Abstract
Objective To estimate the analgesic efficacy of non-steroidal anti-inflammatory drugs (NSAIDs), including selective cyclo-oxygenase-2 inhibitors (coxibs), in patients with osteoarthritis of the knee.
Design Systematic review and meta-analysis of randomised placebo controlled trials.
Studies reviewed 23 trials including 10 845 patients, median age of 62.5 years. 7807 patients received adequate doses of NSAIDs and 3038 received placebo. The mean weighted baseline pain score was 64.2 mm on 100 mm visual analogue scale (VAS), and average duration of symptoms was 8.2 years.
Main outcome measure Change in overall intensity of pain.
Results Methodological quality of trials was acceptable, but 13 trials excluded patients before randomisation if they did not respond to NSAIDs. One trial provided long term data for pain that showed no significant effect of NSAIDs compared with placebo at one to four years. The pooled difference for pain on visual analogue scale in all included trials was 10.1 mm (95% confidence interval 7.4 to 12.8) or 15.6% better than placebo after 2-13 weeks. The results were heterogeneous, and the effect size for pain reduction was 0.32 (0.24 to 0.39) in a random effects model. In 10 trials that did not exclude non-responders to NSAID treatment the results were homogeneous, with an effect size for pain reduction of 0.23 (0.15 to 0.31).
Conclusion NSAIDs can reduce short term pain in osteoarthritis of the knee slightly better than placebo, but the current analysis does not support long term use of NSAIDs for this condition. As serious adverse effects are associated with oral NSAIDs, only limited use can be recommended.
Introduction
Osteoarthritis of the knee is the most common type of osteoarthritis,1 the prevalence of which is rising in parallel with the increasing age of the population.2 The condition is associated with pain and inflammation of the joint capsule,3-5 impaired muscular stability,6,7 reduced range of motion,8 and functional disability.9 Treatment guidelines for knee osteoarthritis recommend pharmacological intervention, initially with paracetamol and subsequently with a non-steroidal anti-inflammatory drug (NSAID).10 In a recent UK survey, 15% of patients with osteoarthritis used paracetamol, whereas 50% reported regular use of NSAIDs. Of the latter, 32% were using traditional NSAIDs and 18% were using cyclo-oxygenase-2 inhibitors (coxibs).11 This widespread use is one explanation for the interest in tolerability and efficacy issues regarding these drugs.10,12,13 The recent introduction of coxibs seemed to promise a reduction in serious adverse events related to NSAIDs,13,14 but this remains controversial.15-18
Guidelines from the European League Against Rheumatism (EULAR) state that both pharmacological and non-pharmacological interventions are needed for optimal treatment of knee osteoarthritis.19 The various potentially effective pharmacological interventions at the clinicians' disposal19 highlight the need for information regarding treatment efficacy.
Meta-analyses can be used for reliable comparison of the efficacy of different interventions.20 Effect size measures the magnitude of a treatment effect independent of sample size.21 There is no current operational definition for what constitutes a sufficiently large effect size for a therapeutic intervention to be considered as useful, but a value of 0.2 is usually considered small, 0.5 moderate, and 0.8 large.22
A recent systematic review of therapeutic alternatives in knee osteoarthritis gives no effect sizes for paracetamol and an imprecise range (0.47-0.96), derived from a minority of available trials, for NSAIDs.19 Neither other reviewers nor the Cochrane library provide comprehensive and robust effect size data for the efficacy of either of these interventions in osteoarthritis of the knee.10,13,23-25 Calculations of effect size require data for mean change and standard deviation (SD). If not provided, these data can be obtained by indirect means from standard errors, P values, t values, and 95% confidence intervals when sample sizes are known. The lack of data on effect size is surprising because treatment with NSAIDs for knee osteoarthritis is established to the point of being a reference against which other interventions are often compared.
We carried out a meta-analysis of published randomised placebo controlled trials to estimate the analgesic efficacy of NSAIDs, including coxibs, in patients with knee osteoarthritis.
Methods
Protocol specification
We specified a detailed review of protocol before analysis. This included a sequential three step reviewing procedure of identifying relevant randomised placebo controlled trials from Medline, Embase, and the Cochrane central register of controlled trials; evaluating their methodological quality according to predefined criteria (Jadad scale)26; and calculating their pooled effect as the mean difference in change between NSAID groups and placebo groups in mm on a visual analogue scale and as a unitless effect size.
Literature search
We carried out the literature search from 1966 to April 2004. In addition, we crosschecked reference lists in systematic reviews, searched conference abstracts, and talked to clinical experts. We included papers in English, German, and Scandinavian. Our key search terms were knee, osteoarthritis, randomised, controlled, placebo, NSAID, coxib, cox-2 inhibitor.
