Abstract
Sex-related outcome disparities following severe trauma have been demonstrated in human and animal studies however sex hormone status could not fully account for the differences. This study tested whether X-linked cellular mosaicism, which is unique to females, could represent a genetically based mechanism contributing to sex-related immuno-modulation following trauma. Serial blood samples collected for routine laboratory tests were analyzed for ChrX inactivation (XCI) ratios in white blood cells. 39 severely injured (mean ISS 19) female trauma patients on mixed racial and ethnic background were tested for initial (baseline) and trauma-induced changes in XCI-ratios and their associations with severity of injury and clinical outcome. At admission, two third of the patients showed XCI-ratio values between one and three, about a third presented skewed XCI-ratios (3–7 range) and three patients displayed extremely skewed XCI-ratios (8–30 range). Serial blood samples during the clinical course showed additional changes in XCI-ratios ranging between 20–900% over initial. Increasing XCI-ratios during the injury course correlated with the severity of trauma, subsequent need for ventilator support and pneumonia. In contrast, initial XCI-ratios did not show correlations with injury severity or clinical complications. Initial XCI-ratios showed a positive correlation with age but older patients retained the ability to mount trauma-induced secondary XCI changes. These data shows that trauma results in X-linked cell selection in females, which is likely to be driven by polymorphic differences between the parental ChrXs. X-linked white blood cell skewing correlates with injury severity and a complicated post-injury clinical course. Female X-linked cellular mosaicism and its capacity to change dynamically during the injury course compared to the lack of this machinery in males may represent a novel immuno-modulatory mechanism contributing to sex-based outcome differences after injury and infection.
Keywords: X chromosome inactivation, Cellular Mosaicism, Genetic Polymorphism, X-linked, Infection, Injury
Introduction
Although still controversial, several studies have demonstrated that females have better outcomes than males following trauma or sepsis [1–11] which is postulated to be the result of the immuno-modulatory role of sex hormones [1;2;12–14]. However, gender-based outcome differences cannot be exclusively attributed to the effect of sex hormones, as studies on pre-pubertal children also found that boys do clinically worse than girls after burn injury or infections [15–17]. Additionally, gender differences in disease processes including sepsis were also shown to manifest between postmenopausal women and elderly men [18;19]. Lastly, experimental sex hormone replacements only partially diminished sex-based differences in animal models of injury or sepsis with most studies using pharmacological doses of estrogen [1;2;20–22] which may cause alterations in the ACTH axis [23–27]. These data indicate that factors other than sex hormones alone contribute to gender-dimorphic outcomes.
We proposed previously that X-linked genetic polymorphisms may be important in gender-based outcome differences following injury or infection [28]. This hypothesis was based on the fact that females carry two inherently polymorphic X chromosomes (ChrXs) one inherited from the mother and one from the father. In contrast, males carry a single ChrX passed on from the mother. Therefore, polymorphic X-linked genes are present in two copies in females whereas males carry only the maternal variants. Dosage compensation of the potential “double dose of proteins” in females is achieved by random ChrX inactivation at an early embryonic stage when one of the parental ChrXs within an individual cell becomes methylated and stops gene expression through the entire lifespan of the cell or its progenies [28–31]. Thus in females, biologically important polymorphic X-linked genes will produce two cellular phenotypes with potentially different regulatory or functional potentials driven by respective genetic polymorphisms of the maternal and paternal ChrX-s [28–31]. In contrast, genetic polymorphisms of the maternal ChrX affect all cellular phenotypes in males [17;28;32;33].
X-linked cellular phenotype differences in females may alter functional diversity in white blood cells (WBC) which can manifest as differences in mobilization, migration, apoptosis, necrosis or proliferation between mosaic subsets. If such functional and cellular response differences linked to the ChrX exist then this should manifest in skewed cell selection during the innate immune response. This was tested in murine models and it was found that X-linked immune cell skewing occurred in blood as well as in immune-competent organs following sepsis or endotoxemia [34–36]. Importantly, this acute ChrX skewing during the host response is different from the “classical” ChrX skewing which is stable and driven by skewed cell progenitors in the bone marrow and observed frequently in elderly females or carriers of severe X-linked genetic defects [37–40].
It is however unknown whether acute X-linked cell selection exists in humans during the innate immune response. If present, this would reveal a novel adaptive cellular mechanism unique to females and consequently may contribute to gender-based outcome differences. Thus, the current study tested the hypothesis whether X-linked WBC skewing occurs following moderate to severe injury and if so, how this phenomenon correlates with the severity of injury and clinical outcomes. We found that almost all female trauma patients displayed some degree of acute X-linked WBC skewing during the clinical course. Increased X-linked white blood cell skewing from baseline was more pronounced in patients who suffered more severe initial injuries or presented a complicated clinical course.
