INTRODUCTION
Canine ocular onchocercosis is a parasitic disease that is being diagnosed with an increasing frequency in both the United States and Europe.1–15 The causative agent, Onchocerca lupi, is a filarial nematode that was originally isolated from a wolf in Russia in 1967.16 Onchocerca.spp. are typically parasites of ungulates, with the notable exception of O. volvulus, which parasitizes humans. Most cases of canine onchocercosis diagnosed in the United States have been found in dogs from the southwestern United States or California.2,3,10,16,17
Onchocerca spp. are in the same superfamily (Filaroidea) as Dirofilaria immitis (heartworm) and, similarly, the parasite larva must mature in an insect intermediate host. Microfilariae (L1) are ingested by the vector when it feeds upon the host. The microfilariae develop within the insect vector to infective stage 3 larvae (L3). Infective larvae are transmitted when the insect vector again feeds upon the host and the larvae mature into adult worms, mate and release microfilariae within the definitive host. The black fly, Simulium tribulatum, was recently identified as a putative insect vector of O. lupi in California (US).17 Ongoing work also suggests black flies as the putative vector in Albuquerque, New Mexico.(Quan et al., in preparation)
The adult O. lupi worms are long, white and thread-like. Morphologically, the worms can be identified as O. lupi based on the characteristic cuticular ridges of the female worms, the presence of two internal striae per ridge and from the size and morphology of the microfilariae within the uterus of the adult female worms. Additional tools that have been utilized to detect and characterize O. lupi include identification of its Wolbachia endosymbiont via PCR and amplification and sequencing of the O. lupi mitochondrial NADH dehydrogenase subunit 5 (ND5) and cytochrome oxidase subunit 1 (cox1) genes.18
Several case series documenting the diagnosis, treatment, and outcome of dogs with O. lupi infection in Europe have been published.4,11,12 However, the published cases from the United States are more limited with the largest series including eight dogs.3 This report describes a series of 16 dogs in New Mexico diagnosed and managed by a single veterinary ophthalmologist.
MATERIALS AND METHODS
Records of dogs examined and diagnosed with O. lupi at VCA Veterinary Care Animal Hospital Referral Center in Albuquerque, New Mexico between 2011 and 2015 were reviewed. Criteria for inclusion included ophthalmic abnormalities that were clinically consistent with O. lupi infection combined with histologic identification of nematodes or PCR detection of O. lupi ND5 gene. Two cases in which the records could not be accessed from storage were not included. From each case age, breed, gender, weight, eye affected, use of prophylactic year-round heartworm preventative in the twelve months prior to initial presentation, ophthalmic exam, treatment, recurrence, biopsy, PCR-detection of parasite DNA, presence or absence of eosinophilia on complete blood cell count (CBC), results of D. immitis antigen test results, MDR-1 mutation results, travel history and geographic location at the time of diagnosis were recorded, when available.
All cases received a complete ophthalmic exam including applanation tonometry (Tono-Pen XL, Mentor, OH, USA), biomicroscopy (Kowa SL-14 or SL-15 Slit Lamp, Kowa, Torrance, CA, USA) and indirect ophthalmoscopy (Keeler Vantage Plus Indirect Ophthalmoscope, Broomall, PA, USA) at the initial and follow-up examinations. Schirmer tear test measurements (Schering-Plough Animal Health, Union, NJ, USA) and evaluation of corneal fluorescein stain retention (Ful-Glo Strips, Akorn, Lake Forest, IL, USA) were done at the initial examination and repeated at the follow-up examinations when indicated. Dogs were treated with either medical therapy or a combination of medical and surgical therapy.
Diagnosis
In most cases, the diagnosis was suspected at the initial examination based on the characteristic clinical appearance of a pink to tan, smooth, subconjunctival mass. The diagnosis was supported in the majority of cases by obtaining a snip biopsy from the subconjunctival tissue. The biopsies were obtained following the instillation of topical anesthetic (proparacaine hydrochloride ophthalmic solution 0.5%, Henry Schien, Melville, NY). Sedation was not needed except in a single case. The bulbar conjunctiva above the granuloma or in an area of diffuse chemosis was lifted with Colibri forceps. Several pieces of bulbar conjunctiva and underlying subconjunctival tissues were excised with tenotomy scissors. Samples were placed in 10% neutral buffered formalin for histopathologic submission or in 80 % ethanol for molecular identification. If thread-like worms were seen, they were removed and submitted for histopathology and/or molecular identification. Topical antimicrobials or antimicrobial-steroid combinations were administered for 3 to 5 days following the snip biopsy.
Description of medical management
Medical management included: intramuscular administration of melarsomine dihydrochloride (Immiticide, Merial, Lyon, France) 2.5 mg/kg once daily for two consecutive days, subcutaneous administration of ivermectin 1% (Ivomec 1%, Merial, Duluth, GA) 50 or 150 μg/kg administered one month after melarsomine in cases in which melarsomine was administered and, in half of the cases, repeated every 6 months, doxycycline or minocycline 5–10 mg/kg PO BID for 4 weeks and, in eight cases, repeated every 3 months for one or more years and oral prednisone (0.4–1.6 mg/kg/day PO initially then tapered to the lowest effective dose or discontinued). Ancillary medications included topical neomycin, polymyxin, dexamethasone solution or ointment and topical tobramycin 0.3% solution.