Inclusion criteria
Trials had to study patients whose knee osteoarthritis had been verified by clinical examination according to the American College of Rheumatology criteria and by x ray. The symptoms had to have been present for more than three months. All trials had to be randomised, blinded, placebo controlled, and of parallel design. Pain intensity had to be scored on the subscale of pain on Western Ontario and McMaster Universities osteoarthritis index (WOMAC)27 or on a 100 mm visual analogue scale for one or the mean score of two or more pain dimensions. Functional disability had to be measured on the WOMAC subscale for function.
The intervention groups had to have received matched placebo drug or adequate NSAID dose (except indomethacin)—that is, daily drug dose equal to or exceeding celecoxib 200 mg, diclofenac 100 mg, etodolac 400 mg, etoricoxib 30 mg, ibuprofen 2400 mg, meloxicam 7.5 mg, nabumetone 1500 mg, naproxen 1000 mg, oxaprozin 1200 mg, rofecoxib 12.5 mg, tiaprofenic acid 600 mg, or valdecoxib 10 mg.
Extraction of outcome measure
We used the change in overall pain intensity between the NSAID group and placebo to assess differences. Data were primarily obtained as a mean of the five items on the pain subscale of WOMAC. If WOMAC data were registered on non-continuous scales (categorical, Likert) we converted them to 100 mm visual analogue scales and checked them against other subscales and overall WOMAC score, as this has been found to have good internal consistency.28 If WOMAC data were not available, we used the mean score of knee pain on 100 mm visual analogue scales. If none of the above data were available and more than one type of pain was measured (for instance, pain at rest, pain during walking, etc) we used the mean of these scores.
Statistical analysis of pain relief
We included mean differences of change for intervention groups and placebo groups and their respective standard deviations (SD) in a statistical pooling. If variance data were not reported as SDs, we calculated them from the trial data of sample size and other variance data such as P values, t values, SE of mean, or 95% confidence intervals. Results were presented as weighted mean differences between NSAID and placebo with 95% confidence intervals in mm on visual analogue scales—that is, as a pooled estimate of the mean difference in change between the treatment and the placebo groups, weighted by the inverse of the variance for each study.29 We also combined unitless effect sizes—that is, the standardised mean difference in change between NSAIDs and placebo groups for all included trials weighted by the inverse of the variance for each study.19 A statistical software package (Comprehensive Meta-Analysis, ver.1.0.23, Biostat, Englewood, USA) was used for calculations. We computed homogeneity statistics to test the agreement of the individual trial results with the overall meta-analytical summary. If we detected significant heterogeneity (P < 0.1) we calculated random effects estimates.
Appraisal of trial quality
We assessed the quality of the trials according to a predefined list of criteria.26 To assess the potential for bias we evaluated the method of randomisation, concealment of allocation, blinding of trial investigators and patients, handling of dropouts and withdrawals, and analysis according to intention to treat. In addition, we counted selection criteria for patients and evaluated them for possible bias or dissimilarity to an average population with knee osteoarthritis. We did not predefine cut off limits for method scores.
Results
Included studies
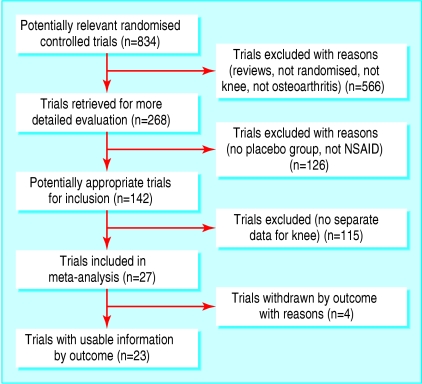
We evaluated 268 randomised controlled trials of NSAIDs for knee osteoarthritis (fig 1). Of these, 4 did not provide pain scale data, 126 did not have a placebo control group, and 115 presented combined data for osteoarthritis in several sites with no separate data for the knee. This provided a final sample of 23 trials that satisfied the inclusion criteria.30-52 Of these, 16 were sponsored by the pharmaceutical industry,31-34,36,39,40,43,44,46-52 while three others did not state sponsorship but gave an address of a pharmaceutical company as the workplace of most of the authors.37,38,41 The final sample included 10 845 patients, of whom 7767 received NSAIDs and 3078 received placebo (table).
Fig 1.
Selection of trials for inclusion in meta-analysis
Table 1.