Materials and Methods
Patients
The cohort represents consecutive female patients admitted to the trauma service at the New Jersey Trauma Center at University Hospital in Newark, a verified Level I Trauma Center. Inclusion criteria were: 1) age >18 years; 2) ISS ≥9); 3) Glasgow Coma Score > 3; and 4) admission to the Progressive care or Surgical Intensive Care Units. Patients who expired within 24h of injury were excluded. Clinical data were obtained from the Trauma Registry including demographics, mechanism of injury, injuries sustained, length of ICU and hospital stays, days on ventilator support, occurrence of pneumonia, unit blood transfusions, organ failures and mortality. The study was approved by the Institutional Review Board of Rutgers-New Jersey Medical School.
Blood Sampling
DNA analyses were performed on the leftover, ready to discard blood drawn at admission into EDTA containing vacutainer tubes and as available after subsequent routine tests. Blood was stored at 4°C and DNA was isolated using QiAamp DNA isolation kit (Qiagen, Germany) according to manufacturer’s instructions within a ten day period after the original blood drawing. The isolated DNA tested by agarose electrophoresis indicated good quality and consistent yield. DNA was stored at −80°C until analyses.
ChrX inactivation (XCI) assay
XCI-ratios were determined by measuring DNA methylation at the androgen receptor locus (HUMARA), which is a variable-length polymorphic site [38;41] and heterozygosity for the variants is common. 1 μg genomic DNA was digested overnight with 2 U of methylation-sensitive and insensitive restriction endonucleases HpaII and RsaI in 50 μl volume at 37◦C (New England BioLabs, Beverly, MA). The analysis and PCR amplifications were performed according to a previously published procedure [41] using fluorescent labeled primers: 56-FAM/TCC AGA ATC TGT TCC AGA GCG TGC-3V; and 5V-GCT GTG AAG GTT GCT GTT CCT CAT-3V for PCR amplification. PCR-amplified fragments were electrophoresed on ABI Prism 3130XL Genetic Analyzer and quantified by the area under the curve for each allele peak, using GeneMapper software (Applied Biosystems). [42]. As expected ratios determined form samples undigested with HpaII were near one in each patient who were heterozygous for Humara length-polymorphisms or resulted in one peak in homozygous patients. In calculating XCI ratios in the first (at admission) sample, we arbitrarily calculated ratios resulting in value of ≥ 1 for consistent presentation and easier reading.
Statistical analyses
Statistical calculations were performed using JMP software (SAS Institute Cary, NC, USA). Clinical data were expressed as frequencies and proportions for categorical variables and means and standard deviations for continuous variables. For continuous variables we used ANOVA followed by T-test for pairwise or Tukey-Kramer for multiple comparisons. Univariate analyses used chi-square or Fisher exact tests for associations. Significant difference was concluded at p<0.05; a trend toward significance at p<0.1.
Results
Patient population
66 female patients met the initial inclusion criteria (Table 1). 27 patients were excluded from the study because homozygosity for HUMARA was found in eight patients, no serial blood samples were available from 18 patients due to uncomplicated clinical course and early discharge and one individual showed XCI-ratio 34 at admission representing an outlier. Mean ISS was 19, mean ICU stay was 10 days and 33% of the patients needed ventilation support of which 40% developed pneumonia. There was one death on day 5 post-trauma in the cohort (Table 1).
Table 1.
Basic and Clinical Parameters of the Female Trauma Cohort
| Subject | Value |
|---|---|
| Initial inclusion (N) | 66 |
| Exclusions: | 27 |
| Homozygous at the HUMARA locus, N (%) | 8 (12) |
| Early discharge or lack of blood specimens | 18 |
| Extreme initial skewing (XCI-ratio >30, see Fig 1C) | 1 |
| Study Population (N) | 39 |
| Age (years) (Mean±S.D.) | 53 ± 23 |
| ISS (Mean±S.D.) | 19 ± 9 |
| Race/Ethnicity (%) | |
| African American | 33 |
| Caucasian | 28 |
| Hispanic | 18 |
| Asian | 3 |
| Mixed or Unknown | 8 |
| Hospital length of stay (days) (Mean±S.D.) | 16 ± 14 |
| Patients admitted to ICU (%) | 27 (69) |
| ICU length of stay (days) (Mean±S.D.) | 10 ± 9 |
| Patients on ventilator (%) | 13 (33) |
| Days on ventilator (±S.D.) | 11 ± 9 |
| Pneumonia (%) | 5 (13) |
| In-hospital mortality, N (%) | 1 (3) |
ISS, Injury Severity Score
ChrX skewing during the trauma course
Fig 1 depicts XCI-ratio changes during the trauma course, which is a reflection of differences in circulating WBC numbers expressing respective parental ChrXs. For clarity of display we stratified patients according to the degree of XCI-ratio change. Fig 1A indicates those patients whose XCI-ratios changed less than 30% during the course over the initial value collected at admission. Interestingly, this relatively stable ratio was readily manifested even in those patients whose initial XCI-ratio was markedly skewed (Y axis scale 1 through 6 Fig 1A). Fig 1B depicts patients who displayed at least a 30% change over initial XCI-ratio during the course indicating dynamically changing WBC ratios expressing respective parental ChrXs. Two patients in the cohort presented markedly skewed XCI-ratios at baseline and one patients showed extreme XCI-ratio changes during the course but twice returning back to baseline (Fig 1C). To better illustrate the XCI dynamics during the clinical course and the general tendency of returning to baseline independent of the initial XCI-ratio we also expressed trauma-induced XCI changes as percent of the initial XCI-ratio measured at admission (Fig 1 D E).