Description of surgical management
Surgical management included removal of nematodes and their associated granuloma(s) or, in a single case, enucleation. Surgical removal of subconjunctival granulomas was performed under general anesthesia. The dogs were positioned in dorsal recumbency and the affected eye was aseptically prepared with dilute betadine solution and saline. An operating microscope (OPMI VISU 210; Zeiss, Germany) was used to provide magnification. A lateral canthotomy was performed to increase exposure to the granuloma(s). One or two scleral stay sutures were placed adjacent to the limbus to facilitate globe manipulation. Unaffected bulbar conjunctiva immediately adjacent to the lesion was incised with tenotomy scissors and blunt dissection was used to dissect to the sclera. A combination of blunt and sharp dissection with tenotomy scissors was used to remove the lesion en bloc, when possible. The sclera was not penetrated in any cases. Hemostasis was achieved with a disposable low-temp microcautery unit and instillation of topical 2.5% phenylephrine. When feasible, the bulbar conjunctiva was then apposed with 8–0 Vicryl using a simple continuous suture pattern with buried knots. The lateral canthotomy was closed with simple interrupted sutures of 5–0 Vicryl. In many cases, the entire lesion could not be removed en bloc because nematodes were located underneath or within the tendons of insertion of the extraocular muscles. In these cases, the nematodes where removed individually with Colibri forceps. In some instances, the granuloma extended too far posteriorly to ensure it was completely excised without performing an orbitotomy, which was not done in any case. In a single case, a routine transconjunctival enucleation was performed due to a large retrobulbar granuloma. In all cases, the surgically removed tissues were placed in 10% neutral buffered formalin solution and submitted for histopathologic evaluation. In some cases, tissue and or nematodes were placed in 80% ethanol for PCR.
Formalin-fixed samples were routinely processed, embedded in paraffin and 5 μm sections were stained with hematoxylin and eosin. Slides were reviewed by a board-certified anatomic pathologist (KMN).
Description of PCR
Ethanol-fixed samples, either tissues with associated nematodes or nematode fragments, were blotted dry using Kim wipes. Tissues were macerated using a scalpel. Genomic DNA was extracted from tissue fragments and or worms, under DNAzol reagent (Thermo Fisher Scientific), using the manufacturer’s protocol. DNA samples were used as a template in a nested PCR protocol aimed at amplification of a sequence fragment of the mitochondrial gene ND5 (employing TaqGold; Thermo Fisher Scientific). The nested PCR used Onchocerca-specific primers (all sequences shown 5′ to 3′) ND5Of (TTGGTTGCCTAAGGCTATGG) and ND5Or (CCCCTAGTAAACAACAAACCACA) followed by inside primers ND5OFi (TTGTTTGGTTCATAGTAGGACTT) and ND5Ori (TCTGTAACTGAACCAAAACAGG), newly designed from ND5 sequence of O. lupi worm derived from an infected dog in NM (GenBank accession JX183105).19 The sequential nested PCR reactions each employed the same thermal cycling profile: 95C, 10min (denaturation and polymerase activation; 30 cycles of 95C 30sec, 55C 30sec, 72 1min; 72C 7min (final extension)). Reaction volumes were 50 ul, with 2 ul of extracted DNA as template for the outside PCR reaction, and 2 ul of the completed first reaction as template for the second (nested) reaction. Positive controls were performed with a template that consisted of DNA extracted previously from O. lupi worms, for which the identity was confirmed by ND5 sequence. Negative controls included PCR reactions without primers, without template and using DNA from non-infected dogs (anonymized surgical skin samples obtained during elective castration). The presence and appropriate size of amplicons (outside PCR: 469bp, inside PCR: 382bp) was checked on Gel red (Biotium)-stained 1.5 % agarose gels. Amplicons obtained from several samples were sequenced to confirm PCR specificity. Results were reported as “no amplicon observed” or “parasite DNA detected” (amplicon present).
RESULTS
Sixteen dogs were included in the study. The clinical characteristics of the dogs are described in Table 1. One eye was affected in seven of the dogs (44%) and there was bilateral involvement nine of the dogs (56%). In the majority of the cases with bilateral involvement, one eye was affected initially with the other eye becoming affected at a later date (7/9). In the other two cases, both eyes were affected at the initial exam. The time lapsed between the first and second eye being affected ranged from 1 to 34 months.
Table 1.
Patient characteristics and eye affected in dogs infected with O. lupi
| Case number | Age (years) | Breed | Gender | Weight (kgs) | Affected eye |
|---|---|---|---|---|---|
| 1 | 7 | Mixed breed | MN | 27 | OD |
| 2 | 2 | Australian cattle dog | MN | 22 | OU |
| 3 | 1 | Border Collie | MN | 20 | OD |
| 4 | 5 | Mixed breed | MN | 31 | OU |
| 5 | 2 | Mixed breed | MN | 30 | OS |
| 6 | 2 | Pit bull | FS | 23 | OD |
| 7 | 7 | Mixed breed | FS | 22 | OU |
| 8 | 4 | Mixed breed | MN | 44 | OU |
| 9 | 5 | Dachshund | FS | 5 | OS |
| 10 | 2 | Pit Bull | MN | 30 | OU |
| 11 | 6 | Labrador Retriever | MN | 34 | OS |
| 12 | 12 | Australian Shepherd | FS | 22 | OU |
| 13 | 5 | Mixed breed | MN | 18 | OS |
| 14 | 5 | Mixed breed | FS | 27 | OU |
| 15 | 3 | Pit bull | FS | 28 | OU |
| 16 | 1 | Siberian Husky | FS | 23 | OU |
MN = male neutered; FS = female spayed; OD = right eye; OS = left eye; OU, = both eyes.
