Abstract
Objective To investigate the relation of diastolic blood pressure in pregnancy with birth weight and perinatal mortality.
Design Prospective study.
Setting 15 maternity units in one London health region, 1988-2000.
Participants 210 814 first singleton births of babies weighing more than 200 g among mothers with no hypertension before 20 weeks' gestation and without proteinuria, delivering between 24 and 43 weeks' gestation.
Main outcome measures Birth weight and perinatal mortality.
Results The mean (SD) birth weight of babies born to mothers with no hypertension before 20 weeks' gestation or proteinuria was 3282 g (545 g) and there were 1335 perinatal deaths, compared with 94 perinatal deaths among women with proteinuria or a history of hypertension. Diastolic blood pressure at booking for antenatal checks was progressively higher from weeks 34 to 40 of gestation. The birth weight of babies being delivered after 34 weeks was highest for highest recorded maternal diastolic blood pressures of between 70 and 80 mm Hg and lower for blood pressures outside this range. Both low and high diastolic blood pressures were associated with statistically significantly higher perinatal mortality. Using a linear quadratic model, 94 of 825 (11.4%) perinatal deaths could be attributed to mothers having blood pressure differing from the optimal blood pressure (82.7 mm Hg) predicted by the fitted model. Most of these excess deaths occurred with blood pressures below the optimal value.
Conclusions Both low and high diastolic blood pressures in women during pregnancy are associated with small babies and high perinatal mortality.
Introduction
Hypertensive disorders in pregnancy are a leading cause of maternal and perinatal mortality in the developed world1,2 and have been extensively studied. Few studies, however, have reported fetal outcomes at lower blood pressures. In long term follow up studies, lower blood pressure (down to at least 115 mm Hg systolic and 75 mm Hg diastolic pressures) was associated with a lower risk of vascular and all cause mortality.3 Low blood pressure has also been associated with symptoms, including unexplained tiredness, chronic fatigue syndrome, and recurrent syncope.4-7 It is particularly common in underweight women with a low muscle mass.8 We hypothesised that the association of a low maternal weight with an increased risk of a low birthweight baby and poor infant survival9 might be due in part to low blood pressure leading to poor placental perfusion. Three small studies reported that low maternal blood pressure in pregnancy is associated with low birth weight and an increased risk of small for gestational age babies.10-12 Another study reported an increased risk of perinatal mortality.13 Since 1988, prospective data have been collected on all pregnancies booked into 15 of the 17 maternity units in the North West Thames region of London using the St Mary's Maternity Information System. We used this database to investigate the association between blood pressure in pregnancy and fetal outcome (birth weight and perinatal mortality) in women without pre-existing hypertension or proteinuria.
Materials and methods
From 1988-2000, the St Mary's database included 585 291 pregnancies, of which 517 381 had a birth weight recorded. Data were entered by trained clerks or midwives, with online validation, prompting, and standard definitions for clinical measurements, giving high quality data.14 We restricted our study to 222 218 singleton births between 24 and 43 weeks' gestation, weighing more than 200 g to nulliparous women (ensuring that only one delivery was included for each woman). For most analyses, we excluded 4733 births to women with pre-existing (chronic) hypertension and a further 6671 births to women with persistent proteinuria at any stage of pregnancy (thus excluding all women who developed pre-eclampsia). This left data on 210 814 mothers and their births.
Blood pressure measurements
Blood pressure was recorded as a clinical routine during the first and subsequent antenatal checks. In 1988, clinicians mostly recorded diastolic blood pressure using the Korotkoff phase IV heart sound, because of reports that heart sounds could be heard almost down to 0 mm Hg in a proportion of pregnant women.15-18 Since 1988 there has been a gradual change to using the Korotkoff phase V heart sound.19-22
When women delivered, the attending midwives determined the diastolic blood pressure and gestational age recorded at the first antenatal check and the highest diastolic blood pressure during pregnancy. To minimise sources of error, including variable maternal positions, inappropriately sized cuffs, rounding of terminal digits, and avoidance of thresholds, the midwives used clinical judgment and recorded a representative value.23
When the database was first introduced, constraints on storage of data limited the number and size of variables that could be entered. At that time in the United Kingdom diastolic blood pressure in pregnancy was generally considered more important than systolic blood pressure.16-24 Some workers still report that diastolic pressure is as informative about hypertension in pregnancy as are systolic and diastolic pressures.25,26
Fetal outcomes
We defined perinatal mortality as a stillbirth (death before birth at, or after, 28 weeks' gestation until October 1992, and 24 weeks thereafter) or the death of a baby in its first week of life. We cross checked deaths against national death registrations and data from the Confidential Enquiry into Stillbirths and Death in Infancy.27
Maternal data
The St Mary's database also includes data on gestational age (weeks) at booking for antenatal checks and birth (using the clinician's best estimate based on the first day of the last menstrual period, or ultrasound measurements, which were offered during the study period); persistent important proteinuria (important according to the clinician's judgment on the basis of measurements of > 300 mg protein/l in a 24 hour urine specimen or at least two dipstick results suggesting albumin concentrations of 1 g/l). Data recorded on the women were ethnic group (based on self classification in discussion with the midwife), smoking status, height (metres), weight (kilograms), age at booking (years), and any major medical disorders.
