Abstract
Pancreatic metastasis from colorectal cancer is rare, and there have been only a few reports of its preoperative diagnosis by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) with immunohistochemical staining. We herein describe the case of a 77-year-old woman in whom a solitary mass in the pancreatic tail was detected 11 years after rectal cancer resection. The patient also had a history of pulmonary tumor resection. We performed EUS-FNA and a histopathological examination showed adenocarcinoma with CD20+, CD7-, and CDX2+ (similar to her rectal cancer). EUS-FNA enabled a histopathological examination, including immunohistochemical staining, which helped to confirm the diagnosis of pancreatic and pulmonary metastasis from rectal cancer.
Keywords: colorectal cancer, EUS-FNA, immunohistochemical staining, pancreatic metastasis, pulmonary metastasis, rectal cancer
Introduction
Metastatic tumors of the pancreas are rare and approximately 2% of pancreatic cancers are metastases from other primary sites (1,2). With regard to pancreatic metastasis from colorectal cancer, solitary metastasis to the pancreas is less common than multiple metastases to other organs - metachronous metastases to both the lungs and the pancreas are rare (3,4).
The preoperative histopathological diagnosis of pancreatic metastases has traditionally been difficult, thus in most of these cases the diagnosis is confirmed after pancreatic resection (3,5). However, recent reports have shown that endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is a feasible and safe method for definitively diagnosing pancreatic metastases and for planning the therapeutic approach (6). Moreover, several reports have suggested the effectiveness of EUS-FNA in diagnosing pancreatic metastases from colorectal cancer (4,7-11). However, to the best of our knowledge, there are few reports on the histopathological and immunohistochemical findings.
We herein describe a case of metachronous pulmonary and pancreatic metastasis from rectal cancer. We were able to confirm the preoperative diagnosis of pancreatic metastasis by EUS-FNA with immunohistochemical staining. We were finally able to obtain a definitive diagnosis with a histopathological comparison of the EUS-FNA specimens from the patient's metastatic lung tumor and the primary rectal cancer.
Case Report
A 77-year-old woman was admitted to our department for further examination. She had undergone surgical resection for rectal adenocarcinoma (stage IIIB; pT3N1M0 according to the 7th edition of the Union for International Cancer Control [UICC]) 11 years previously, and left lower lobectomy for pulmonary cancer, which was considered to be primary pulmonary adenocarcinoma (stage IIA; pT2bN0M0 according to the 7th edition of UICC) 3 years previously. In the follow-up examination, the patient's carcinoembryonic antigen (CEA) level was found to be elevated and 18 fluoro-2-deoxy glucose positron emission tomography/computed tomography (18F-FDG PET/CT) showed a high level of accumulation (SUVmax 9.7) in the pancreatic tail.
The laboratory data on the day of admission revealed an elevated CEA level (Table 1). Computed tomography (CT) showed a hypodense mass in the pancreatic tail of 30 mm in the largest diameter, with delayed enhancement (Fig. 1). Magnetic resonance imaging (MRI) revealed an area of low signal intensity on T1WI and high signal intensity on T2WI. MRI also detected an area of high signal intensity on diffusion-weighted imaging. EUS showed a hypoechoic mass in the pancreatic tail with an irregular lesion border (Fig. 2). From these findings, we considered the pancreatic lesion to be a primary pancreatic cancer and performed EUS-FNA using a 22-gauge needle (SonoTipⓇ Pro Control, Medi-Globe, Rosenheim, Germany). Sufficient specimens were obtained after 2 needle passes. Histopathologically, the EUS-FNA specimens showed adenocarcinoma with tall columnar epithelial cells, sparse nuclear stratification and necrosis. An immunohistochemical examination revealed CK20- and CDX2-positive, and CK7- and TTF-1-negative tumor cells, which were morphologically similar to the primary rectal cancer cells. Thus, we reached a final diagnosis of metachronous pancreatic metastasis from rectal cancer and distal pancreatectomy was performed. A histopathological examination of the resected pancreatic specimen, which included immunohistochemical staining, showed the same findings as the EUS-FNA findings (Fig. 3). We re-evaluated the resected pulmonary specimen. The histopathological and immunohistochemical staining findings were consistent with the patient's rectal cancer (Fig. 4). Recurrence was found in the patient's liver at 6 months after pancreatectomy, and chemotherapy was performed with tegafur-gimeracil-oteracil potassium and bevacizumab. The patient remains alive at 17 months after pancreatectomy.
Table 1.