In all dogs, some degree of conjunctival hyperemia or episcleral injection was present; the severity varied from minimal to moderate. The most common clinical presentation that was more specific for the disease was a variably sized, non-painful, discrete, pink to tan, subconjunctival mass beneath the bulbar conjunctiva (14/16, 82%) (Fig. 1). Retinal detachment secondary to compression of the posterior sclera from a granuloma was also a common finding (7/16, 43%). Corneal vascularization was noted in several cases; in the majority of these cases, the vessels were located immediately adjacent to a subconjunctival mass and extended 1 to 5 mm axially from the limbus. When present, focal corneal edema was associated with the corneal vessels (4/16, 25%). Diffuse conjunctival chemosis (3/16, 19%) and focal crystalline anterior stromal corneal opacities (2/16, 12%) were noted in a few animals. Additionally, one dog each had: secondary glaucoma attributed to severe globe compression, ventral displacement of the globe, blepharospasm, vitreal degeneration and a focal chorioretinal scar. A complete blood cell count was performed in 7/16 dogs with the only notable finding being eosinophilia in 2/7 dogs.
Figure 1.
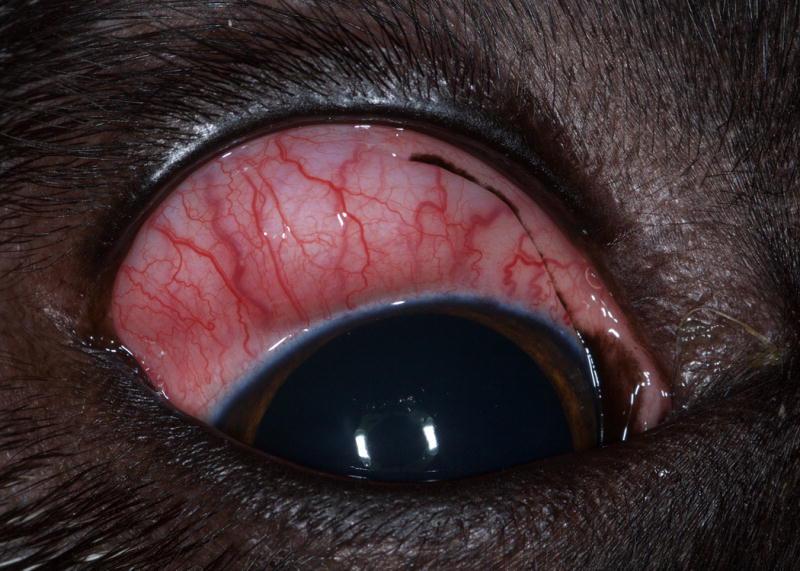
Dorsal bulbar subconjunctival mass in the right eye of a dog with confirmed ocular onchocerciasis.
Only 3/16 (19%) dogs had been on reliable, year-round heartworm prophylaxis in the six months prior to their initial exam. Two were treated with monthly milbemycin oxime and the third was treated first with milbemycin oxime and then switched to ivermectin. An antigen test for D. immitis was performed in 11/16 dogs and 2/11 dogs tested positive. Both dogs that tested positive for D. immitis had radiographic evidence of heartworm disease and their positive antigen test was not thought to be a cross-reaction from infection with O. lupi. Testing for MDR-1 gene mutation was performed in dogs that were herding breeds or herding mixes prior to the administration of ivermectin to decrease the risk of a drug reaction. Five dogs (dogs 2, 3, 5, 7 and 12 in Table 1) were tested for MDR-1 mutation and each dog tested negative. All dogs lived in New Mexico and only one dog had a significant travel history. The cities in which the dogs resided within New Mexico included Albuquerque or surrounding communities (12/16), Santa Fe (2), Capitan (1), and Ponderosa (1). Five of the dogs were strays that belonged to or had been recently been adopted from a New Mexico shelter. The shelters were located in Albuquerque and Espanola, New Mexico.
Diagnosis
A snip biopsy of conjunctival and subconjunctival tissues was obtained in 13/16 cases and the tissue was submitted for histopathology in 12/13 cases and PCR in 9/13 cases. In one case, the sample was submitted for neither histopathology nor PCR because a large number of thread-like worms were seen grossly. The diagnosis was supported by histologic identification of nematodes anatomically consistent with O. lupi from a conjunctival snip biopsy in 6/13 cases. PCR was positive from a snip biopsy in 8/9 cases tested and the remaining case had inconclusive results. In four of the cases with positive PCR results, nematodes were not seen with histopathology. White, thread-like worms were seen grossly at surgery or when the snip biopsy was performed in 10/16 cases. Nematodes were identified in surgical biopsies in 7/9 samples.
Pathologic findings
In most cases, the periocular tissues were expanded by varying combinations and concentrations of epithelioid macrophages and multinucleated giant cells with fewer eosinophils, neutrophils, lymphocytes and plasma cells. There was often peripheral fibrosis. In 10 cases, a nematode was present within the previously described granulomatous inflammation (Fig. 2). The nematodes were approximately 120 μm in diameter with coelomyarian musculature, two reproductive tracts and a small intestinal tract. The uterus often contained microfilaria. In longitudinal sections, regularly spaced cuticular ridges that ran cirumferentially around the nematode were apparent. Occasionally, nematodes lacked differential staining (necrosis) or were surrounded by basophilic granular material (mineralization).