Fig 1. Female patients present ChrX skewing during the clinical course of injury.
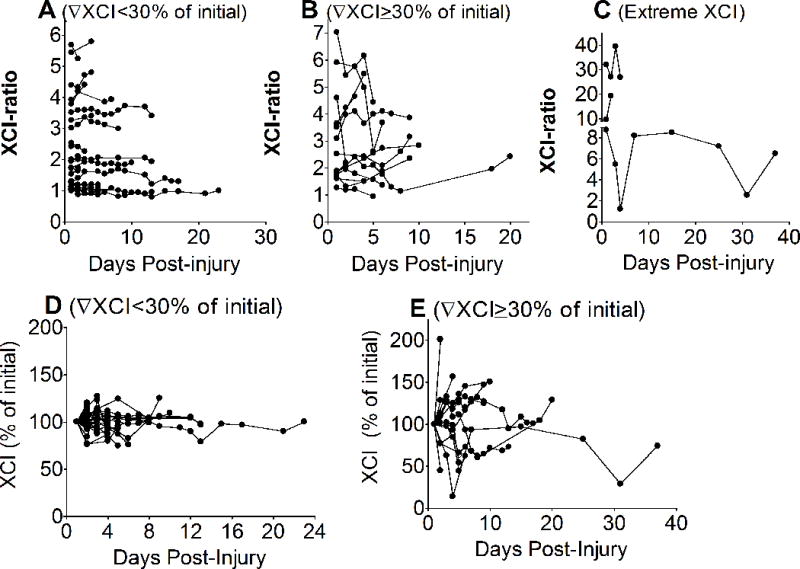
White blood cell DNA isolated from whole blood collected during the clinical course was tested for methylation at the polymorphic HUMARA locus as described in the material and methods section in order to determine ChrX inactivation ratios (XCI). For clarity of presentation, patients were stratified by degree of the observed XCI-ratio changes in response to trauma showing XCI-ratio changes less than 30% of initial (A); more or equal than 30% of initial (B); or extreme XCI (C). Panels D and E display the same patients showing XCI-ratio changes as percent of initial after starification by XCI changes less than 30% (D) or equal or more than 30% (E).
ChrX skewing and clinical outcomes
In order to assess the potential pathophysiological significance of trauma-induced XCI we examined whether changes in XCI-ratios during the trauma course correlate with clinical parameters. Associations were tested after stratification by 20, 30 and 50% changes in trauma-induced XCI-ratios (change over initial anytime during the course). Stratifications at these levels were chosen, as they indicate that half, a third or a fifth of WBCs expressing a particular parental ChrX are affected. First we tested how the severity of injury is related to XCI. Fig 2 indicates that patients who displayed trauma-induced XCI at 20–30% over baseline were more severely injured than patients with less pronounced XCI (Fig 2 A,B,C). Importantly, there was no association between injury severity and the initial XCI measured at admission (Fig 2 D,E,F). Testing XCI-ratios from healthy volunteers showed less than 20% differences within an individual with a variance of 3.1% (N=11, 18 month period from 4 subjects). Intra-assay variance of XCI repeats was 2.6% (n=8).
Fig 2. Association between initial injury severity and trauma-induced XCI-ratio changes.
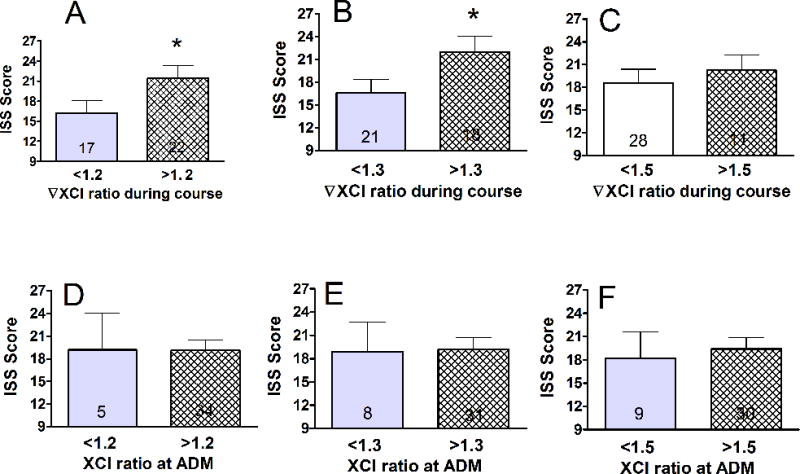
Associations were tested after stratification by 20, 30 and 50% of XCI changes over initial as this indicates that half, a third or a fifth of WBCs expressing a particular parental ChrX are affected. Trauma-induced XCI-ratio changes (A, B, C) were greater in patients with more severe injuries (*p<0.05, see also logistic fit analysis on supplementary Fig 1). In contrast, XCI-ratios measured at admission did not correlate with injury severity (D,E,F).