Statistical analysis
We calculated the body mass index of each woman and her Carstairs' deprivation score, derived from residential postcodes.28 For diastolic blood pressure and birth weight we adjusted for mother's ethnic group, smoking status, age at booking for antenatal checks, Carstairs' score, and height and weight. We adjusted blood pressure measurements for calendar year because of the secular shift from the Korotkoff phase IV to phase V heart sound.19,20
We fitted log linear models to explore the relations between diastolic blood pressure and gestational age at booking and between highest maternal diastolic blood pressure and birth weight (figs 1 and 2). Logistic models were used to model perinatal mortality in relation to highest maternal diastolic blood pressure (figs 4 and 5). Because the residual distributions for the log linear models had much lighter tails than expected, we used bootstrap methods29 to evaluate the confidence intervals on the fitted variables and to assess statistical significance (999 bootstrap samples were used to evaluate 95% confidence intervals in the figures). We used 9999 bootstrap samples to evaluate the tests for heterogeneity (F test, χ2 test).30 Our analyses include only those records for which all variables included in the model were available.
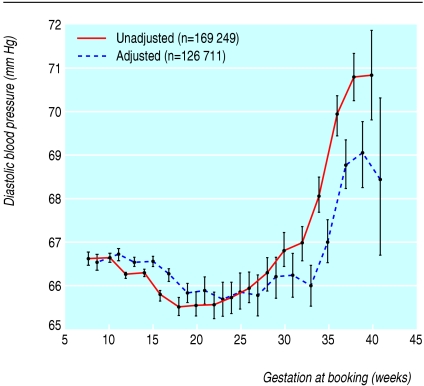
Fig 1.
Diastolic blood pressures of women booking for antenatal checks among mothers without chronic hypertension and no proteinuria between 24 and 43 weeks at delivery. Values adjusted for calendar year and women's ethnic group, smoking status, height, weight, age at booking, and Carstairs' deprivation score
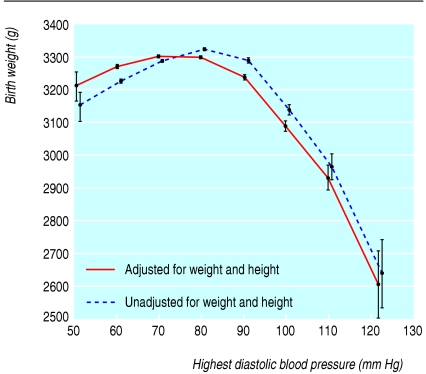
Fig 2.
Birth weight and highest diastolic blood pressure, unadjusted or adjusted for maternal height and weight, among mothers without chronic hypertension, chronic renal disease, or proteinuria and gestation between 34 and 43 weeks at delivery. Values adjusted for calendar year and women's ethnic group, smoking status, height, weight, age at booking, and Carstairs' deprivation score
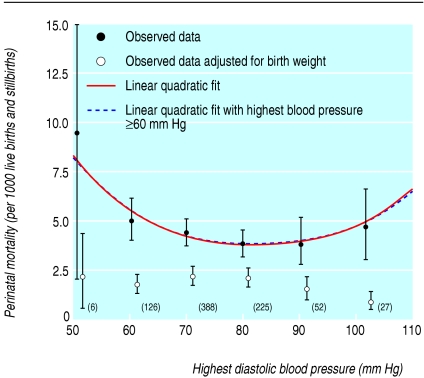
Fig 4.
