
| Myocardial infarction | 1 (1%)) | 6 (8%)) | 7 (4%)) |
| Coronary artery bypass graft surgery | 0 | 5 (7%)) | 5 (3%)) |
Key: Cohort 1: Patients originally diagnosed, treated, and followed by the Kawasaki Disease Research Center at the University of California, San Diego Medical Center and Rady Children’s Hospital
Cohort 2: All other patients
Remodeled aneurysm: Artery that is acutely aneurysmal but undergoes myointimal proliferation with filling in of the aneurysm such that the lumen is no longer dilated on follow-up imaging
Hyperlipidemia: Subject report of having this diagnosis
ACE-I: Angiotensin-converting enzyme inhibitors
ARB: Angiotensin receptor blocker
NOAC: Novel oral anticoagulant (rivaroxaban, apixaban, edoxaban, dabigatran).
Values for continuous variables are listed as median (IQR).
Values for discrete variables are listed as n (%).
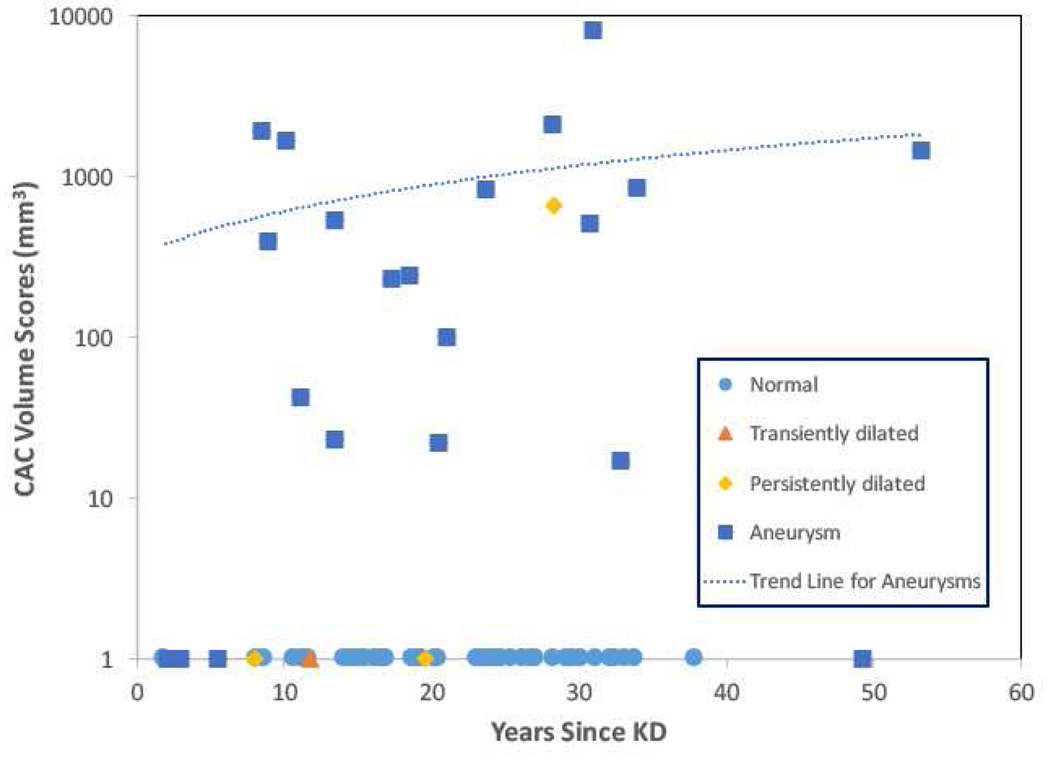
CAC volume scores are summarized in Table 2 and shown graphically in Figure 1 for those subjects in Cohort 2. All but one subject in Cohort 1 had zero CAC. All subjects (n=100) with normal coronary arteries during the acute and subacute illness, had zero CAC. Similarly, all subjects with transient coronary artery dilation, (n=23) and remodeled aneurysms (n=9), had zero CAC. For subjects with persistently dilated coronary artery arteries dilation (n=10), one subject, a 33 yo male described previously2 had a CAC of 666 and all others had zero CAC. The one subject with the elevated CAC presented with unstable angina and required emergent bypass surgery for a critical stenosis that was heavily calcified in the left main coronary artery.
Table 2.