Characteristics of trials of NSAIDs for pain relief in patients with knee osteoarthritis
| Drug | No of patients (n=7767) | Method quality | Trial exclusion criteria | Mean baseline pain (mm VAS) | Mean difference (95% CI) of change over placebo (mm VAS) | No (%) of adverse events (n=687, 9.2%) | |
|---|---|---|---|---|---|---|---|
| Bensen39 | Celecoxib-naproxen | 597 | 5 | 9 | 54.1 | 8.0 (2.3 to 13.7) | 43 (5.4) |
| Case35 | Diclofenac | 25 | 4 | 14 | 39.6 | 11.7 (6.2 to 17.2) | 3 (12) |
| Dore37 | Naproxen-etodolac | 168 | 3 | 12 | NA | 16.3 (4.8 to 27.7) | 15 (8.9) |
| Ehrich46 | Rofecoxib | 147 | 5 | 10 | 61.9 | 21.9 (15 to 28.7) | 26 (17.6) |
| Fleischmann32 | Nabumetone-Naprelan | 185 | 3 | 14 | 59.9 | 1.3 (−7.0 to 10.5) | 10 (5.4) |
| Gibofsky33 | Celecoxib-rofecoxib | 379 | 5 | 14 | 67.7 | 11.6 (3.4 to 19.8) | 21 (5.5) |
| Gottesdiener34 | Eterocoxib | 326 | 5 | 21 | 68.4 | 18.4 (16.6 to 20.2) | 17(5.2) |
| Kivitz36 | Valdecoxib-naproxen | 408 | 5 | 18 | 71.9 | 5.5 (2.3 to 8.8) | 77 (10.5) |
| Kivitz47 | Rofecoxib-nabumetone | 834 | 5 | 13 | 74.5 | 15.1 (4.9 to 25.3) | 49 (5.8) |
| Lee49 | Diflunisal | 279 | 3 | 4 | 57 | 8.5 (−2.9 to 19.5) | 33 (11.8) |
| Lund41 | Meloxicam | 134 | 3 | Not stated | 48.2 | 6.6 (1.4 to 11.8) | 16 (5.8) |
| McKenna40 | Celecoxib-diclofenac | 400 | 3 | Not stated | 69.1 | 8.8 (5.2 to 12.3) | 36 (18) |
| McKenna31 | Celecoxib-rofecoxib | 122 | 3 | 15 | 73.3 | 14.5 (2.7 to 26.3) | 8 (6.6) |
| Schnitzer38 | Nabumetone-etodolac | 180 | 3 | 19 | 57.5 | 13.2 (5.4 to 21) | 18 (10) |
| Scott42 | Tiaprofenic acid | 307 | 4 | Not stated | 55.1 | 4.1 (4.0 to 4.2) | 61 (12) |
| Makarowski52 | Nabumetone-oxaprozin | 231 | 3 | 17 | NA | NA | 20 (8.5) |
| Simon50 | Celecoxib | 222 | 4 | 8 | 67.8 | 6.0 (−1.1 to 12.1) | 6 (3.0) |
| Tannenbaum48 | Lumiracoxib-celecoxib | 1459 | 4 | Not stated | 65.2 | 8.0 (2.7 to 13.3) | 132 (9.1) |
| Uzun30 | Flurbiprofen-tiaprofenic acid | 26 | 3 | 6 | 61 | 17.0 (3.9 to 37.9) | 0 |
| Weaver51 | Nabumetone-oxaprozin | 219 | 3 | 15 | NA | 6 (−0.1 to 12.1) | 11 (5.0) |
| Williams44 | Celecoxib | 472 | 4 | 15 | 66.4 | 7.5 (2.9 to 12.1) | 15 (3.5) |
| Williams45 | Etodolac | 50 | 3 | 10 | 76 | 7.3 (0 to 14.6) | NA |
| Zhao43 | Celecoxib | 597 | 5 | 9 | 53.9 | 7.5 (4.8 to 10.2) | 70 (10.7) |
| Overall | 3.8* | 14† | 64.1‡ | 10.1‡ (7.4 to 12.8) | 687 (9.2) |
NA=not available.
Mean.
Median.
Weighted mean.
Patients and possible selection bias
The included patients had a median age of 62.5 years, and three trials had an upper age limit of 75 years.35,45,49 There were more women (67.9%) than men, and the median duration of symptoms was 8.2 years. The weighted mean baseline pain of 64.1 mm on a visual analogue scale was calculated from all but three trials.37,51,52 Six reports provided data of body mass index with a median mean value of 31.2.32,35,38,43,44,48 Most trials excluded individuals with concomitant health disorders by a median of 14 exclusion criteria. All trials had a minimum limit for pain intensity or disease activity for inclusion, and they all used a pretreatment washout period of 3-14 days for previous pharmacotherapy. Thirteen trials used an additional criterion by requiring a predefined minimum flare of symptoms when NSAID treatment was discontinued in the pretreatment wash out period.31,33-35,39,40,43,44,46,47,50-52 Five of these trials reported the proportion of regular NSAIDs users, ranging from 66% to 100% (median 90.5%).31,34,35,40,47
Trial quality
The methodological quality was adequate or good (table). All trials were randomised and double blinded, but adequate randomisation procedure, concealed allocation to groups, and blinding procedure were described satisfactorily in only eight studies.33,34,36,39,43,44,46,47 All trials described dropouts and withdrawals well, but one trial did not perform intention to treat analyses.45
Presentation of data on outcome measures
Only four trials presented outcome data as the mean difference of change with SD between NSAIDs and placebo groups.34,42,46,48 Fourteen trials presented the mean difference of change for each group with P values, SE of mean, or 95% confidence intervals but not mean differences between groups.31,33,35-39,41,43,47,49-52 Four trials did not present mean change data but only before and after means and P values.30,32,44,45 Eleven trials presented data on overall pain on 100 mm visual analogue scales or on a categorical five point scale,30-32,37,38,41,42,47,50-52 while the 12 remaining trials presented either categorical or continuous data from the WOMAC subscale for pain. All 23 trials reported data on pain intensity, and 11 trials reported data on functional disability.