Figure 2.
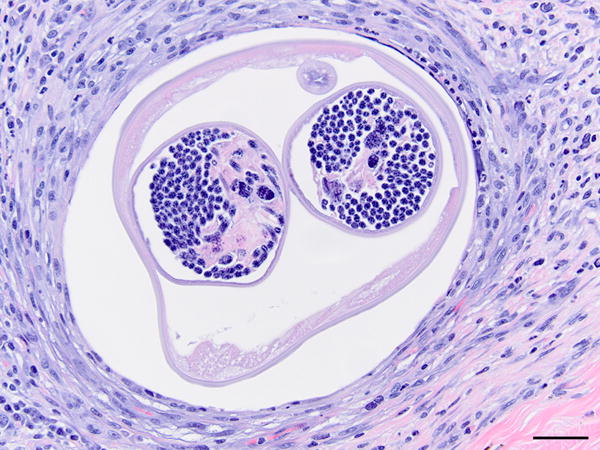
Nematode cross section embedded in a marked mixed inflammatory infiltrate. Note the paired uteri with microfilaria. 400X, H&E stain, bar = 20μm.
PCR findings
At first, PCR experiments and sequencing helped to confirm the initial identification of the surgically collected nematodes as filarial worms of the species O. lupi. All biopsy samples from dogs initially diagnosed based on clinical symptoms, yielded specific PCR amplicons for the ND5 sequence of O. lupi. Although the nested PCR protocol provided increased sensitivity and specificity over the regular PCR, ND5-specific amplicons were usually observed after the first (outside) PCR reaction. Only from some biopsy samples, that were not observed to include worms upon inspection by dissection microscopy, was parasite DNA detected exclusively after the second, nested PCR reaction. Overall, the PCR assay supported the initial diagnosis and it may be feasible to use PCR detection of parasite DNA toward more routine diagnosis of O. lupi infections.
Treatment
Surgical excision of granulomas was initially recommended in all dogs with accessible lesions. However, initial medical management led to resolution of the granulomas in several cases and surgery was canceled and, in other cases, medical management was successful in cases in which surgery was declined due to financial limitations. Medical management was recommended in cases in which an identifiable granuloma was not visible (e.g. in cases with diffuse chemosis or lesions in the posterior segment).
A summary of each dog’s medical treatment, surgical treatment, follow-up time and outcome is provided in Table 2. Surgical excision of subconjunctival granuloma(s) was performed in 8/16 dogs. Surgery was performed in both eyes in one dog and surgical excision of granulomas was repeated on the same eye of another dog. Enucleation of the left eye was performed in one dog in which retrobulbar disease had led to exophthalmia, retinal detachment and secondary glaucoma.
Table 2.
Treatment, follow-up times and outcomes for canine patients infected with O. lupi.
| Case number | Medical treatment | Surgical treatment | Follow-up time | Outcome |
|---|---|---|---|---|
| 1 | Doxycyclinea | Granuloma excision OD | 19 months | No recurrences. |
| 2 | Melarsomine, ivermectina, doxycyclinea, prednisone | None | 11 months | New granuloma in contralateral eye 9 months after initial exam, resolved with medical management. |
| 3 | Melarsomine, ivermectina, doxycyclinea, oral prednisone | Granuloma excision OD | 18 months | Initial granuloma resolved with medical management then recurred and was excised with no post-op recurrences. |
| 4 | Melarsomine, ivermectina, doxycyclinea | Granuloma excision OU | 23 months | No recurrences. |
| 5 | Melarsomine, ivermectina, doxycyclinea, prednisone | Granuloma excision OS | 15 months | No recurrences. |
| 6 | Melarsomine. ivermectinb, doxycyclineb, prednisone | Granuloma excision OD | 5 months | No recurrences. |
| 7 | Melarsomine, ivermectina, doxycyclinea, prednisone | None | 5 months | Symptoms recurred and eyes were enucleated OU by referring veterinarian. |
| 8 | Ivermectina, doxycyclinea, ivermectina, prednisone | Granuloma excision OU | 4 months | Symptoms recurred OU and dog was euthanized. |
| 9 | Melarsomine, ivermectinab, doxycyclineb, prednisone | Enucleation OS | 13 months | Developed exophthalmia OD one month after OS was enucleated. Resolved with oral amoxicillin/clavulanic acid and prednisone. |
| 10 | Melarsomine, ivermectinb, minocyclineb, prednisone | None | 6 months | Presented initially OD, 6 months later developed lesions OS, resolved with medical therapy. |
| 11 | Minocycline 10mg/kg PO BID for 30 days, prednisone | None | No follow-up | Owner communicated by phone that the eye appeared normal after 1 week of minocycline and prednisone. |
| 12 | Melarsomine, ivermectinb, doxycycline b, prednisone | None | 3 months | No recurrences; pet died 3 months after diagnosis from liver disease. |
| 13 | Melarsomine (4 doses), ivermectin b, minocycline, | Granuloma excision OS | 18 months | Initial granuloma treated with medical therapy. Granuloma recurred 6 months later and was excised. |
| 14 | Melarsomine, ivermectina b, doxycyclinea, doxycyclineb, minocycline, prednisone | None | 38 months | Initial granuloma OD resolved with doxycyclinea and ivermectin a. Disease recurred 33 months later OU and was managed medically. |
| 15 | Melarsomine, ivermectin b, doxycyclineb, minocycline, prednisone | Surgical excision of granuloma OD X 2 | 8 months | Granulomas recurred two months after surgery. Surgery repeated with no additional recurrences. |
| 16 | Melarsomine, ivermectin b, minocycline, prednisone | None | 7 months | Subtle granulomas reappeared OU and resolved with medical therapy. |
Doxycyclinea = doxycycline 5 mg/kg PO BID for 14–30 days.