Need for ventilator support and the development of pneumonia are important determinants of post-injury complications potentially affecting short and long-term outcomes. There were more patients on ventilator support who displayed trauma-induced XCI at 20–30% over baseline as compared to patients with less pronounced XCI changes during the clinical course (Fig 3 A,B). There was no association between need for ventilator support and initial XCI-ratios measured at admission (not shown). Testing the relationship between ChrX skewing and pneumonia showed a tendency of association at greater than 1.5 fold change in XCI-ratio (Fig 3F).
Fig 3. Association between pulmonary complications and trauma-induced XCI.
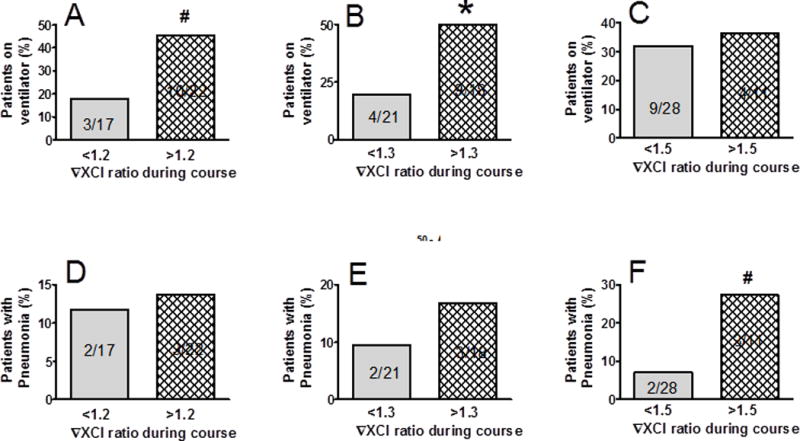
After the same stratification as described for Fig 2, we also tested associations between XCI-ratio changes and need for vetilattor support (A,B,C) or development of pneumonia (D,E,F). Trauma-induced changes in XCI-ratios were dependent on ventilator support and inicated a trend for association with pneumonia. *Statistically significant difference at p<0.05; #trend for significant difference at p<0.1. See also Supplemental Digital Content-Fig 1.
Age is an important confounder in the clinical course of trauma. It was also shown that spontaneous and in-itself non-pathological ChrX skewing is frequently observed in healthy elderly females [37–39]. Testing this relationship we found that patients with greater than 1.3–1.5 initial XCI-ratios were about double the age than patients with lower initial XCI-ratios (Fig 4A,B,C). Interestingly however, stratification by greater than 100% initial XCI did not reveal any age difference (Supplemental Digital Content – Fig 1). Importantly, the trauma-induced secondary changes in XCI did not reveal any association with age (Fig 4 D,E,F) indicating that acute X-linked cell selection readily occurs in elderly females.
Fig 4. Relationship between age and initial XCI-ratio measured at admission.
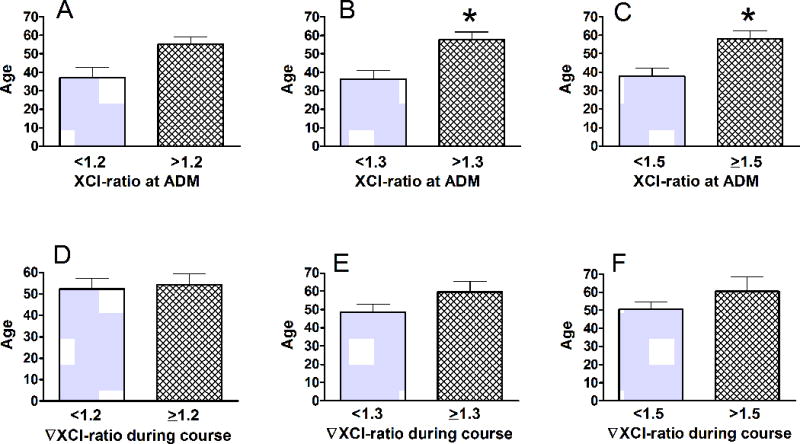
After the same stratification as described for Fig 2, we found that advanced age correlates with elevated XCI at admission up to a ratio of 1.5 (A,B, C). However, trauma-induced acute XCI changes does not correlate with advanced age (D,E,F). *Statistically significant difference at p<0.05. See also Supplemental Digital Content–Fig 1.