Effect size for reduction in functional disability and pain
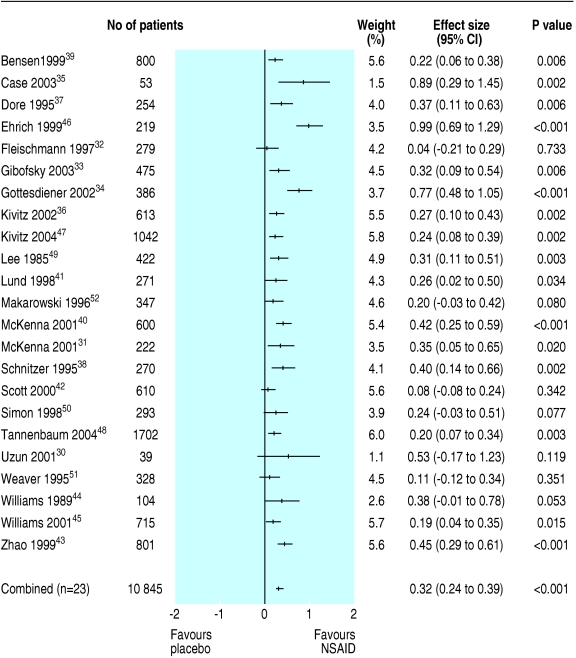
We excluded from analysis six intervention groups (n = 867) in five trials because patients did not receive an adequate NSAID dose.34-36,39,43 As most trials with multiple time points showed rather stable results from 2-13 weeks, we pooled data. Tests for heterogeneity showed positive results for reduction in functional disability (Q = 39.9, P < 0.01) and reduction in pain (Q = 56.6, P < 0.01). For this reason, we decided to use a random effects model. Eleven trials with 7433 patients provided separate scores for reduction in functional disability,33,35,36,39-41,43,44,46-48 and their combined effect size was 0.29 (95% confidence interval 0.18 to 0.40). One trial reported long term effects on pain but found no significant difference between tiaprofenic acid and placebo at one, two, three, and four years after start of treatment.42 For short term effects (2-13 weeks) the pooled effect size of all included trials was 10.1 mm on visual analogue scale (7.4 to 12.8) or 15.6% better than placebo, and the effect size was 0.32 (0.24 to 0.39) (fig 2).
Fig 2.
Effect size (pain) for all trials on NSAID use in knee osteoarthritis
Subgroup analyses
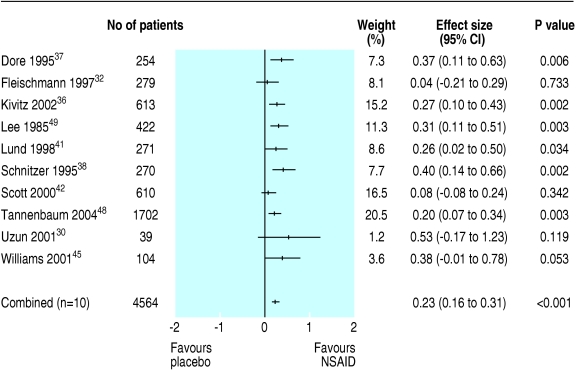
We carried out post hoc subgroup analyses as heterogeneity was evident and trial procedures differed in selection of patients, duration of treatment, and pain scales. For subgroups of trials that used short durations of treatment (< 6 weeks) or WOMAC subscale for pain, neither effect size (0.35 or 0.39) nor heterogeneity (Q = 48.3, P < 0.01, or Q = 43.0, P < 0.01) changed significantly from the results of the total material. For the subgroup of 10 trials (n = 4565) that did not require patients to have a minimum flare of symptoms after treatment with NSAIDs was stopped before the trial, trial results were homogeneous both for function (Q = 2.6, P = 0.275) and pain (Q = 10.0, P = 0.263).30,32,36-38,41,42,45,48,49 Three of these trials (n = 2928) provided data for reduction in functional disability36,41,48 and we calculated effect size by a fixed effects model to be 0.20 (0.09 to 0.30). For pain reduction, we a used fixed effect model to calculate a pooled effect size of 0.23 (0.16 to 0.31) or 5.9 mm on visual analogue scale (3.8 to 7.9) (fig 3).
Fig 3.