Calcium Score Results
| Coronary Artery Status | Calcium Volume (mm3) | |||
|---|---|---|---|---|
| 0 | 1–100 | 101–1000 | >1000 | |
| Normal | 100 | 0 | 0 | 0 |
| Transiently Dilated | 22 | 0 | 1 | 0 |
| Persistently Dilated | 10 | 0 | 0 | 0 |
| Remodeled Aneurysm | 9 | 0 | 0 | 0 |
| Aneurysm | 5 | 5 | 8 | 6 |
Figure 1.
CT calcium volume scores (on a logarithmic scale) for Cohort 2 subjects as a function of time since KD onset, coded by coronary artery status (n= 74 subjects; 2 subjects are not included because the interval since KD onset is unknown). A linear fit to the CT calcium scores is shown for subjects with aneurysms. Key: Assessment of coronary artery status by echocardiography during the acute and subacute phase. Normal = no coronary artery dilation, transiently dilated = dilated internal dimension of at least one coronary artery with subsequent normalization.
For subjects with aneurysms, 19 of 24 (79%) patients had calcification (median volume score 542 mm3; range 17 mm3 to 8,218 mm). Of the 5 subjects with aneurysms and zero calcification, four of the five were imaged within 6 years of KD onset.
For the subjects (n=20) with non-zero CAC scores there was no significant correlation between the CAC scores and times since KD onset (r2 = 0.05, p = NS). Of the 20 subjects with non-zero calcium scores, nine had zero CAD risk factors, five had one risk factor, and six had two or more risk factors. The patients in this group with zero CAD risk factors were younger than those with one or more CAD risk factors (median age 20.8 vs 33.2 years, p=0.01). The most common risk factor for patients in this group was hyperlipidemia (n=8).
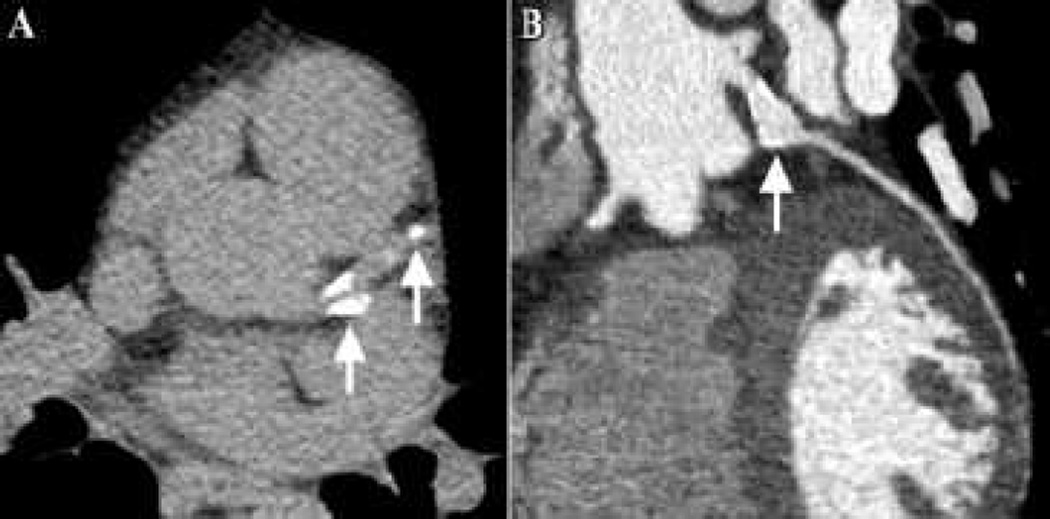
Figure 2 shows an example of calcification of the right coronary artery (RCA) artery from a subject with giant aneurysms. Figure 3 shows images from a 34 year old male with a history of KD at age 1 but unknown coronary artery status. CT calcium scoring detected calcification of the subject’s LAD and ramus intermedius arteries. Therefore a CT angiogram was obtained that demonstrated a 7 mm aneurysm of the proximal ramus intermedius artery for which the patient was initiated on aspirin and warfarin with a target INR of 2.0–3.0.
Figure 2.
CT images from a 28 year old male with a history Kawasaki disease (KD) at age 14 and coronary aneurysms. (A) Image from CT calcium scan demonstrating coronary calcification of the right coronary artery (arrows). (B) Curved oblique images from CT angiogram demonstrating a large fusiform aneurysm (arrows) of the right coronary artery.
Figure 3.
CT images from a 34 year old male with a history Kawasaki Disease (KD) at age 1 and unknown coronary artery status. (A) Image from CT calcium scan demonstrating coronary calcification of the left anterior descending and ramus intermedius arteries (arrows). (B) Curved oblique images from CT angiogram demonstrating a large focal proximal aneurysm (arrow) of the ramus intermedius artery.