Perinatal mortality per 1000 live births and stillbirths in relation to highest diastolic blood pressure in mothers and gestation between 24 and 43 weeks at delivery, unadjusted and adjusted for birth weight, among women without chronic hypertension and no proteinuria (numbers of deaths are in brackets). Values adjusted for calendar year and women's ethnic group, smoking status, height, weight, age at booking, and Carstairs' deprivation score
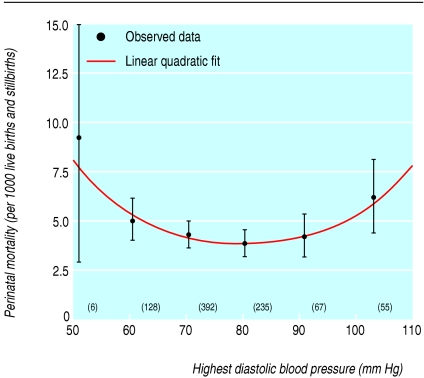
Fig 5.
Perinatal mortality per 1000 live births and stillbirths in relation to highest diastolic blood pressure in mothers and gestation between 24 and 43 weeks at delivery, including women with proteinuria and chronic hypertension. Values adjusted for calendar year and women's ethnic group, smoking status, height, weight, age at booking, and Carstairs' deprivation score
Results
We identified 210 814 first singleton births of babies weighing more than 200 g among women with no hypertension before 20 weeks' gestation and with no proteinuria, delivering between 24 and 43 weeks' gestation. The mean (SD) birth weight of the babies was 3282 g (545 g), and there were 1335 perinatal deaths. Recorded mean diastolic blood pressure at booking fell by an average of 0.23 mm Hg per year from 67.9 mm Hg in 1988 to 65.2 mm Hg in 2000. Table 1 shows the mean highest diastolic blood pressures during pregnancy and perinatal death rates for selected variables. We found statistically significant differences between groups for blood pressure by age at booking for antenatal checks; weeks of gestation at booking; mother's body mass index, ethnic group, Carstairs' score, and smoking status; birth weight, and year of birth. We also found statistically significant differences between groups for perinatal mortality for all variables except year of birth. Overall, there were 46 perinatal deaths in women excluded from the analysis with a history of hypertension and 58 perinatal deaths for those with proteinuria (including all cases of pre-eclampsia). In 10 of these women there was a history of hypertension and proteinuria; thus 94 perinatal deaths occurred in the excluded group; 93.4% of all perinatal deaths (1335 of 1429 deaths) therefore occurred in women without a history of hypertension and who did not develop pre-eclampsia.
Table 1.
Mean highest diastolic blood pressure in mothers during pregnancy and perinatal death rates for selected variables in cohort of 210 814 first singleton births in North West Thames region, London*
| Variable | No (%) of women | Mean (SD) highest diastolic blood pressure (mm Hg) | P value | Perinatal deaths per 1000 births (No of deaths)† | P value |
|---|---|---|---|---|---|
| Mother's age at booking (years‡): | |||||
| 15-24
|
65 246 (33.6)
|
72.9 (9.2)
|
<0.0001 | 6.26 (437)
|
0.0001 |
| 25-29
|
69 940 (36.0)
|
74.5 (9.3)
|
5.35 (402)
|
||
| 30-34
|
44 743 (23.0)
|
74.9 (9.3)
|
7.14 (340)
|
||
| ≥35 | 14 310 (7.4) | 75.6 (9.5) | 8.56 (130) | ||
| Gestation at booking (weeks): | |||||
| 0-20
|
153 569 (89.0)
|
74.4 (9.3)
|
<0.0001 | 5.26 (867)
|
<0.0001 |
| ≥21 | 18 936 (11.0) | 73.1 (9.1) | 12.95 (265) | ||
| Mother's body mass index at first pregnancy check (kg/m2): | |||||
| <20
|
21 084 (13.4)
|
71.2 (8.4)
|
<0.0001 | 4.70 (108)
|
<0.0001 |
| 20-24.99
|
89 566 (57.1)
|
73.5 (8.9)
|
5.37 (521)
|
||
| ≥25 | 46 276 (29.5) | 77.3 (9.7) | 7.52 (372) | ||
| Mother's ethnic group: | |||||
| White European
|
139 565 (73.0)
|
74.8 (9.3)
|
<0.0001 | 5.54 (838)
|
<0.0001 |
| Black African
|
6038 (3.2)
|
72.5 (9.0)
|