Effect size (pain) for trials on NSAID use in knee osteoarthritis without identified selection bias
Discussion
NSAIDs can reduce short term pain in osteoarthritis of the knee slightly better than placebo, but the current analysis does not support the long term use of these drugs. Several NSAIDs, of which rofecoxib is the most recent example, have been withdrawn because of adverse effects.53 We initially included rofecoxib in our investigation, but did not include it in the final subgroup analysis. As use of oral NSAIDs may incur serious adverse effects, they can only be recommended for limited use in osteoarthritis of the knee. Although NSAIDs have been used for nearly three decades, most trials included in this review were from the past decade. This is mainly due to the inclusion in older studies of patients with multiple joint osteoarthritis and the lack of separate data for osteoarthritis of the knee.
Strengths and weaknesses of analysis
The strengths of the present study include the acceptable methodological quality of the separate trials on which the analysis is based, as well as the considerable number of trials and patients included. We also translated categorical WOMAC data and P values, t test results, standard error of mean values, and before and after values to mean differences in change between groups to avoid bias. For instance, we excluded all groups with less than adequate NSAID doses from the efficacy calculations.
One possible limitation of our study is that we included nine trials in which outcomes were recorded with fewer than the five pain dimensions covered by the WOMAC pain subscale.31,32,37,38,41,42,45,47,49 We thought that this, as well as the different time points in the individual studies (2, 3, 4, 6, 12, and 13 weeks) for registering outcome measures, could explain the heterogeneity that was found in the trial results, but heterogeneity persisted after we performed relevant subgroup analyses. Heterogeneity was not apparent, however, when we performed a subgroup analysis of trials that did not exclude non-responders to NSAIDs. The validity of requiring a certain increase in symptoms after discontinuation of NSAIDs before inclusion in an NSAID trial seems dubious. Indeed, our results show that this procedure significantly inflates effect sizes in favour of the trial drug. In a clinical setting, it may nevertheless be useful to have information about the magnitude of effect to be expected in patients who are known responders to NSAIDs. In comparisons of various treatments, however, the selective exclusion of people who do not respond to NSAIDs among patients given this type of therapy will introduce bias in favour of NSAID efficacy and may hence be inappropriate.
Another possible source of selection bias in patients is that the average age of the participants was low (62.5 years) for people with osteoarthritis of the knee, reflecting the exclusion of patients above 75 years of age in some trials.35,45,49 Data on efficacy and tolerability as a function of age were reported in only one comparatively small trial.45
Benefits of NSAIDs
NSAIDs significantly reduce pain in acute conditions.54,55 In chronic conditions, however, patients have reported that pain has to be reduced by about 30% to be considered meaningful.56 For knee osteoarthritis in particular, an effect size of 0.4 or 17-22% change from baseline has been calculated from empirical data and suggested as the minimal clinically important change.57 Other authors have found that the least perceptible change in pain from osteoarthritis of the knee is 9.7 mm measured by the WOMAC subscale for pain.58 In accordance with this, the effect size of 0.32 or 10.1 mm on visual analogue scale for pain reduction and the effect size of 0.29 for disability reduction may be considered too small to be clinically significant. This may in turn explain non-compliance with prescribed drug therapy in 29% of patients and the use of non-conventional drug therapy by one in four patients with osteoarthritis.11
The widespread and long term use of NSAIDs among elderly people with osteoarthritis is associated with considerable side effects. NSAIDs cause serious gastrointestinal complications such as bleeding or perforation in one in 50-100 patient years, and this risk increases with age, concurrent use of other medications, and probably also duration of treatment.12 Substantial epidemiological and experimental data show that NSAIDs may increase blood pressure,59 and NSAID use has been linked to the development and acceleration of congestive heart failure.60 Elderly patients also have an increased risk for development of associated renal failure.61 In addition, NSAID users are at risk of interactions, including pharmacodynamic interactions with anti-hypertensive drugs59 and pharmacokinetic interactions with compounds eliminated by renal excretion, such as lithium.62 These important caveats were not considered in the short term studies of NSAIDs that we included. Thus, it may be reasonable to assume that the benefits of NSAIDs may be less and the harmful effects more common in an unselected population of patients with knee osteoarthritis compared with the patients in these studies.