Doxycclineb, = doxycycline 10 mg/kg PO BID for 28 days repeated every 3 months for at least one year.
Melarsomine = melarsomine dihydrochloride 2.5 mg/kg IM twice, 24 hours apart.
Ivermectina = 50 mcg/kg SQ one time.
Ivermectinb = 150 mcg/kg SQ every 6 months for at least one year.
Prednisone = oral prednisone at various doses and for various lengths of time.
Medical management alone was carried out in 6/16 dogs. Oral doxycycline and minocycline were typically prescribed initially while awaiting histopathology and/or PCR results to confirm a final diagnosis, usually in conjunction with oral prednisone. This led to a rapid resolution of conjunctival hyperemia, subconjunctival masses, corneal blood vessels and retinal detachments in all dogs; typically the lesions dramatically improved or resolved within one week. Melarsomine dihydrochloride (2.5 mg/kg IM) was administered in the epaxial muscles for two consecutive days in 13/16 dogs. One dog (#13) had this course repeated one time because moving worms were noted at surgery 6 months after the initial treatment. Two other dogs (#4, 16) received more than two doses of melarsomine as part of treatment for concurrent D. immitis infection. Doxycycline or minocycline were initially prescribed at a lower dose (5 mg/kg PO BID for 14–30 days) in 8/16 dogs. In an effort to limit recurrences in later cases, a higher dose of doxycycline or minocycline was prescribed in 9/16 dogs (10 mg/kg PO BID for 28 days) and the four week course was repeated every 3 months for at least one year. One dog (#14) was initially treated one time with the lower dose and then was treated repeatedly with the higher dose when a recurrence of disease was diagnosed 33 months after the initial diagnosis. Eleven dogs were treated with doxycycline, 4 dogs were treated with minocycline and one dog was treated with both at different times. Similarly, ivermectin was administered to 14/16 dogs and it was initially administered at a lower dose (50 μg/kg SQ) in 7/14 dogs. The dose was later increased (150 μg/kg SQ) in 8/14 dogs and repeated every 6 months. One dog (#14) was treated with the lower dose initially and the higher dose after a recurrence of disease. No dogs showed increased periocular swelling or intense itching following treatment with any of the above medications. The only side-effect noted was muscular pain following the melarsomine injections.
Outcomes
The follow-up time from the initial visit to the last recheck examination ranged from 2 to 38 months, with an average follow-up time of 12 months. One dog did not return for any follow-up exams. Ten dogs (67%) had known or suspected recurrences of disease after the initial treatment. Of those ten, one was euthanized due to severity of its ocular disease and one had bilateral enucleations performed by the referring veterinarian. The remainder of dogs with recurrences were successfully treated with either medical therapy alone (5/8) or a combination of medical and surgical therapy (3/8). Of the dogs with known or suspected recurrence of disease, 6/10 were treated initially with medical therapy alone and 4/10 were initially treated with a combination of medical and surgical therapy. Of the dogs with known or suspected recurrences, 9/10 were treated with melarsomine dihydrochloride, all were treated with ivermectin and all were treated with doxycycline or minocycline. 5/15 dogs had no recurrence of disease. In these dogs, 4/5 were initially treated with medical and surgical therapy and one was treated with medical therapy, alone. Of these five, three were treated with melarsomine, 4/5 were treated with subcutaneous ivermectin, and 5/5 were treated with oral doxycycline or minocycline.
Discussion
This case series represents the largest case series of canine ocular onchocerciasis from the United States and illustrates that canine ocular onchocerciasis is an endemic disease of growing importance in New Mexico. Veterinarians should be aware of the disease and consider it as a differential diagnosis in dogs with subconjunctival masses, retrobulbar disease, chemosis and retinal detachment.
Although there are many similarities between the cases in this report and previously reported cases, there are some interesting differences when compared to the case series from dogs in Greece.11,12 In a report of 23 cases in Greece, dogs were treated with surgical excision of granulomas, melarsomine and ivermectin.12 The dogs were followed for at least one year and there were no recurrences in any dogs.12 This outcome contrasts starkly with the results in this study in which 67% of dogs had recurrent disease. Interestingly, in another cases series of dogs from the United States, recurrences were also noted in 3/8 dogs.3 This suggests that the particular strain of O. lupi endemic in the United States, which represents a single haplotype of those described in Europe, may be more difficult to control.2,3 Alternatively, differences in medical and/or surgical treatment may explain the different recurrence rates, or, dogs in the United States may be more likely to suffer recurrent infection by O. lupi. Differences in the clinical presentations of the dogs were also noted. In Kommenenou et al, ocular discomfort was noted in all 23 dogs, anterior uveitis was noted in nearly half of the dogs and scleral indentation and/or retinal detachments were not noted in any dogs.12 In this series, ocular discomfort was notably absent in nearly all dogs, anterior uveitis was not seen in any dogs and scleral indentation leading to retinal detachment was noted in nearly half of the dogs. Periorbital edema and pruritis were noted in all dogs following melarsomine administration in Komenenou et al and were documented in no dogs in this report.12
In most reported cases of canine O. lupi, surgery has been the mainstay of therapy. Because the symptoms of the disease are caused by the adult worms and adult Onchocerca spp. are notoriously difficult to kill, it stands to reason that surgical excision of the worms will remain a crucial part of therapy as our understanding of this parasite, and its appropriate treatment, evolves. However, as this case series illustrates, surgical therapy does not prevent recurrent disease in many dogs and dogs with significant retrobulbar involvement pose a considerable challenge when one is planning surgical intervention. Many dogs will have a dramatic and sustained response to the combination of oral tetracyclines and a low dose of oral prednisone. Thus, medical therapy alone may be a reasonable approach in dogs in which surgical excision of the granulomas would be challenging or impossible or in cases in which financial limitations prevent surgical intervention.