8.76 (55)
|
||
| Black Caribbean
|
5235 (2.7)
|
72.8 (8.8)
|
10.25 (56)
|
||
| Indian, Pakistani, Bangladeshi
|
23 278 (12.2)
|
72.2 (9.2)
|
9.31 (227)
|
||
| Oriental
|
3396 (1.8)
|
71.8 (9.0)
|
4.74 (17)
|
||
| Mediterranean
|
5135 (2.7)
|
72.2 (8.5)
|
6.91 (37)
|
||
| Other | 8615 (4.5) | 72.7 (9.2) | 7.55 (68) | ||
| Deprivation fifths§: | |||||
| 1 (most affluent)
|
35 419 (20.0)
|
75.0 (9.4)
|
<0.0001 | 5.66 (218)
|
0.0244 |
| 2
|
38 616 (21.8)
|
74.7 (9.3)
|
6.03 (250)
|
||
| 3
|
43 328 (24.4)
|
74.2 (9.3)
|
6.12 (283)
|
||
| 4
|
36 343 (20.5)
|
73.7 (9.3)
|
6.61 (256)
|
||
| 5 (most deprived) | 23 706 (13.4) | 72.8 (9.1) | 7.64 (192) | ||
| Maternal smoking (cigarettes/d): | |||||
| 0
|
161 011 (82.2)
|
74.4 (9.4)
|
<0.0001 | 6.11 (1056)
|
0.0223 |
| 1-9
|
19 339 (9.9)
|
72.8 (8.9)
|
6.54 (137)
|
||
| 10-19
|
11 983 (6.1)
|
72.8 (8.6)
|
7.82 (102)
|
||
| ≥20 | 3432 (1.8) | 73.1 (8.6) | 8.81 (33) | ||
| Birth weight (g): | |||||
| <2500
|
12 435 (6.3)
|
74.3 (11.7)
|
0.0314 | 62.56 (831)
|
<0.0001 |
| ≥2500 | 183 685 (93.7) | 74.1 (9.1) | 2.55 (504) | ||
| Year of birth: | |||||
| 1988-90
|
41 136 (21.0)
|
75.6 (9.5)
|
<0.0001 | 6.19 (303)
|
0.3923 |
| 1991-3
|
48 010 (24.5)
|
74.8 (9.3)
|
6.10 (314)
|
||
| 1994-6
|
46 399 (23.7)
|
73.9 (9.1)
|
6.95 (330)
|
||
| 1997-8
|
30 219 (15.4)
|
73.1 (9.3)
|
6.37 (197)
|
||
| 1999-2000 | 30 356 (15.5) | 72.6 (9.0) | 5.97 (191) |
Tests of statistical significance are based on measures of heterogeneity (ϰ2 for perinatal mortality, F test for blood pressure).
Mothers without chronic hypertension, gestation 24-43 weeks at delivery, and no proteinuria.
Stillbirths (deaths in utero before 28 weeks until October 1992, thereafter before 24 weeks), plus deaths in first postnatal week, as proportion of total births.
Booking for antenatal checks.
Carstairs' deprivation categories.
Figure 1 shows diastolic blood pressures at booking for antenatal checks and weeks of gestation among 169 249 women when both were recorded. Substantial numbers of women had their first antenatal check at all gestations from eight to 40 weeks, thus providing cross sectional data on blood pressures through pregnancy. Mean diastolic blood pressure was 66.6 mm Hg in the first trimester and 66.3 mm Hg in the second trimester. It was progressively higher after 34 weeks' gestation, reaching 68.4 mm Hg by 40 weeks' gestation or more. This was after adjustment for the mother's ethnic group, smoking status, height and weight, calendar year, age at booking, and Carstairs' score; similar changes were also observed in the unadjusted data.
Before 34 weeks' gestation there was no substantial correlation between highest blood pressure in pregnancy and birth weight (data not shown). At 34 weeks' gestation or more, however, there was an inverted U shaped relation between birth weight and blood pressure, with a maximum birth weight at around 80 mm Hg (fig 2). When birth weight was adjusted for maternal height and weight, the relation remained similar but shifted slightly towards lower blood pressures. The relation of birth weight with blood pressure could not be explained by confounding by gestational age; analysis of the Z scores of birth weights (by gestational age) showed the same relation (data not shown). To account for the effects of obstetric interventions on relation between highest antenatal blood pressure and birth weight, we reanalysed the data only for women with spontaneous onset of labour at term (37-42 weeks' gestation). We found no material change in the results (data not shown).
