Abstract
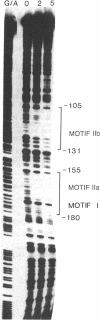
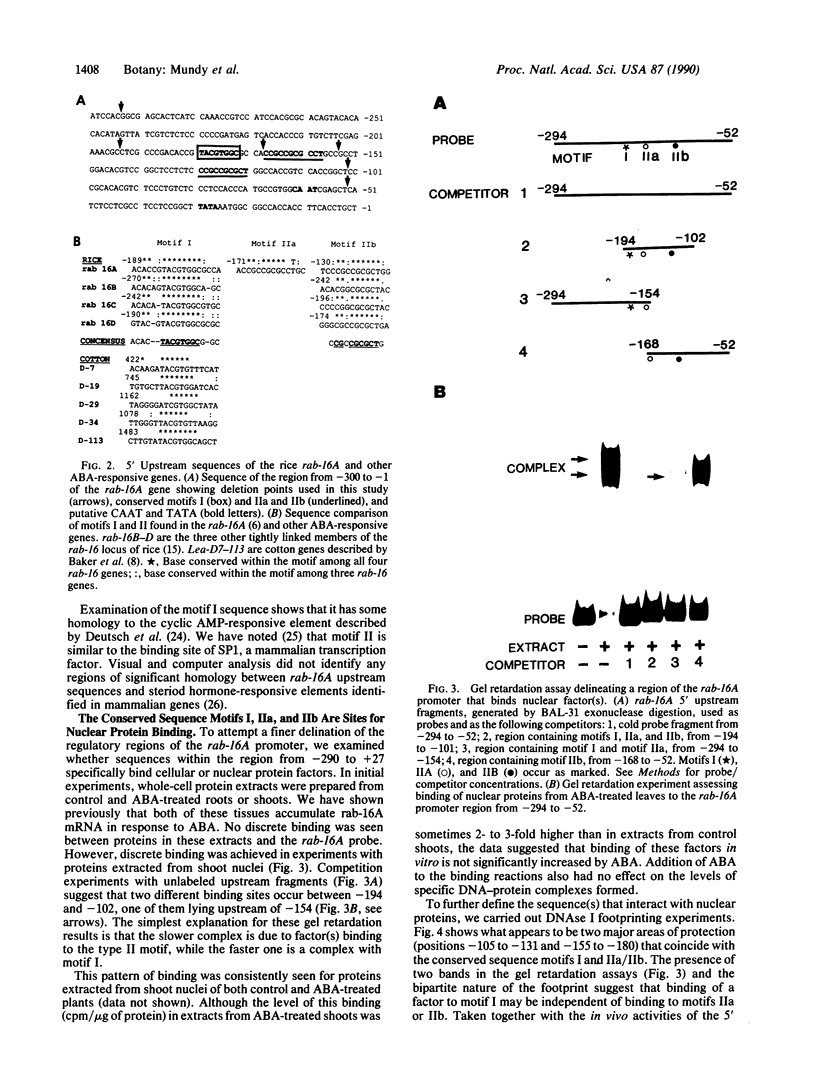
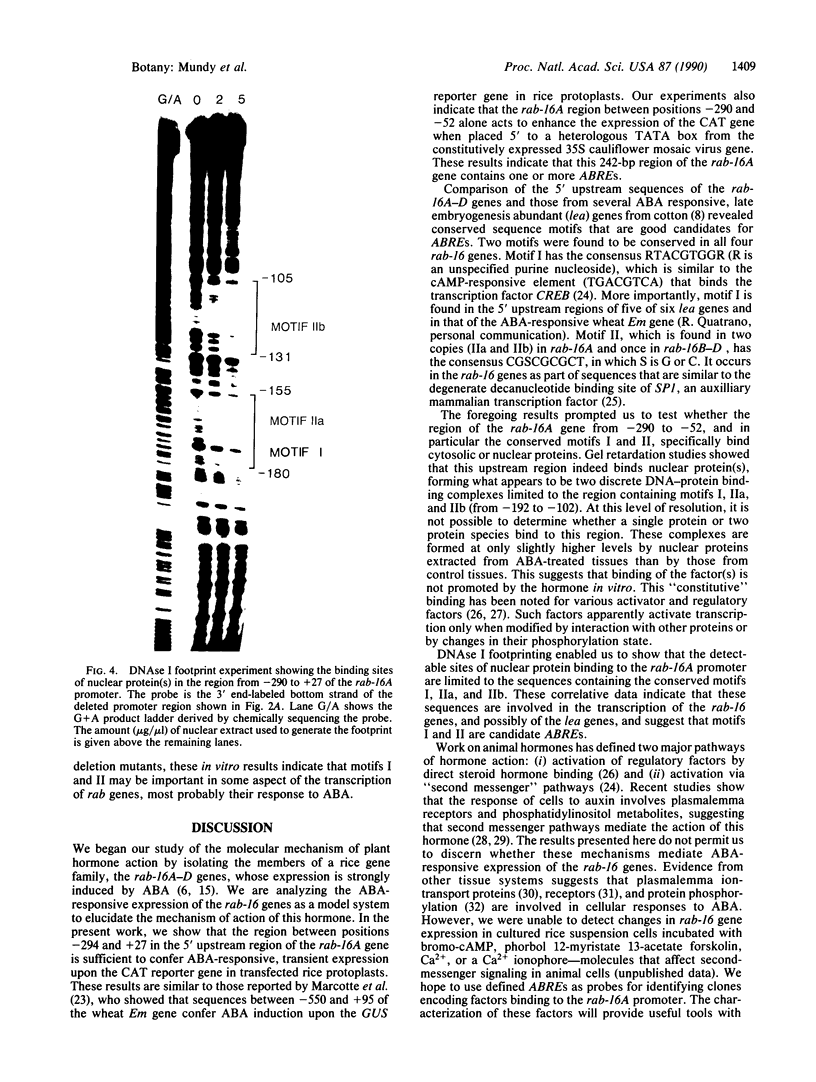
We have previously shown that the expression of a rice gene, rab-16A, is responsive to abscisic acid (ABA) and osmotic stress in plant tissues and cultured suspension cells. We demonstrate here that transcriptional elements between -294 and -52 of this gene are sufficient to confer ABA-dependent expression on the chloramphenicol acetyltransferase reporter gene in rice protoplasts. Sequence motifs within this 242-base-pair region of the rab-16A gene are conserved among the 5' upstream regions of other ABA-responsive genes. Gel retardation and DNAse I experiments show nuclear factor(s) binding to these sequences. This correlative data indicate that these motifs are involved in the transcription of the rab genes and suggest that they may be ABA-responsive-elements (ABREs).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Habener J. F. Cyclic AMP and phorbol ester-stimulated transcription mediated by similar DNA elements that bind distinct proteins. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7922–7926. doi: 10.1073/pnas.85.21.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettlinger C., Lehle L. Auxin induces rapid changes in phosphatidylinositol metabolites. Nature. 1988 Jan 14;331(6152):176–178. doi: 10.1038/331176a0. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. R., Tenbarge K. M., Shumway J. E., Crouch M. L. Role of ABA in Maturation of Rapeseed Embryos. Plant Physiol. 1985 Jul;78(3):630–636. doi: 10.1104/pp.78.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goday A., Sánchez-Martínez D., Gómez J., Puigdomènech P., Pagès M. Gene Expression in Developing Zea mays Embryos: Regulation by Abscisic Acid of a Highly Phosphorylated 23- to 25-kD Group of Proteins. Plant Physiol. 1988 Nov;88(3):564–569. doi: 10.1104/pp.88.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez J., Sánchez-Martínez D., Stiefel V., Rigau J., Puigdomènech P., Pagès M. A gene induced by the plant hormone abscisic acid in response to water stress encodes a glycine-rich protein. Nature. 1988 Jul 21;334(6179):262–264. doi: 10.1038/334262a0. [DOI] [PubMed] [Google Scholar]