Like most filariae, O. lupi harbors a Wolbachia bacterial endosymbiont.20–22 The role of Wolbachia in filarial disease has been shown to be multifaceted. The Gram-negative bacteria promotes the survival of microfilaria but it has also been shown to exacerbate inflammation in the host through interactions with monocytes, dendritic cells and neutrophils.20 The presence of Wolbachia within O. lupi provides a potential target for therapy. For instance, anti-Wolbachia treatment of people with onchocerciasis or lymphatic filariasis with doxycycline results in a decrease in filarial growth, sterilization of the adult female worms, blockage of the first and third moults and death of the adult worms.20 Destruction of Wolbachia may reduce inflammation in the host, decrease microfilariae embryogenesis, prevent development of microfilariae and may eventually act as a macrofilaricide and eradicate adult worms.20 Use of doxycycline as an anti-Wolbachia therapy requires a prolonged treatment course of 4 to 6 weeks.
A doxycycline dose of 5 mg/kg PO BID for 4 weeks has been previously recommended to target the Wolbachia of O. lupi. However, this dose may be too low given that a 10 mg/kg PO BID dose is recommended for targeting Wolbachia in dogs infected with D. immitis.23,24 This concern led to an increase in the dose of doxycycline and minocycline to 10 mg/kg PO BID administered to the dogs that presented later in this series.
In the treatment of O. volvulus, ivermectin has been shown to not only kill the larvae but repeated doses will accelerate the death of adult worms when given two to four times annually.25,26 Given that multiple recurrences were noted in the first several treated dogs when the previously recommended dose of 50 ug/kg was administered, the standard dose administered to human patients infected with O. volvulus of 150 ug/kg was administered every 6 months in subsequent cases, in hopes that it would provide long-term macrofilaricide effects.
The fact that a single strain of O. lupi has been identified in the United States makes it likely that O. lupi established recently in the United States. The genetic uniformity of the parasites encountered suggests that only a single strain of O. lupi was introduced into the United States, perhaps by means of an infected dog or blackfly vector. The ensuing population of O. lupi parasites may not yet have diversified if this introduction was a recent event. Alternatively, selection pressures from environmental conditions and/or less permissive insect hosts may have restricted the diversity of introduced O. lupi parasites to a single remaining strain.
Several of the dogs in this report had been adopted by their owners or acquired by an animal shelter within a few months of being diagnosed with O. lupi. It stands to reason that stray animals in endemic areas may have increased exposure to the insect vector and be at an increased risk of infection with O. lupi. Screening of dogs for O. lupi at shelters in endemic areas should be considered. Most of the dogs in this series were not treated with monthly prophylaxis for D. immitis and, the two dogs that were infected despite the administration of monthly prophylaxis, received milbemycin oxime. The ability of various prophylactics for heartworm disease to affect the risk of infection with O. lupi in dogs is unknown and deserves further study.
Molecular amplification and sequencing of the mitochondrial O. lupi ND5 gene from conjunctival snip biopsies was a highly useful tool in the evaluation dogs in this case series. Snip biopsies of the bulbar conjunctiva can be easily obtained from most dogs following the instillation of a topical anesthetic and without the use of sedation. Submitting these samples for PCR and histopathology can support the diagnosis and rule out diseases that may cause similar clinical signs. Reliable tests for O. lupi are needed. A serologic test investigated to identify O. lupi antigens in infected dogs was positive in only 50% of the cases.27 Identification of microfilariae from skin snips is another means of identifying infected dogs.28 Microfilariae can be found in the skin of infected dogs; microfilariae have been reported to be at the highest concentration in the skin of the head and their numbers are highest in the evenings.28,29
The importance of O. lupi lies not only in its ability to produce potentially blinding disease in dogs, but also in its role as a zoonotic agent and as a potential animal model for O. volvulus.30–38 A better understanding of the life cycle of O. lupi, as well as treatment and prevention strategies, is needed. Although it is difficult to provide concrete treatment guidelines given our limited understanding of this parasite, the current treatment strategy used by these authors includes an initial six week course of doxycycline or minocycline (10 mg/kg PO BID) followed by a four week course of the same dose repeated every three months for at least one year, melarsomine dihydrochloride (2.5 mg/kg IM) for two consecutive days, ivermectin (150 μg/kg SQ) one month after melarsomine administration and then every six months indefinitely, oral prednisone (0.5 mg/kg PO daily then tapered or discontinued), and surgical excision of the adult nematodes if the lesions are still present one month after the administration of melarsomine or if they recur following the discontinuation of prednisone. The role of vector control, topical insect repellents effective against black flies and the role of heartworm prophylaxis in diminishing the risk of canine ocular onchocercosis also deserves further study.