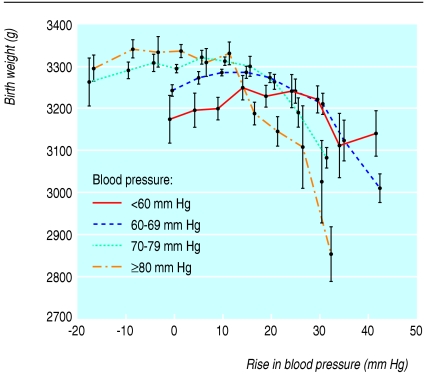
Birth weight in relation to rises in blood pressure during pregnancy depended on the blood pressure at booking (fig 3). A blood pressure of 70 mm Hg or more at booking was associated with the highest birth weights as long as the blood pressure rise during pregnancy did not exceed 10 mm Hg; with rises greater than this, birth weight fell sharply. If the blood pressure at booking was less than 70 mm Hg, birth weight rose as the rise became greater, but started to fall again if the rise exceeded 30 mm Hg.
Fig 3.
Birth weight and rise in blood pressure after booking for antenatal checks, by blood pressure at booking and gestation between 34 and 43 weeks at delivery, among women without chronic hypertension and no proteinuria. Values adjusted for calendar year and women's ethnic group, smoking status, height, weight, age at booking, and Carstairs' deprivation score
Perinatal mortality showed a strong curvilinear association with highest diastolic blood pressure (fig 4). In particular, if linear quadratic models were fitted to the data, the quadratic term was highly statistically significantly different from zero and remained so even when we excluded women with highest diastolic pressures of less than 60 mm Hg. This relation largely disappeared if corrected for birth weight (fig 4). Of the 824 perinatal deaths contributing to this analysis, we estimated that 94.3 (11.4%) were attributable to women with blood pressure differing from the optimal blood pressure (82.7 mm Hg) predicted by the linear quadratic model; most (91.2%) of these excess perinatal deaths (86.1) occurred among women with lower blood pressures (table 2), mainly in the ranges 70-79 mm Hg (51.2 excess deaths) and 60-69 mm Hg (30.9 excess deaths; table 2). Perinatal mortality still exhibited a strong curvilinear association with highest diastolic blood pressure even when we included women with proteinuria or chronic hypertension (fig 5); in linear quadratic models, the quadratic term was still highly statistically significantly different from zero. In this larger group, 98.7 perinatal deaths (11.2%) were attributable to mothers with blood pressure differing from the optimal blood pressure (80.4 mm Hg) predicted by the linear quadratic model; most (70.1%) of these excess perinatal deaths (69.2) occurred among mothers with lower blood pressures (see table 2).
Table 2.
Number of perinatal deaths, by blood pressure range, attributed to mother's highest blood pressure during antenatal check being more or less than optimal value predicted by linear quadratic model
| Highest blood pressure (mm Hg) | Overall No of deaths | No of attributable deaths* | No of women |
|---|---|---|---|
| Excluding women with chronic hypertension or proteinuria | |||
| <60 | 6 | 3.6 | 474 |
| 60-69 | 126 | 30.9 | 18 511 |
| 70-79 | 388 | 51.2 | 65 179 |
| 80-82.71 | 198 | 0.4 | 36 582 |
| 82.72-89 | 27 | 2.4 | 4365 |
| 90-99 | 52 | 0.7 | 9029 |
| 100-109 | 16 | −2.3 | 3164 |
| ≥110 | 11 | 7.4 | 621 |
| Total in relation to blood pressure (mm Hg): | |||
| <82.71 | 718 | 86.1 | 120 746 |
| ≥82.71 | 106 | 8.3 | 17 179 |
| Overall total | 824 | 94.4 | 137 925 |
| Including women with chronic hypertension or proteinuria | |||
| <60 | 6 | 3.5 | 477 |
| 60-69 | 128 | 29.1 | 18 651 |
| 70-79 | 392 | 41.0 | 65 980 |
| 80-80.37 | 204 | −4.4 | 37 394 |
| 80.38-89 | 31 | 2.9 | 4832 |
| 90-99 | 67 | 5.7 | 10 346 |
| 100-109 | 28 | 1.6 | 4378 |
| ≥110 | 27 | 19.3 | 1245 |
| Total in relation to blood pressure (mm Hg): | |||
| <80.37 | 730 | 69.2 | 122 502 |
| ≥80.37 | 153 | 29.5 | 20 801 |
| Overall total | 883 | 98.7 | 143 303 |
Attributable to highest blood pressure being different from optimum.