For all subjects, MACE occurred in a total of 13 subjects, all but one of whom had aneurysms. The one subject with a MACE who did not have an aneurysm was the subject with persistent dilation and CAC of 666. For subjects with aneurysms, MACE occurred in 2 of 5 (40%) subjects with CAC = 0. One subject, a 57 yo Filipino male, had a history consistent with missed KD in childhood and presented with an ST elevation myocardial infarction associated with a thrombus in a distal RCA aneurysm.4 The other subject, a 19 yo male, was treated with IVIG for acute KD at age 13 years. Despite treatment, he developed giant aneurysms of the RCA and LAD and was placed on warfarin and aspirin. Warfarin therapy was discontinued at age 17 years and he presented two years later with a myocardial infarction associated with occlusion of the aneurysmal RCA. His CAC score was obtained within six years of the onset of his KD. MACE occurred in 11 of 20 (55%) patients with CAC > 0 (range 17–8218) and included five myocardial infarctions and five coronary artery bypass graft surgeries. For subjects imaged 9 or more years after their acute KD (n =144), the presence of CAC on CT had a sensitivity of 95%, specificity of 100%, negative predictive value of 99%, and positive predictive value of 100% for detecting coronary artery abnormalities.
HsCRP was measured in 85 subjects. For 51 of these subjects, hsCRP was drawn the same day as the CT calcium score, and for the remaining 32 subjects the median time between phlebotomy and CT calcium score was 15 days (IQR 1–224 days). For subjects with CAC = 0, the median hsCRP was 0.51 mg/L (n=69, IQR 0.31–1.27 mg/L) and for subjects with CAC > 0, the median hsCRP was 0.47 mg/L (n=14, IQR 0.25–2.21 mg/L). There was no significant difference in hsCRP between these two groups. For subjects with CAC > 0, the CAC volume scores did not correlate with hsCRP values (R2 = 0.05, p = NS).
Discussion
All 100 subjects with normal coronary arteries by echocardiography during the acute and subacute illness had CAC scores of zero. These data provide strong evidence that patients with normal coronary arteries acutely do not develop late coronary artery calcium within three decades of the onset of KD and supports the concept that these patients have no evidence of coronary artery abnormalities. In the one individual who was told he had normal coronary arteries by echocardiogram, the elevated CAC score prompted discovery of a heavily calcified aneurysm followed by initiation of systemic anticoagulation to prevent thrombosis of the aneurysm, as well as surveillance by a cardiologist for potential ischemic complications.
In the present study, of the patients with transient dilation, persistent dilation, and remodeled aneurysms, all but one had CAC scores of zero. The one subject was classified as persistently dilated and had coronary artery calcification with associated severe stenosis. The echocardiogram in this individual was performed in 1978 at an outside institution and was not available for review. Clearly, the resolution and precision of echocardiography for detecting coronary artery dilation has improved over the ensuing four decades. Many young adults presenting today with a history of KD had their initial echocardiography during an era when the imaging was limited due to technical issues. Hence, the possibility of clinically important coronary abnormalities cannot be excluded in patients with echocardiograms performed in past decades, and a CT calcium scan may be reasonable to evaluate for the presence of coronary artery abnormalities that might have been missed in these patients.
Of the nine subjects with remodeled aneurysms documented by echocardiogram or CT angiography, all were from Cohort 1 and the original echocardiograms were not available for review. Five of these subjects were less than one year of age when they developed acute KD and three of the five had giant aneurysms that remodeled over the first two years after disease onset. This is consistent with published literature suggesting that young age at KD onset is associated with a greater likelihood of aneurysm remodeling.5 However, the long-term outcome for such patients has not yet been determined.
This study provides further evidence that patients with a history of KD and coronary artery aneurysms virtually always develop late coronary artery calcification. The degree of calcification did not correlate with the interval since acute KD; however, our study suggests that calcification is a process that is often not detected in the first decade after disease onset. It is not known why some patients develop more severe calcification and whether the degree of calcification has prognostic significance, as is the case for atherosclerotic CAD.6
The findings of coronary calcification on chest radiographs in patients with a history of KD has been well-reported.7–11 We did not have chest radiographs on the subjects in this study, and hence were unable to compare data between chest radiographs and CT. Some of the subjects in this study had dense calcification that would be expected to be apparent on chest radiographs. Compared with chest radiographs, we expect that CT calcium scoring provides a more sensitive and quantitative measure of coronary calcification.