What is already known on this topic
Current guidelines recommend the use of oral non-steroidal anti-inflammatory drugs (NSAIDs) in the treatment of osteoarthritis of the knee
Oral NSAIDs are used regularly by half of all patients with painful osteoarthritis
What this study adds
The advantage of oral NSAIDs over placebo for short term pain relief is small and probably clinically insignificant
Evidence of long term effects from oral NSAIDs is still lacking
Supplementary Material
Contributors: JMB had the original idea and designed the review together with LS. JMB and AK performed the literature search. AEL and JMB assessed methodological trial quality. JMB, LS, and AK analysed the statistical data. AEL, JMB, and LS wrote the report, while AK contributed in layout and proof reading. JMB is the guarantor.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Andrianakos A, Trontzas P, Christoyannis F, Dantis P, Voudouris C, Georgountzos A, et al. Prevalence of rheumatic diseases in Greece: a cross-sectional population based epidemiological study. The ESORDIG study. J Rheumatol 2003;30: 1589-601. [PubMed] [Google Scholar]
- 2.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med 2000;133: 635-46. [DOI] [PubMed] [Google Scholar]
- 3.Vaatainen U, Lohmander LS, Thonar E, Hongisto T, Agren U, Ronkko S, et al. Markers of cartilage and synovial metabolism in joint fluid and serum of patients with chondromalacia of the patella. Osteoarthr Cartil 1998;6: 115-24. [DOI] [PubMed] [Google Scholar]
- 4.Suenaga S, Abeyama K, Indo H, Shigeta K, Noikura T. Temporomandibular disorders: MR assessment of inflammatory changes in the posterior disk attachment during the menstrual cycle. J Comput Assist Tomogr 2001;25: 476-81. [DOI] [PubMed] [Google Scholar]
- 5.Speldewinde GC, Bashford GM, Davidson IR. Diagnostic cervical zygapophyseal joint blocks for chronic cervical pain. Med J Aust 2001;174: 174-6. [DOI] [PubMed] [Google Scholar]
- 6.Radebold A, Cholewicki J, Polzhofer GK, Greene HS. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine 2001;26: 724-30. [DOI] [PubMed] [Google Scholar]
- 7.Cowan SM, Bennell KL, Hodges PW, Crossley KM, McConnell J. Delayed onset of electromyographic activity of vastus medialis obliquus relative to vastus lateralis in subjects with patellofemoral pain syndrome. Arch Phys Med Rehabil 2001;82: 183-9. [DOI] [PubMed] [Google Scholar]
- 8.Steultjens MP, Dekker J, van Baar ME, Oostendorp RA, Bijlsma JW. Range of joint motion and disability in patients with osteoarthritis of the knee or hip. Rheumatology (Oxford) 2000;39: 955-61. [DOI] [PubMed] [Google Scholar]
- 9.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the American Rheumatism Association. Arthritis Rheum 1986;29: 1039-49. [DOI] [PubMed] [Google Scholar]
- 10.Wegman A, van der Windt D, van Tulder M, Stalman W, de Vries T. Nonsteroidal anti-inflammatory drugs or acetaminophen for osteoarthritis of the hip or knee? A systematic review of evidence and guidelines. J Rheumatol 2004;31: 344-54. [PubMed] [Google Scholar]
- 11.OA Nation survey. http://oanation.arthritiscare.org.ukfileadmin/oanation/downloads/OA_Nation_report.pdf (accessed 10 Sept 2004).
- 12.Tramer MR, Moore RA, Reynolds DJ, McQuay HJ. Quantitative estimation of rare adverse events which follow a biological progression: a new model applied to chronic NSAID use. Pain 2000;85: 169-82. [DOI] [PubMed] [Google Scholar]
- 13.Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ 2002;325: 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zabinski RA, Burke TA, Johnson J, Lavoie F, Fitzsimon C, Tretiak R, et al. An economic model for determining the costs and consequences of using various treatment alternatives for the management of arthritis in Canada. Pharmacoeconomics 2001;19(suppl 1): 49-58. [DOI] [PubMed] [Google Scholar]
- 15.Juni P, Rutjes AW, Dieppe PA. Are selective COX 2 inhibitors superior to traditional non-steroidal anti-inflammatory drugs? BMJ 2002;324: 1287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerezo J, Hristov RL, Sansuan AJ, Rodriguez JJV. Outcome trials of COX-2 selective inhibitors: global safety evaluation does not promise benefits. Eur J Clin Pharmacol 2003;59: 169-75. [DOI] [PubMed] [Google Scholar]
- 17.Wright JM. The double-edged sword of COX-2 selective NSAIDs. CMAJ 2002;167: 1131-7. [PMC free article] [PubMed] [Google Scholar]
- 18.Sturkenboom MC, Romano F, Simon G, Correa-Leite ML, Villa M, Nicolosi A, et al. The iatrogenic costs of NSAID therapy: a population study. Arthritis Rheum 2002;47: 132-40. [DOI] [PubMed] [Google Scholar]
- 19.Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis 2003;62: 1145-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 2003;326: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care 1989;27:suppl: S178-89. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. Statistical power analysis for behavioural sciences. New York: Academic Press, 1977.