Acknowledgments
CMA acknowledges support from NIH grant number P20GM103452 from the National Institute of General Medical Sciences (NIGMS).
References
- 1.Labelle AL, Maddox CW, Daniels JB, et al. Canine ocular onchocercosis in the United States is associated with Onchocerca lupi. Veterinary Parasitology. 2013;193:297–301. doi: 10.1016/j.vetpar.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Otranto D, Giannelli A, Scotty Trumble N, et al. Clinical case presentation and a review of the literature of canine onchocercosis by Onchocerca lupi in the United States. Parasites & Vectors. 2015;8:89. doi: 10.1186/s13071-015-0699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otranto D, Giannelli A, Latrofa MS, et al. Canine infections with Onchocerca lupi nematodes, United States, 2011–2014. Emerging Infectious Diseases. 2015;21:868–871. doi: 10.3201/eid2105.141812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermosilla C, Hetzel U, Bausch M, et al. First autochthonous case of canine ocular onchocercosis in Germany. The Veterinary Record. 2005;156:450–452. doi: 10.1136/vr.156.14.450. [DOI] [PubMed] [Google Scholar]
- 5.Zarfoss MK, Dubielzig RR, Eberhard ML, et al. Veterinary Ophthalmology. 2005;8:51–57. doi: 10.1111/j.1463-5224.2005.00348.x. [DOI] [PubMed] [Google Scholar]
- 6.Széll Z, Erdélyi I, Sréter T, et al. Canine ocular onchocercosis in Hungary. Veterinary Parasitology. 2001;97:243–249. doi: 10.1016/s0304-4017(01)00397-1. [DOI] [PubMed] [Google Scholar]
- 7.Sréter T, Széll Z, Egyed Z, et al. Ocular onchocercosis in dogs: a review. The Veterinary Record. 2002;151:176–180. doi: 10.1136/vr.151.6.176. [DOI] [PubMed] [Google Scholar]
- 8.Sréter T, Széll Z. Onchocercosis: a newly recognized disease in dogs. Veterinary Parasitology. 2008;151:1–13. doi: 10.1016/j.vetpar.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Gardiner CH, Dick EJ, Jr, Meininger AC, et al. Onchocerciasis in two dogs. Journal of the American Veterinary Medical Association. 1993;203:828–830. [PubMed] [Google Scholar]
- 10.Eberhard ML, Ortega Y, Dial S, et al. Ocular Onchocerca infections in two dogs in western United States. Veterinary Parasitology. 2000;90:333–338. doi: 10.1016/s0304-4017(00)00252-1. [DOI] [PubMed] [Google Scholar]
- 11.Komnenou A, Egyed Z, Sréter T, et al. Canine onchocercosis in Greece: report of further 20 cases and molecular characterization of the parasite and its Wolbachia endosymbiont. Veterinary Parasitology. 2003;118:151–155. doi: 10.1016/j.vetpar.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Komnenou A, Eberhard ML, Kaldrymidou E, et al. Subconjunctival filariasis due to Onchocerca sp. in dogs: report of 23 cases in Greece. Veterinary Ophthalmology. 2002;5:119–126. doi: 10.1046/j.1463-5224.2002.00235.x. [DOI] [PubMed] [Google Scholar]
- 13.Franchini D, Giannelli A, Di Paola G, et al. Image diagnosis of zoonotic onchocercosis by Onchocerca lupi. 2014;203:91–95. doi: 10.1016/j.vetpar.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Komnenou AT, Thomas ALN, Papadopoulos E, et al. Intraocular localization of Onchocerca lupi adult worm in a dog with anterior uveitis: a case report. Veterinary Ophthalmology. 2015;19:245–249. doi: 10.1111/vop.12277. [DOI] [PubMed] [Google Scholar]
- 15.Faísca P, Morales-Hojas R, Alves M, et al. A case of canine ocular onchocercosis in Portugal. Veterinary Ophthalmology. 2010;13:117–121. doi: 10.1111/j.1463-5224.2010.00763.x. [DOI] [PubMed] [Google Scholar]
- 16.Rodonaja TE. A new species of nematode, Onchocerca lupi sp. nov., from Canis lupus cubanensis. Soobshcheniya Akademii Nauk, Gruzinksaya SSR. 1967;45:715–719. in Russian. [Google Scholar]
- 17.Hassan HK, Bolcen S, Kubofcik J, et al. Isolation of Onchocerca lupi in dogs and black flies, California, USA. Emerging Infectious Diseases. 2015;5:789–796. doi: 10.3201/eid2105.142011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sréter-Lancz Z, Széll Z, Sréter T. Molecular genetic comparison of Onchocerca sp. Infecting dogs in Europe with other spirurid nematodes including Onchocerca lienalis. Veterinary Parasitology. 2007;148:365–370. doi: 10.1016/j.vetpar.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Morales-Hojas R, Cheke RA, Post RJ. Molecular systematics of five Onchocerca species (Nematoda: Filarioidea) including the human parasite, O. volvulus, suggest symatric speciation. Journal of Helminthology. 2006;80:281–290. [PubMed] [Google Scholar]
- 20.Bouchery T, Lefoulon E, Karadjian G, et al. The symbiotic role of Wolbachia and its impact on filariasis. Clinical Microbiology and Infection. 2013;19:131–140. doi: 10.1111/1469-0691.12069. [DOI] [PubMed] [Google Scholar]