As increasing numbers of patients with a history of KD reach adulthood and transfer their care to physicians who care for adults, clinicians will encounter patients with a history of KD for whom original records may be difficult to obtain and for whom the coronary artery status during the acute illness is uncertain. For these patients, CT calcium scoring to assess for coronary calcification seems ideally suited. When performed approximately one decade or more after acute KD, this test appears to be very sensitive and specific for the presence of coronary artery abnormalities.
In this study we used a modified CT protocol with relatively low radiation dose (median dose equivalent to 2.4 months of background radiation). The exact risk of this low amount of radiation is unknown, but is likely to be small and hence the risk to benefit ratio for this inexpensive test seems favorable for patients with a history of KD. A limitation of the study is that the original echocardiograms could not be directly evaluated.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health, Heart, Lung, and Blood Institute to JCB (RO1-HL69413), a grant from the American Heart Association National Affiliate (09SDG2010231) to LBD, and a grant from the Gordon and Marilyn Macklin Foundation to JCB. A Ziostation workstation was provided to the University of California, San Diego, without charge, via a research agreement with Ziosoft, Inc (now QI Imaging Inc.).
Disclosures
Author M.J.B. receives grant support from GE HealthCare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK, Smith SC, Jr, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Nishimura R, Ohman EM, Page RL, Stevenson WG, Tarkington LG, Yancy CW American College of Cardiology F, American Heart A. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–e103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Kahn AM, Budoff MJ, Daniels LB, Jimenez-Fernandez S, Cox AS, Gordon JB, Burns JC. Calcium scoring in patients with a history of Kawasaki disease. JACC Cardiovasc Imaging. 2012;5:264–272. doi: 10.1016/j.jcmg.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakazato R, Dey D, Gutstein A, Le Meunier L, Cheng VY, Pimentel R, Paz W, Hayes SW, Thomson LE, Friedman JD, Berman DS. Coronary artery calcium scoring using a reduced tube voltage and radiation dose protocol with dual-source computed tomography. J Cardiovasc Comput Tomogr. 2009;3:394–400. doi: 10.1016/j.jcct.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Gordon JB, Daniels LB, Kahn AM, Jimenez-Fernandez S, Vejar M, Numano F, Burns JC. The Spectrum of Cardiovascular Lesions Requiring Intervention in Adults After Kawasaki Disease. JACC Cardiovasc Interv. 2016;9:687–696. doi: 10.1016/j.jcin.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi M, Mason W, Lewis AB. Regression of coronary aneurysms in patients with Kawasaki syndrome. Circulation. 1987;75:387–394. doi: 10.1161/01.cir.75.2.387. [DOI] [PubMed] [Google Scholar]
- 6.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 7.Doi YL, Hamashige N, Odawara H, Kuzume O, Chikamori T, Ozawa T. Ring-calcification of coronary artery aneurysms in an adolescent. Chest. 1987;92:1118–1120. doi: 10.1378/chest.92.6.1118. [DOI] [PubMed] [Google Scholar]
- 8.Nakada T, Yonesaka S, Sunagawa Y, Tomimoto K, Takahashi T, Matsubara T, Furukawa H, Kamimura K, Naka S. Coronary arterial calcification in Kawasaki disease. Acta Paediatr Jpn. 1991;33:443–449. doi: 10.1111/j.1442-200x.1991.tb02569.x. [DOI] [PubMed] [Google Scholar]
- 9.Muneuchi J, Joo K, Morihana E, Mizushima A. Detectable silent calcification in a regressed coronary artery aneurysm of a young adult with a history of Kawasaki disease. Pediatr Cardiol. 2008;29:195–197. doi: 10.1007/s00246-007-9062-6. [DOI] [PubMed] [Google Scholar]
- 10.Lapierre C, Bitsch A, Guerin R, Garel L, Miro J, Dahdah N. Follow-up chest X-ray in patients with Kawasaki disease: the significance and clinical application of coronary artery macro-calcification. Pediatr Cardiol. 2010;31:56–61. doi: 10.1007/s00246-009-9548-5. [DOI] [PubMed] [Google Scholar]
- 11.Salamat M, Khan MS. Ring-calcification of giant coronary artery aneurysm of an 11-year-old child with history of Kawasaki disease. Pediatr Cardiol. 2010;31:558–559. doi: 10.1007/s00246-009-9594-z. [DOI] [PubMed] [Google Scholar]