- 23.Eccles M, Freemantle N, Mason J. North of England evidence based guideline development project: summary guideline for non-steroidal anti-inflammatory drugs versus basic analgesia in treating the pain of degenerative arthritis. The north of England non-steroidal anti-inflammatory drug guideline development group. BMJ 1998;317: 526-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson MC, Brookes ST, Kirwan JR, Faulkner A Non-aspirin, non-steroidal anti-inflammatory drugs for treating osteoarthritis of the knee. Cochrane Database Syst Rev 2004;(3): CD000142. [DOI] [PMC free article] [PubMed]
- 25.Zhang W, Jones A, Doherty M. Does paracetamol (acetaminophen) reduce the pain of osteoarthritis? A meta-analysis of randomised controlled trials. Ann Rheum Dis 2004;63: 901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jadad AR, McQuay HJ. Meta-analyses to evaluate analgesic interventions: a systematic qualitative review of their methodology. J Clin Epidemiol 1996;49: 235-43. [DOI] [PubMed] [Google Scholar]
- 27.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of hip or knee. J Rheumatol 1988;15: 1833-40. [PubMed] [Google Scholar]
- 28.Roos EM, Klassbo M, Lohmander LS. WOMAC osteoarthritis index. Reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis. Western Ontario and MacMaster Universities. Scand J Rheumatol 1999;28: 210-5. [DOI] [PubMed] [Google Scholar]
- 29.Fleiss J. The statistical basis of meta-analysis. Stat Methods Med Res 1993;2: 121-45. [DOI] [PubMed] [Google Scholar]
- 30.Uzun H, Tuzun S, Ozaras N, Aydin S, Ozaras R, Dondurmaci S, et al. The effect of flurbiprofen and tiaprofenic acid on serum cytokine levels of patients with osteoarthrosis. Acta Orthop Scand 2001;72: 499-502. [DOI] [PubMed] [Google Scholar]
- 31.McKenna F, Weaver A, Fiechtner JJ, Bello AE, Fort J. COX-2 Specific inhibitors in the management of osteoarthritis of the knee: A placebo-controlled, randomized double-blind study. J Clin Rheumatol 2001;7: 151-9. [DOI] [PubMed] [Google Scholar]
- 32.Fleischmann RM, Flint K, Constantine G, Kolecki B. A double-masked comparison of Naprelan and nabumetone in osteoarthritis of the knee. Naprelan study group. Clin Ther 1997;19: 642-55. [DOI] [PubMed] [Google Scholar]
- 33.Gibofsky A, Williams GW, McKenna F, Fort JG. Comparing the efficacy of cyclooxygenase 2-specific inhibitors in treating osteoarthritis: appropriate trial design considerations and results of a randomized, placebo-controlled trial. Arthritis Rheum 2003;48: 3102-11. [DOI] [PubMed] [Google Scholar]
- 34.Gottesdiener K, Schnitzer T, Fisher C, Bockow B, Markenson J, Ko A, et al. Results of a randomized, dose-ranging trial of etoricoxib in patients with osteoarthritis. Rheumatology (Oxford) 2002;41: 1052-61. [DOI] [PubMed] [Google Scholar]
- 35.Case JP, Baliunas AJ, Block JA. Lack of efficacy of acetaminophen in treating symptomatic knee osteoarthritis: a randomized, double-blind, placebo-controlled comparison trial with diclofenac sodium. Arch Intern Med 2003;163: 169-78. [DOI] [PubMed] [Google Scholar]
- 36.Kivitz A, Eisen G, Zhao WW, Bevirt T, Recker DP. Randomized placebo-controlled trial comparing efficacy and safety of valdecoxib with naproxen in patients with osteoarthritis. J Fam Pract 2002;51: 530-7. [PubMed] [Google Scholar]
- 37.Dore R, Ballard I, Constantine G, McDonald P. Efficacy and safety of etodolac and naproxen in patients with osteoarthritis of the knee: a double-blind, placebo-controlled study. Clin Ther 1995;17: 656-66. [DOI] [PubMed] [Google Scholar]
- 38.Schnitzer TJ, Ballard IM, Constantine G, McDonald P. Double-blind, placebo-controlled comparison of the safety and efficacy of orally administered etodolac and nabumetone in patients with active osteoarthritis of the knee. Clin Ther 1995;17: 602-12. [DOI] [PubMed] [Google Scholar]
- 39.Bensen WG, Fiechtner JJ, McMillen JI, Zhao WW, Yu SS, Woods EM, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc 1999;74: 1095-105. [DOI] [PubMed] [Google Scholar]
- 40.McKenna F, Borenstein D, Wendt H, Wallemark C, Lefkowith JB, Geis GS. Celecoxib versus diclofenac in the management of osteoarthritis of the knee. Scand J Rheumatol 2001;30: 11-8. [DOI] [PubMed] [Google Scholar]
- 41.Lund B, Distel M, Bluhmki E. A double-blind, randomized, placebo-controlled study of efficacy and tolerance of meloxicam treatment in patients with osteoarthritis of the knee. Scand J Rheumatol 1998;27: 32-7. [DOI] [PubMed] [Google Scholar]