Discussion
During pregnancy, both low and high diastolic blood pressures in women are associated with small for gestational age babies and high perinatal mortality. Blood pressure in women without pre-existing hypertension or pre-eclampsia falls slightly during the first half of pregnancy, but then rises from around 34 weeks onwards, suggesting a physiological mechanism.31-34 Our analysis was based on a single blood pressure measurement for women at booking for antenatal care. The findings are similar to longitudinal observations on women followed through pregnancy, but with smaller datasets.35 We adjusted for multiple potential confounders since booking late for antenatal care is associated with adverse socioeconomic factors that might influence blood pressure.
From 34 weeks' gestation onwards, birth weight was maximal when the highest recorded blood pressure during pregnancy was between 70 and 90 mm Hg diastolic. The relation was an inverted U shape, so that both higher and lower blood pressures were associated with lower birth weights. The association of blood pressure with birth weight remained, even allowing for the effects of gestational age and maternal height and weight (some of the association of body mass with birth weight might itself be mediated through blood pressure).
A positive quadratic (U shaped) association was also seen with perinatal mortality, largely mediated through the association of blood pressure with birth weight. The size of our database confers high statistical power. We included over 1300 perinatal deaths, which is five and 14 times larger than the two largest previous studies.13,36 The first of these studies found a threefold increased risk of perinatal death with low blood pressure and a fourfold increased risk with non-proteinuric hypertension, whereas the second study found an increase only with hypertension.
Our findings support two previous small studies of women with low blood pressure in pregnancy that found lower birth weights and an increase in preterm deliveries12 and small for gestational age babies.10 In another study, birth weight was related to low blood pressure as well as high blood pressure in the third trimester.13 In a controlled trial to prevent pre-eclampsia, atenolol produced a significant reduction in the incidence of preeclampsia but also reduced mean birth weight by 440 g,37 which the authors attributed to the drug's lowering effect on blood pressure; in a meta-analysis of antihypertensives in pregnancy, a mean diastolic blood pressure lower by 10 mm Hg or more was associated with an increased risk of small for gestational age babies.38
The effect of a rise in blood pressure during pregnancy depends on the level at booking for antenatal care. A high blood pressure at booking is advantageous for birth weight as long as there is only a small rise (less than 15 mm Hg) in blood pressure thereafter. If the blood pressure at booking is low (70 mm Hg or less), however, a rise of 15-30 mm Hg is beneficial. This supports the concept that the rise in blood pressure in the third trimester is advantageous for fetal growth and may be a response to placental function failing to keep pace with fetal growth.
Strengths of study
The strengths of our study include the large numbers of women studied and their geographical coherence (only two maternity units in the region chose not to contribute data). A potential weakness is that the data were collected as part of routine obstetric practice, with the variability that implies. Women with high blood pressure in pregnancy are more likely to have received medical or obstetric interventions such as early induction of labour and caesarean section, thereby reducing the incidence of high blood pressure and increasing the risk of low birthweight babies. This would increase the incidence of low birthweight babies at the higher end of the blood pressure distribution, thus overestimating the effect of high blood pressure on low birth weight. Separate analysis of data from women with spontaneous onset of labour at term, however, gave essentially unchanged results. Women with low blood pressure were more likely to have booked late for antenatal care and therefore to have fewer hospital antenatal checks, but the differences were slight. The small decrease in mean diastolic blood pressure by calendar year, suggesting a shift in measurement from the Korotkoff phase IV to phase V heart sound, was corrected for in the regression models by adjusting for calendar year. The size of this decrease was small.
What is already known on this topic
The measurement of maternal blood pressure is a key part of antenatal care
Hypertension is associated with low birth weight and increased perinatal mortality, but data on the effects on the fetus of low maternal blood pressure are scarce
What this study adds
Birth weight is maximal and perinatal mortality lowest when the highest maternal blood pressure during pregnancy is between 70 and 90 mm Hg
Low as well as high blood pressures are associated with low birth weight and increased perinatal mortality
We thank the midwives who entered the data and the supportive clinicians and data guardians at the participating maternity units.