Lastly, because baseline ChrX skewing may impact subsequent trauma-induced changes in XCI, we also tested this relationship. We found that a greater initial XCI-ratio was associated with a greater degree of trauma-induced XCI when stratified at the 50% response level (Supplemental Digital Content – Fig 1).
Discussion
This study demonstrates for the first time that female patients present acute ChrX skewing in response to injury. The degree of ChrX skewing during the trauma course is associated with injury severity and post-traumatic clinical complications. The capacity to manifest acute trauma-induced ChrX skewing is independent of age and also occurs in individuals with marked ChrX skewing at baseline. Based on these observations, it is likely that increased and uniquely female X-linked cellular diversity contributes to gender-based outcome differences.
Acute, trauma-induced ChrX skewing is a reflection of differences between X-linked mosaic WBC subsets in rates of cell trafficking between the blood and periphery [35;36]. In turn, X-linked changes in WBC trafficking are most likely the result of differences in rates of cell migration, metabolism, apoptosis, necrosis or proliferation at the periphery or release from and retention at the bone marrow. At this point, it is unknown which combinations of these cellular processes and inflammatory mechanisms are determinants in causing ChrX skewing in circulating blood. The fact however that the trauma-induced ChrX skewing tends to be modest and reversible suggests that X-linked cell selection takes place at the periphery in contrast with the “classical” ChrX skewing, which is marked and stable due to skewed progenitor selection in the bone marrow [37–40;43]. As the current study tested mixed WBCs in whole blood, additional work is needed to elucidate whether trauma-induced cell skewing differently affects circulating and tissue resident immune cells or if WBC composition changes during the clinical course confound observations.
ChrX is rich in immune-competent genes including members of the TLR-NFkB signaling pathway, multiple redox, pro and anti-apoptotic proteins and key regulatory proteins of T cell activation [17;28;32]. Whereas severe X-linked immune deficiencies are well-studied in humans [17;32;33], studies investigating common X-linked polymorphism are less abundant [17;28]. This, in part, is the result of analytical difficulties associated with the X-linked genetic patterns and the apparent lack of heterozygosity in the population [44;45]. Nonetheless, the fact that almost all female patients manifested some degree of ChrX skewing during the trauma course suggests prevailing immuno-modulatory impacts of common, otherwise non-pathological, X-linked polymorphisms. This acute variability in XCI under a pathophysiological challenge is in sharp contrast to stable XCI ratios in healthy females observed in this study and as demonstrated earlier [46].
The facts that more severely injured patients and those who subsequently required additional ventilator support or developed pneumonia presented greater degrees of ChrX skewing than patients with fewer injuries or uncomplicated clinical course suggest that prevailing pathophysiological challenges promote polarization of cellular phenotypes. However, it remains unknown whether this X-linked cell polarization is an adaptive response or it is simply a reflection of a greater systemic inflammatory response. Nevertheless, based on the accepted notion that females show improved outcomes after injuries [1–10], it is expected that increased X-linked cell variability provides clinical benefits in females over males.
However, our study also reveals that X-linked cell selection may be disadvantageous in selected individuals. For example, some of the patients showed marked initial ChrX skewing indicating an imbalance between WBC mosaic phenotypes. Thus, in these patients, it is determined only by chance whether the prevailing WBC phenotype is advantageous or not during the early trauma response. Importantly however, even in those patients who had marked initial ChrX skewing, the secondary skewing was readily manifested in response to trauma. In support of this notion, the patient who died on day 5 post-injury showed an initial XCI-ratio of 9.5 and also displayed a more than 50% change in XCI-ratio during the clinical course. Therefore, it is improbable that XCI changes associated with severe trauma or clinical complications will be uniformly advantageous or disadvantageous. It is more likely that skewed X-liked cell selection of mosaic populations will be determined by the presence of particular X-linked mutations in individual patients.
Our findings reinforce the analytical difficulties of studies testing associations between X-linked polymorphisms and clinical outcome [44]. This is because the gender-biased genotype frequencies and lack of heterozygosity is further complicated by the possible presence of XCI skewing in some patients causing a discrepancy between X-linked genotype and its presumed phenotype. These possible genotype-phenotype discrepancies may confound conclusions of association studies when outcomes are analyzed solely on the X-linked genotype.
Another limitation in the interpretation of these observations is that the initial XCI-ratios were from blood collected upon admission to the trauma center. It is possible that some of the trauma-induced XCI changes may have already occurred during transportation to the hospital. As these studies were carried out in an urban trauma center with a mean transport time of 15 minutes and time from injury of less than 30 minutes, we do not believe that prolonged transport influenced baseline XCl data. Lastly the fact that trauma-induced XCI-ratios showed a tendency of returning to initial values (Fig 1 and 2) supports our assertion that the XCI-ratio reported upon admission represents an acceptable baseline level.