Laboratory Data on Admission.
| [Peripheral Blood] | ||
| WBC | 3,810 | /μL |
| RBC | 407 | ×104/μL |
| Hb | 12.1 | g/dL |
| Plt | 15.3 | ×104/μL |
| [Biochemistry] | ||
| T-Bil | 1.0 | mg/dL |
| AST | 21 | IU/L |
| ALT | 19 | U/L |
| ALP | 183 | IU/L |
| GTP | 24 | IU/L |
| BUN | 14.0 | mg/dL |
| Cre | 0.53 | mg/dL |
| CRP | 0.14 | mg/dL |
| [Tumor Maker] | ||
| CEA | 8.1 | ng/mL |
| CA19-9 | 27.6 | U/mL |
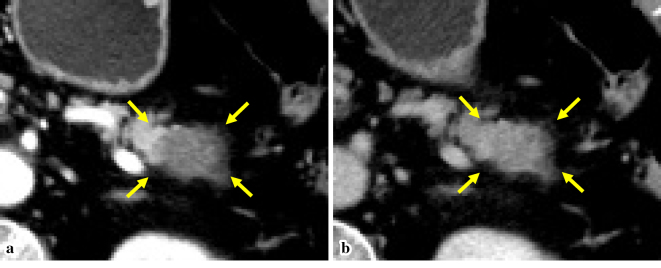
Figure 1.
A CT image: A hypodense mass of 30 mm in diameter in the pancreatic tail in the arterial phase (arrows) (a), with increasing enhancement in the equilibrium phase (arrows) (b).
Figure 2.
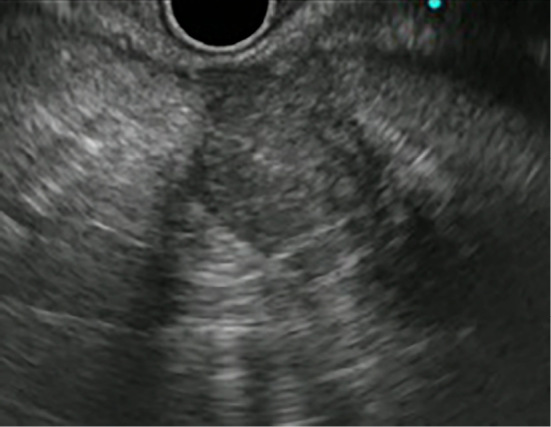
An EUS image: A hypoechoic mass in the pancreatic tail with an irregular lesion border.
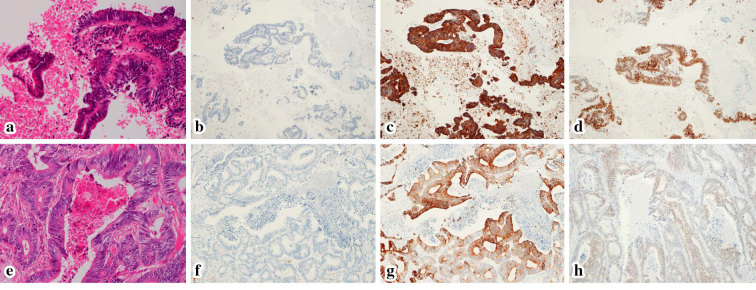
Figure 3.
Histopathological sections: In the EUS-FNA specimen, Hematoxylin and Eosin (H&E) staining showing adenocarcinoma with tall columnar epithelial cells, sparse nuclear stratification and necrosis (a), CK7- (b), CK20+(c), and CDX2+(d). These findings were similar to the H&E staining findings (e), CK7- (f), CK20+(g), and CDX2+(h) in the resected primary rectal cancer specimen.
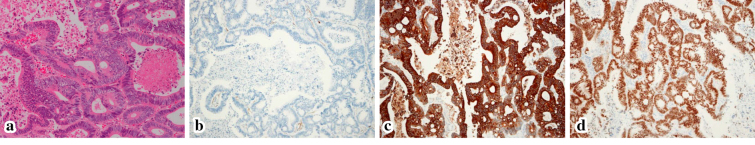
Figure 4.
Histopathological sections from the metastatic pulmonary tumor: Hematoxylin and Eosin staining showing adenocarcinoma (a), CK7- (b), CK20+(c), and CDX2+(d). These findings were the same as those in the primary rectal cancer.