- 42.Scott DL, Berry H, Capell H, Coppock J, Daymond T, Doyle DV, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology (Oxford) 2000;39: 1095-101. [DOI] [PubMed] [Google Scholar]
- 43.Zhao SZ, McMillen JI, Markenson JA, Dedhiya SD, Zhao WW, Osterhaus JT, et al. Evaluation of the functional status aspects of health-related quality of life of patients with osteoarthritis treated with celecoxib. Pharmacotherapy 1999;19: 1269-78. [DOI] [PubMed] [Google Scholar]
- 44.Williams GW, Hubbard RC, Yu SS, Zhao W, Geis GS. Comparison of once-daily and twice-daily administration of celecoxib for the treatment of osteoarthritis of the knee. Clin Ther 2001;23: 213-27. [DOI] [PubMed] [Google Scholar]
- 45.Williams PI, Hosie J, Scott DL. Etodolac therapy for osteoarthritis: a double-blind, placebo-controlled trial. Curr Med Res Opin 1989;11: 463-70. [DOI] [PubMed] [Google Scholar]
- 46.Ehrich EW, Bolognese JA, Watson DJ, Kong SX. Effect of rofecoxib therapy on measures of health-related quality of life in patients with osteoarthritis. Am J Manag Care 2001;7: 609-16. [PubMed] [Google Scholar]
- 47.Kivitz AJ, Greenwald MW, Cohen SB, Polis AB, Najarian DK, Dixon ME, et al. Efficacy and safety of Rofecoxib 12.5 mg versus Nabumetone 1,000 mg in patients with osteoarthritis of the knee: a randomized controlled trial. J Am Geriatr Soc 2004;52: 666-74. [DOI] [PubMed] [Google Scholar]
- 48.Tannenbaum H, Berenbaum F, Reginster JY, Zacher J, Robinson J, Poor G, et al. Lumiracoxib is effective in the treatment of osteoarthritis of the knee: a 13-week, randomized, double-blind study versus placebo and celecoxib. Ann Rheum Dis 2004;63: 1419-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee P, Davis P, Prat A. The efficacy of diflunisal in osteoarthritis of the knee. A Canadian multicenter study. J Rheumatol 1985;12: 544-8. [PubMed] [Google Scholar]
- 50.Simon LS, Lanza FL, Lipsky PE, Hubbard RC, Talwalker S, Schwartz BD, et al. Preliminary study of the safety and efficacy of SC-58635, a novel cyclooxygenase 2 inhibitor: efficacy and safety in two placebo-controlled trials in osteoarthritis and rheumatoid arthritis, and studies of gastrointestinal and platelet effects. Arthritis Rheum 1998;41: 1591-602. [DOI] [PubMed] [Google Scholar]
- 51.Weaver A, Rubin B, Caldwell J, McMahon FG, Lee D, Makarowski W, et al. Comparison of the efficacy and safety of oxaprozin and nabumetone in the treatment of patients with osteoarthritis of the knee. Clin Ther 1995;17: 735-45. [DOI] [PubMed] [Google Scholar]
- 52.Makarowski W, Weaver A, Rubin B, Caldwell J, McMahon FG, Noveck RJ, et al. The efficacy, tolerability, and safety of 1200 mg/d of oxaprozin and 1500 mg/d of nabumetone in the treatment of patients with osteoarthritis of the knee. Clin Ther 1996;18: 114-24. [DOI] [PubMed] [Google Scholar]
- 53.Dieppe PA, Ebrahim S, Martin RM, Juni P. Lessons from the withdrawal of rofecoxib. BMJ 2004;329: 867-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oxford league table of analgesics in acute pain. www.jr2.ox.ac.uk/bandolier/booth/painpag/Acutrev/Analgesics/Leagtab.html (accessed 10 Sep 2004).
- 55.Barden J, Edwards JE, McQuay HJ, Moore RA. Single dose oral celecoxib for postoperative pain. Cochrane Database Syst Rev 2003;(2): CD004233. [DOI] [PubMed]
- 56.Rowbotham MC. What is a “clinically meaningful” reduction in pain? Pain 2001;94: 131-2. [DOI] [PubMed] [Google Scholar]
- 57.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol 2002;29: 131-8. [PubMed] [Google Scholar]
- 58.Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol 2000;27: 2635-41. [PubMed] [Google Scholar]
- 59.Johnson AG. NSAIDs and increased blood pressure. What is the clinical significance? Drug Saf 1997;17: 277-89. [DOI] [PubMed] [Google Scholar]
- 60.Bleumink GS, Feenstra J, Sturkenboom MC, Stricker BH. Nonsteroidal anti-inflammatory drugs and heart failure. Drugs 2003;63: 525-34. [DOI] [PubMed] [Google Scholar]
- 61.Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med 1999;106(5B): 13-24S. [DOI] [PubMed] [Google Scholar]
- 62.Phelan KM, Mosholder AD, Lu S. Lithium interaction with the cyclooxygenase 2 inhibitors rofecoxib and celecoxib and other nonsteroidal anti-inflammatory drugs. J Clin Psychiatry 2003;64: 1328-34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.