Heterozygosity of the HUMARA locus was reported between 80–90 % in various female populations [37–39;43], which is similar to our findings (88 %). It is also known that healthy females over age sixty frequently present bone marrow-derived spontaneous otherwise non-pathological ChrX skewing, which is defined by convention as XCI-ratio greater than three [37–39]. Interestingly, we did not find a correlation with age when stratified by XCI-ratio greater than three however, stratification at lower degrees of XCI-ratios (30–50%) showed a positive correlation with age. Importantly, age did not seem to affect the trauma-induced changes indicating that the elderly did not lose the capacity to present XCI-dynamics during the clinical course.
Lastly, it cannot be ruled out that acute epigenetic changes may have also contributed to our findings, as inflammation may acutely alter global DNA methylation [47]. Furthermore, it was also reported that in addition to the unmethylated PAR regions of the ChrXs, an additional 15% of X-liked genes may escape methylation and permanent silencing on the “inactive” ChrX [29;48]. Nonetheless, if epigenetic changes differently impact parental ChrXs following trauma then these would further increase cellular diversity in females as compared to males.
In summary, our observations indicating X-linked functional impacts of circulating WBCs indicate that common X-linked polymorphisms are modulators of the host response to injury. Trauma-induced X-linked WBC skewing is more pronounced in the more severely ill. This fact, together with the X-linked inheritance pattern and the unique functionality of X-linked cellular mosaicism may explain continuing controversies in clinical studies testing outcome differences between males and females. The cellular interplay in mosaic females could “buffer” the inflammatory response by down-regulating hyper-active cell populations during excessive inflammation or in contrast, may compensate for immuno-paralysis thereby improving the clinical course. Evidently, this potentially beneficial X-linked balancing mechanism by mosaic sub-populations is absent in single-ChrX males. In some cases however, ChrX skewing may be disadvantageous, as marked skewing can polarize cell responses driven only by one of the ChrXs similarly to that of males. We conclude that separation of clinical cohorts by sex and hormonal status may not be sufficient enough in aiming full elucidation of sex-specific outcome differences. This question can be answered only in combination with information on the accompanying status of X-linked polymorphisms, cellular skewing and its inherent differences between males and females as well as among individual females within a cohort.
Supplementary Material
References
- 1.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs EJ, Messingham KA, Gregory MS. Estrogen regulation of immune responses after injury. Mol Cell Endocrinol. 2002;193:129–135. doi: 10.1016/s0303-7207(02)00106-5. [DOI] [PubMed] [Google Scholar]
- 3.Choudhry MA, Bland KI, Chaudry IH. Gender and susceptibility to sepsis following trauma. Endocr Metab Immune Disord Drug Targets. 2006;6:127–135. doi: 10.2174/187153006777442422. [DOI] [PubMed] [Google Scholar]
- 4.Offner PJ, Moore EE, Biffl WL. Male gender is a risk factor for major infections after surgery. Arch Surg. 1999;134:935–938. doi: 10.1001/archsurg.134.9.935. [DOI] [PubMed] [Google Scholar]
- 5.Wichmann MW, Inthorn D, Andress HJ, Schildberg FW. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intensive Care Med. 2000;26:167–172. doi: 10.1007/s001340050041. [DOI] [PubMed] [Google Scholar]
- 6.Schroder J, Kahlke V, Book M, Stuber F. Gender differences in sepsis: genetically determined? Shock. 2000;14:307–310. [PubMed] [Google Scholar]
- 7.Sperry JL, Friese RS, Frankel HL, West MA, Cuschieri J, Moore EE, Harbrecht BG, Peitzman AB, Billiar TR, Maier RV, Remick DG, Minei JP. Male gender is associated with excessive IL-6 expression following severe injury. J Trauma. 2008;64:572–578. doi: 10.1097/TA.0b013e3181650fdf. [DOI] [PubMed] [Google Scholar]