Contributors: PJS conceived this study, participated in the data analysis, and wrote the first draft of the paper. He is also the guarantor. MPL refined the hypotheses, directed and carried out most of the data analysis, contributed to the writing of the paper, and took the lead in writing the sections on data analysis. TK-J participated in the formulation of the hypotheses and writing of the paper. JC was responsible for setting up the database and advising about its use and was involved in correcting drafts of the paper. PE advised on the analysis, contributed knowledge about the effects of blood pressure, and participated in the writing of the paper.
Funding: None.
Competing interests: None declared.
Ethical approval: This study was approved by the St Mary's NHS Trust local research ethics committee.
References
- 1.American College of Obstetricians and Gynecologists. Hypertension in pregnancy. ACOG, 1996.
- 2.Anon. Report on confidential enquiries into maternal deaths in the United Kingdom 1994-1996. London: Stationery Office, 1998.
- 3.Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2004;360: 1903-13. [DOI] [PubMed] [Google Scholar]
- 4.Pilgrim JA, Stansfeld S, Marmot M. Low blood pressure, low mood? BMJ 1992;304: 75-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowe PC. Orthostatic intolerance and chronic fatigue syndrome: new light on an old problem. J Pediatr 2002;140: 387-9. [DOI] [PubMed] [Google Scholar]
- 6.Streeten DH, Thomas D, Bell DS. The roles of orthostatic hypotension, orthostatic tachycardia, and subnormal erythrocyte volume in the pathogenesis of the chronic fatigue syndrome. Am J Med Sci 2000;320: 1-8. [DOI] [PubMed] [Google Scholar]
- 7.Mathias CJ, Deguchi K, Schatz I. Observations on recurrent syncope and presyncope in 641 patients. Lancet 2001;357: 348-53. [DOI] [PubMed] [Google Scholar]
- 8.Owens PE, Lyons SP, O'Brien ET. Arterial hypotension: prevalence of low blood pressure in the general population using ambulatory blood pressure monitoring. J Hum Hypertens 2000;14: 243-7. [DOI] [PubMed] [Google Scholar]
- 9.Cogswell ME, Yip R. The influence of fetal and maternal factors on the distribution of birthweight. Sem Perinatol 1995;19: 222-40. [DOI] [PubMed] [Google Scholar]
- 10.Grunberger W, Leodolter S, Parschalk O. Maternal hypotension: fetal outcome in treated and untreated cases. Gynecologic Obstetric Invest 1979;10: 32-8. [DOI] [PubMed] [Google Scholar]
- 11.Margulies M, Voto LS, Fescina R, Lastra L, Lapidus AM, Schwarcz R. Arterial blood pressure standards during normal pregnancy and their relation with mother-fetus variables. Am J Obstet Gynecol 1987;156: 1105-9. [DOI] [PubMed] [Google Scholar]
- 12.Ng PH, Walters WA. The effects of chronic maternal hypotension during pregnancy. Aust NZ J Obstet Gynaecol 1992;32: 14-6. [DOI] [PubMed] [Google Scholar]
- 13.Friedman EA, Neff RK. Hypertension-hypotension in pregnancy. Correlation with fetal outcome. JAMA 1978;239: 2249-51. [PubMed] [Google Scholar]
- 14.Cleary R, Beard RW, Coles J, Devlin HB, Hopkins A, Roberts S, et al. The quality of routinely collected maternity data. Br J Obstet Gynaecol 1994;101: 1042-7. [DOI] [PubMed] [Google Scholar]
- 15.MacGillivray I, Rose GA, Rowe B. Blood pressure survey in pregnancy. Clin Sci 1969;37: 395-407. [PubMed] [Google Scholar]
- 16.Davey DA, MacGillivray I. The classification and definition of the hypertensive disorders of pregnancy. Am J Obstet Gynecol 1988;158: 892-8. [DOI] [PubMed] [Google Scholar]
- 17.De Swiet M. Blood pressure measurement in pregnancy. Br J Obstet Gynaecol 1991;98: 239-40. [DOI] [PubMed] [Google Scholar]