- 21.Egyed A, Sréter T, Széll Z, et al. Molecular phylogenetic analysis of Onchocerca lupi and its Wolbachia endosymbiont. Veterinary Parasitology. 2002;108:153–161. doi: 10.1016/s0304-4017(02)00186-3. [DOI] [PubMed] [Google Scholar]
- 22.Egyed Z, Sréter T, Széll Z, et al. Electron microscopic and molecular identification of Wolbachia endosymbionts from Onchocerca lupi: implications for therapy. Veterinary Parasitology. 2002;106:75–82. doi: 10.1016/s0304-4017(02)00029-8. [DOI] [PubMed] [Google Scholar]
- 23.McCall JW, Kramer L, Genchi C, et al. Effects of doxycycline on heartworm embryogenesis, transmission, circulating microfilaria, and adult worms in microfilaremic dogs. Veterinary Parasitology. 2014;206:5–13. doi: 10.1016/j.vetpar.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 24.McCall JW, Genchi C, Kramer L, et al. Heartworm and Wolbachia: therapeutic implications. Veterinary Parasitology. 2008;158:204–214. doi: 10.1016/j.vetpar.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Cupp EW, Cupp MS. Short report: impact of ivermectin community-level treatments on elimination of adult Onchocerca volvulus when individuals receive multiple treatments per year. American Journal of Tropical Medicine and Hygiene. 2005;73:1159–1161. [PubMed] [Google Scholar]
- 26.Basáñez MG, Pion SD, Boakes E, et al. Effect of single-dose ivermectin on Onchocerca volvulus: a systematic review and meta-analysis. The Lancet Infectious Diseases. 2008;8:310–322. doi: 10.1016/S1473-3099(08)70099-9. [DOI] [PubMed] [Google Scholar]
- 27.Giannelli A, Cantacessi, Graves P, et al. A preliminary investigation of serological tools for the detection of Onchocerca lupi infection in dogs. Parasitology Research. 2014;113:1989–1991. doi: 10.1007/s00436-014-3844-6. [DOI] [PubMed] [Google Scholar]
- 28.Otranto D, Dantas-Torres F, Giannelli A, et al. Cutaneous distribution and circadian rhythm of Onchocerca lupi microfilariae in dogs. PLOS Neglected Tropical Diseases. 2013;7:e2585. doi: 10.1371/journal.pntd.0002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Széll Z, Sréter T, Erdélyi I, et al. Ocular onchocercosis in dogs: aberrant infection in an accidental host or lupi onchocercosis. Veterinary Parasitology. 2001;101:115–125. doi: 10.1016/s0304-4017(01)00507-6. [DOI] [PubMed] [Google Scholar]
- 30.Otranto D, Dantas-Torres F, Cebeci Z, et al. Human ocular filariasis: further evidence on the zoonotic role of Onchocerca lupi. Parasites & Vectors. 2012;5:84. doi: 10.1186/1756-3305-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eberhard ML, Gholamabbas AO, Chundu K, et al. Zoonotic Onchocerca lupi infection in a 22-month-old child in Arizona: first report in the United States and a review of the literature. The American Journal of Tropical Medicine and Hygiene. 2013;88:601–605. doi: 10.4269/ajtmh.12-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen T, Moon K, DeMello DE, et al. Case report of an epidural cervical Onchocerca lupi infection in a 13-year-old boy. Journal of Neurosurgery Pediatrics. 2015;16:217–221. doi: 10.3171/2014.12.PEDS14462. [DOI] [PubMed] [Google Scholar]
- 33.Dudley RW, Smith C, Dishop M, et al. A cervical mass caused by Onchocerca lupi. Lancet. 2015;386:1372. doi: 10.1016/S0140-6736(14)62255-8. [DOI] [PubMed] [Google Scholar]
- 34.Bergua A, Hohberger B, Held J, et al. Human case of Onchocerca lupi infection, Germany, August 2014. Eurosurveillance. 2015;20 doi: 10.2807/1560-7917.es2015.20.16.21099. pii=21099. [DOI] [PubMed] [Google Scholar]
- 35.Otranto D, Sakru N, Testini G, et al. Case report: first evidence of human zoonotic infection by Onchocerca lupi (Spirurida, Onchocercidae) American Journal of Tropical Medicine and Hygiene. 2011;84:55–58. doi: 10.4269/ajtmh.2011.10-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ilhan HD, Yaman A, Morishima Y, et al. Onchocerca lupi infection in Turkey: a unique case of a rare human parasite. Acta Parasitologica. 2013;58:384–388. doi: 10.2478/s11686-013-0152-8. [DOI] [PubMed] [Google Scholar]
- 37.Santos Grácio AJ, Richter J, Komnenou AT, et al. Onchocerciasis caused by Onchocerca lupi: an emerging zoonotic infection. Systematic review. Parasitology Research. 2015;114 doi: 10.1007/s00436-015-4535-7. [DOI] [PubMed] [Google Scholar]
- 38.Mowlavi G, Farzbod F, Kheirkhah A, et al. Human ocular onchocercosis caused by Onchocerca lupi (Spirurida, Onchocercidae) in Iran. Journal of Helminthology. 2014;88:250–255. doi: 10.1017/S0022149X13000060. [DOI] [PubMed] [Google Scholar]