- 8.Deitch EA, Livingston DH, Lavery RF, Monaghan SF, Bongu A, Machiedo GW. Hormonally active women tolerate shock-trauma better than do men: a prospective study of over 4000 trauma patients. Ann Surg. 2007;246:447–453. doi: 10.1097/SLA.0b013e318148566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidry CA, Swenson BR, Davies SW, Dossett LA, Popovsky KA, Bonatti H, Evans HL, Metzger R, Hedrick TL, Tache-Leon CA, Hranjec T, Chaudry IH, Pruett TL, May AK, Sawyer RG. Sex- and diagnosis-dependent differences in mortality and admission cytokine levels among patients admitted for intensive care. Crit Care Med. 2014;42:1110–1120. doi: 10.1097/CCM.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spolarics Z. In gender-based outcomes, sex hormones may be important but it is in the genes*. Crit Care Med. 2014;42:1294–1295. doi: 10.1097/CCM.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawasaki T, Chaudry IH. The effects of estrogen on various organs: therapeutic approach for sepsis, trauma, and reperfusion injury. Part 2: liver, intestine, spleen, and kidney. J Anesth. 2012;26:892–899. doi: 10.1007/s00540-012-1426-2. [DOI] [PubMed] [Google Scholar]
- 12.Maggio M, Basaria S, Ceda GP, Ble A, Ling SM, Bandinelli S, Valenti G, Ferrucci L. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest. 2005;28:116–119. [PubMed] [Google Scholar]
- 13.Weitzmann MN, Pacifici R. Estrogen regulation of immune cell bone interactions. Ann N Y Acad Sci. 2006;1068:256–274. doi: 10.1196/annals.1346.030. [DOI] [PubMed] [Google Scholar]
- 14.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 15.Barrow RE, Herndon DN. Incidence of mortality in boys and girls after severe thermal burns. Surg Gynecol Obstet. 1990;170:295–298. [PubMed] [Google Scholar]
- 16.Wells JC. Natural selection and sex differences in morbidity and mortality in early life. J Theor Biol. 2000;202:65–76. doi: 10.1006/jtbi.1999.1044. [DOI] [PubMed] [Google Scholar]
- 17.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- 18.Adrie C, Azoulay E, Francais A, Clec’h C, Darques L, Schwebel C, Nakache D, Jamali S, Goldgran-Toledano D, Garrouste-Org, Timsit JF. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest. 2007;132:1786–1793. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 19.Crimmins EM, Hayward MD, Saito Y. Differentials in active life expectancy in the older population of the United States. J Gerontol B Psychol Sci Soc Sci. 1996;51:S111–S120. doi: 10.1093/geronb/51b.3.s111. [DOI] [PubMed] [Google Scholar]
- 20.Abdelfattah KR, Gatson JW, Maass DL, Wolf SE, Minei JP, Wigginton JG. 17beta-Estradiol reappropriates mass lost to the hypermetabolic state in thermally injured rats. J Surg Res. 2013;181:136–141. doi: 10.1016/j.jss.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angele MK, Pratschke S, Hubbard WJ, Chaudry IH. Gender differences in sepsis: cardiovascular and immunological aspects. Virulence. 2014;5:12–19. doi: 10.4161/viru.26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperry JL, Nathens AB, Frankel HL, Vanek SL, Moore EE, Maier RV, Minei JP. Characterization of the gender dimorphism after injury and hemorrhagic shock: are hormonal differences responsible? Crit Care Med. 2008;36:1838–1845. doi: 10.1097/CCM.0b013e3181760c14. [DOI] [PubMed] [Google Scholar]
- 23.Redei E, Li L, Halasz I, McGivern RF, Aird F. Fast glucocorticoid feedback inhibition of ACTH secretion in the ovariectomized rat: effect of chronic estrogen and progesterone. Neuroendocrinology. 1994;60:113–123. doi: 10.1159/000126741. [DOI] [PubMed] [Google Scholar]
- 24.Sharma A, Aoun P, Wigham J, Weist S, Veldhuis JD. Gender determines ACTH recovery from hypercortisolemia in healthy older humans. Metabolism. 2013;62:1819–1829. doi: 10.1016/j.metabol.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plechner AJ. Cortisol abnormality as a cause of elevated estrogen and immune destabilization: insights for human medicine from a veterinary perspective. Med Hypotheses. 2004;62:575–581. doi: 10.1016/j.mehy.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Sharma AN, Aoun P, Wigham JR, Weist SM, Veldhuis JD. Estradiol, but not testosterone, heightens cortisol-mediated negative feedback on pulsatile ACTH secretion and ACTH approximate entropy in unstressed older men and women. Am J Physiol Regul Integr Comp Physiol. 2014;306:R627–R635. doi: 10.1152/ajpregu.00551.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams TE, Sakurai H, Adams BM. Effect of stress-like concentrations of cortisol on estradiol-dependent expression of gonadotropin-releasing hormone receptor in orchidectomized sheep. Biol Reprod. 1999;60:164–168. doi: 10.1095/biolreprod60.1.164. [DOI] [PubMed] [Google Scholar]
- 28.Spolarics Z. The X-files of inflammation: cellular mosaicism of X-linked polymorphic genes and the female advantage in the host response to injury and infection. Shock. 2007;27:597–604. doi: 10.1097/SHK.0b013e31802e40bd. [DOI] [PubMed] [Google Scholar]