- 18.Perry IJ, Wilkinson LS, Shinton RA, Beevers DG. Conflicting views on the measurement of blood pressure in pregnancy. Br J Obstet Gynaecol 1991;98: 241-3. [DOI] [PubMed] [Google Scholar]
- 19.Johenning AR, Barron WM. Indirect blood pressure measurement in pregnancy: Korotkoff phase 4 versus phase 5. Am J Obstet Gynecol 1992;167: 577-80. [DOI] [PubMed] [Google Scholar]
- 20.De Swiet M, Shennan A. Blood pressure measurement in pregnancy. Br J Obstet Gynaecol 1996;103: 862-3. [DOI] [PubMed] [Google Scholar]
- 21.Shennan A, Gupta M, Halligan A, Taylor DJ, de Swiet M. Lack of reproducibility in pregnancy of Korotkoff phase IV as measured by mercury sphygmomanometry. Lancet 1996;347: 139-42. [DOI] [PubMed] [Google Scholar]
- 22.Lenfant C. Working group report on high blood pressure in pregnancy. J Clin Hypertens (Greenwich) 2001;3: 75-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steer PJ. The definition of pre-eclampsia. Br J Obstet Gynaecol 1999;106: 753-5. [DOI] [PubMed] [Google Scholar]
- 24.Redman CW, Jefferies M. Revised definition of pre-eclampsia. Lancet 1988;1: 809-12. [DOI] [PubMed] [Google Scholar]
- 25.Biswas A, Choolani MA, Anandakumar C, Arulkumaran S. Ambulatory blood pressure monitoring in pregnancy induced hypertension. Acta Obstet Gynecol Scand 1997;76: 829-33. [DOI] [PubMed] [Google Scholar]
- 26.Helewa ME, Burrows RF, Smith J, Williams K, Brain P, Rabkin SW. Report of the Canadian Hypertension Society Consensus Conference: 1. Definitions, evaluation and classification of hypertensive disorders in pregnancy. CMAJ 1997;157: 715-25. [PMC free article] [PubMed] [Google Scholar]
- 27.CESDI. Confidential enquiry into stillbirths and deaths in infancy. London: Maternal and Child Health Consortium, 1998. (5th Annual Report.)
- 28.Carstairs V, Morris R. Deprivation and health in Scotland. Aberdeen: Aberdeen University Press, 1991.
- 29.Davison AC, Hinkley DV. Bootstrap methods and their application. Cambridge: Cambridge University Press, 1997.
- 30.McCullagh P, Nelder JA. Generalized linear models, second edition London: Chapman and Hall, 1989.
- 31.Reiss RE, O'Shaughnessy RW, Quilligan TJ, Zuspan FP. Retrospective comparison of blood pressure course during preeclamptic and matched control pregnancies. Am J Obstet Gynecol 1987;156: 894-8. [DOI] [PubMed] [Google Scholar]
- 32.Clapp JF, Seaward BL, Sleamaker RH, Hiser J. Maternal physiologic adaptations to early human pregnancy. Am J Obstet Gynecol 1988;159: 1456-60. [DOI] [PubMed] [Google Scholar]
- 33.Halligan A, O'Brien E, O'Malley K, Mee F, Atkins N, Conroy R, et al. Twenty-four-hour ambulatory blood pressure measurement in a primigravid population. J Hypertens 1993;11: 869-73. [DOI] [PubMed] [Google Scholar]
- 34.Brown MA, Robinson A, Bowyer L, Buddle ML, Martin A, Hargood JL, et al. Ambulatory blood pressure monitoring in pregnancy: what is normal? Am J Obstet Gynecol 1998;178: 836-42. [DOI] [PubMed] [Google Scholar]
- 35.Moutquin JM, Rainville C, Giroux L, Raynauld P, Amyot G, Bilodeau R, et al. A prospective study of blood pressure in pregnancy: prediction of preeclampsia. Am J Obstet Gynecol 1985;151: 191-6. [DOI] [PubMed] [Google Scholar]
- 36.Page EW, Christianson R. The impact of mean arterial pressure in the middle trimester upon the outcome of pregnancy. Am J Obstet Gynecol 1976;125: 740-6. [DOI] [PubMed] [Google Scholar]
- 37.Easterling TR, Brateng D, Schmucker B, Brown Z, Millard SP. Prevention of preeclampsia: a randomized trial of atenolol in hyperdynamic patients before onset of hypertension. Obstet Gynecol 1999;93: 725-33. [DOI] [PubMed] [Google Scholar]
- 38.Von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet 2000;355: 87-92. [DOI] [PubMed] [Google Scholar]