- Hicks G. R., Rayle D. L., Jones A. M., Lomax T. L. Specific photoaffinity labeling of two plasma membrane polypeptides with an azido auxin. Proc Natl Acad Sci U S A. 1989 Jul;86:4948–4952. doi: 10.1073/pnas.86.13.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Koornneef M., Hanhart C. J., Hilhorst H. W., Karssen C. M. In Vivo Inhibition of Seed Development and Reserve Protein Accumulation in Recombinants of Abscisic Acid Biosynthesis and Responsiveness Mutants in Arabidopsis thaliana. Plant Physiol. 1989 Jun;90(2):463–469. doi: 10.1104/pp.90.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Benfey P. N., Gilmartin P. M., Fang R. X., Chua N. H. Site-specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7890–7894. doi: 10.1073/pnas.86.20.7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S. S., Poole R. J., Dhindsa R. S. Abscisic Acid-regulated gene expression in relation to freezing tolerance in alfalfa. Plant Physiol. 1988 Jun;87(2):468–473. doi: 10.1104/pp.87.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson E., Dreyfuss G. RNP in maize protein. Nature. 1989 Jan 26;337(6205):312–312. doi: 10.1038/337312b0. [DOI] [PubMed] [Google Scholar]
- Mundy J., Chua N. H. Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J. 1988 Aug;7(8):2279–2286. doi: 10.1002/j.1460-2075.1988.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy J., Hejgaard J., Hansen A., Hallgren L., Jorgensen K. G., Munck L. Differential synthesis in vitro of barley aleurone and starchy endosperm proteins. Plant Physiol. 1986 Jun;81(2):630–636. doi: 10.1104/pp.81.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Hedrich R. Involvement of ion channels and active transport in osmoregulation and signaling of higher plant cells. Trends Biochem Sci. 1989 May;14(5):187–192. doi: 10.1016/0968-0004(89)90272-7. [DOI] [PubMed] [Google Scholar]
- Singh N. K., Larosa P. C., Handa A. K., Hasegawa P. M., Bressan R. A. Hormonal regulation of protein synthesis associated with salt tolerance in plant cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):739–743. doi: 10.1073/pnas.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]