- 29.Heard E, Disteche CM. Dosage compensation in mammals: fine-tuning the expression of the X chromosome. Genes Dev. 2006;20:1848–1867. doi: 10.1101/gad.1422906. [DOI] [PubMed] [Google Scholar]
- 30.Goto T, Monk M. Regulation of X-chromosome inactivation in development in mice and humans. Microbiol Mol Biol Rev. 1998;62:362–378. doi: 10.1128/mmbr.62.2.362-378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latham KE. X chromosome imprinting and inactivation in preimplantation mammalian embryos. Trends Genet. 2005;21:120–127. doi: 10.1016/j.tig.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Migeon BR. The role of X inactivation and cellular mosaicism in women’s health and sex-specific diseases. JAMA. 2006;295:1428–1433. doi: 10.1001/jama.295.12.1428. [DOI] [PubMed] [Google Scholar]
- 33.Migeon BR. Why females are mosaics, X-chromosome inactivation, and sex differences in disease. Gend Med. 2007;4:97–105. doi: 10.1016/s1550-8579(07)80024-6. [DOI] [PubMed] [Google Scholar]
- 34.Chandra R, Federici S, Hasko G, Deitch EA, Spolarics Z. Female X-chromosome mosaicism for gp91phox expression diversifies leukocyte responses during endotoxemia. Crit Care Med. 2010;38:2003–2010. doi: 10.1097/CCM.0b013e3181eb9ed6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandra R, Federici S, Nemeth ZH, Horvath B, Pacher P, Hasko G, Deitch EA, Spolarics Z. Female X-chromosome mosaicism for NOX2 deficiency presents unique inflammatory phenotype and improves outcome in polymicrobial sepsis. J Immunol. 2011;186:6465–6473. doi: 10.4049/jimmunol.1100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chandra R, Federici S, Nemeth ZH, Csoka B, Thomas JA, Donnelly R, Spolarics Z. Cellular mosaicism for X-linked polymorphisms and IRAK1 expression presents a distinct phenotype and improves survival following sepsis. J Leukoc Biol. 2014;95:497–507. doi: 10.1189/jlb.0713397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abkowitz JL, Taboada M, Shelton GH, Catlin SN, Guttorp P, Kiklevich JV. An X chromosome gene regulates hematopoietic stem cell kinetics. Proc Natl Acad Sci U S A. 1998;95:3862–3866. doi: 10.1073/pnas.95.7.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Busque L, Paquette Y, Provost S, Roy DC, Levine RL, Mollica L, Gilliland DG. Skewing of X-inactivation ratios in blood cells of aging women is confirmed by independent methodologies. Blood. 2009;113:3472–3474. doi: 10.1182/blood-2008-12-195677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatakeyama C, Anderson CL, Beever CL, Penaherrera MS, Brown CJ, Robinson WP. The dynamics of X-inactivation skewing as women age. Clin Genet. 2004;66:327–332. doi: 10.1111/j.1399-0004.2004.00310.x. [DOI] [PubMed] [Google Scholar]
- 40.Koker MY, Sanal O, de Boer M, Tezcan I, Metin A, Tan C, Ersoy F, Roos D. Skewing of X-chromosome inactivation in three generations of carriers with X-linked chronic granulomatous disease within one family. Eur J Clin Invest. 2006;36:257–264. doi: 10.1111/j.1365-2362.2006.01619.x. [DOI] [PubMed] [Google Scholar]
- 41.Delabesse E, Aral S, Kamoun P, Varet B, Turhan AG. Quantitative non-radioactive clonality analysis of human leukemic cells and progenitors using the human androgen receptor (AR) gene. Leukemia. 1995;9:1578–1582. [PubMed] [Google Scholar]
- 42.Goldsmith JG, Ntuen EC, Goldsmith EC. Direct quantification of gene expression using capillary electrophoresis with laser-induced fluorescence. Anal Biochem. 2007;360:23–29. doi: 10.1016/j.ab.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gale RE. Evaluation of clonality in myeloid stem-cell disorders. Semin Hematol. 1999;36:361–372. [PubMed] [Google Scholar]
- 44.Clayton DG. Sex chromosomes and genetic association studies. Genome Med. 2009;1:110. doi: 10.1186/gm110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaffner SF. The X chromosome in population genetics. Nat Rev Genet. 2004;5:43–51. doi: 10.1038/nrg1247. [DOI] [PubMed] [Google Scholar]
- 46.van Dijk JP, Heuver L, Stevens-Linders E, Jansen JH, Mensink EJ, Raymakers RA, de W T. Acquired skewing of Lyonization remains stable for a prolonged period in healthy blood donors. Leukemia. 2002;16:362–367. doi: 10.1038/sj.leu.2402379. [DOI] [PubMed] [Google Scholar]
- 47.Sinclair SH, Yegnasubramanian S, Dumler JS. Global DNA methylation changes and differential gene expression in Anaplasma phagocytophilum-infected human neutrophils. Clin Epigenetics. 2015;7:77. doi: 10.1186/s13148-015-0105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