Discussion
In a large autopsy series, the incidence of pancreatic metastasis from other primary malignant tumors has been reported to range from 1.6-15% (12-14); solitary metastasis to the pancreas occurs even less frequently. Sperti et al. (15) reviewed 108 studies involving 418 patients who underwent radical resection of secondary pancreatic tumors, which showed that pancreatic metastases that were treated by pancreatectomy had mainly metastasized from renal cell carcinoma (RCC), followed by melanoma, colorectal cancer, breast cancer, sarcoma, and lung cancer. Furthermore, the previous review evaluated 24 studies involving 37 patients who received surgical treatment for pancreatic metastasis from colorectal cancer. According to this review, 24 patients had metastasis from a primary neoplasm of the colon and 11 had metastasis from rectal cancer. Twenty-eight patients presented with single pancreatic metastasis; in 9 cases additional surgery was required to treat metastatic disease in other sites. In some case reports on pancreatic metastasis from rectal cancer, a history of metachronous pulmonary metastasis prior to the diagnosis of pancreatic lesions was reported (3,4,16,17). Inagaki et al (3) suggested that pulmonary tumors may metastasize to the pancreas by hematogenous spread. Most of the metastases were solitary metastasis to the lungs and were adequately controlled by resection of the metastatic pulmonary tumor. In the present case, there was no recurrence in the other parts of the lungs at 3 years after the performance of left lower lobectomy.
Pancreatic metastases are often detected during follow-up examinations after surgery for a primary lesion (18). Enhanced CT of pancreatic metastases from RCC usually shows intense enhancement during arterial phase imaging, which can easily be differentiated from primary pancreatic cancer (19). However, in metastasis from colorectal cancer, CT shows a hypodense mass that is occasionally accompanied by dilatation of the distal main pancreatic duct, similarly to primary pancreatic cancer (20). Thus, it is usually difficult to differentiate metastatic tumors from primary pancreatic cancer by imaging alone. In the present case, CT showed a hypodense mass with delayed enhancement, similarly to primary pancreatic cancer, and no dilatation of the distal main pancreatic duct due to the location of the mass in the pancreatic tail. On the other hand, the EUS findings of pancreatic metastases occasionally differ from those of primary pancreatic cancer (21,22). Hijioka et al. (22) reported that the presence of a regular lesion border, together with the absence of a retention cyst and main pancreatic duct dilation in an EUS examination were indicative of pancreatic metastasis rather than primary pancreatic cancer. However, their study only included 1 case of pancreatic metastasis from colon cancer; thus, these EUS findings may not apply to metastasis from colorectal cancer. In the present case, EUS showed a hypoechoic mass with an irregular lesion border that was difficult to distinguish from primary pancreatic cancer.
In previous years, the preoperative histopathological diagnosis of pancreatic metastases has been difficult, and in most cases, the diagnoses of most pancreatic metastases were confirmed after pancreatic resection (3,5). However, more recently, several reports have been published regarding the use of EUS-FNA in the diagnosis of pancreatic metastases (4,7-11). In the diagnosis of pancreatic metastases, the sensitivity, specificity, positive and negative predictive values, and the accuracy of the histological analysis of EUS-FNA specimens are reported to be 93.8%, 60%, 93.8%, 60%, and 89%, respectively (11). EUS-FNA is useful for diagnosing pancreatic metastases from RCC due to the features of clear cell (conventional type) RCC and the immunohistochemical findings, which include vimentin and CD10 positivity (23). These histopathological findings are significantly different from those of primary pancreatic cancer. Colorectal cancer also shows a characteristic immunohistochemical staining pattern (CK20+, CK7-, and CDX2+) that differs from that of primary pancreatic cancer (24). A previous immunohistochemical study revealed that the rates of CK20 and CK7 positivity were 100% and 5%, respectively, in colorectal cancer, and 62% and 92% in pancreatic cancer (24). It has also been reported that the rates of CK20 and CK7 positivity in pancreatic cancer were 63% (weak, 43%; moderate, 15%; strong 5%) and 96%, respectively (25). CDX2 is expressed uniformly in the majority of colorectal adenocarcinomas. In contrast, the rate of CDX2 positivity in pancreatic adenocarcinoma is reported to be from 0-50% (26). Although it is difficult to diagnose all cases by immunohistochemistry alone, the immunohistochemical findings are helpful for differentiating between pancreatic metastasis and primary pancreatic cancer. In the present case, the histopathological examination of the EUS-FNA specimen revealed cells that were morphologically similar to the primary rectal cancer cells, and the diagnosis was confirmed from the immunohistochemical findings (CD20+, CK7-, and CDX2+).
It is reported that the diagnostic sensitivity of EUS-FNA (when performed by well-experienced endoscopists) in the diagnosis of pancreatic lesions is not affected by the location or size of the lesions and that the accuracy in all subgroups is more than 90% (27). It is therefore possible to diagnose most pancreatic lesions by EUS-FNA with a considerably high rate of probability. Furthermore, as adequate specimens are needed to perform a histopathological assessment by immunohistochemical staining, it is important to obtain sufficient samples by EUS-FNA using a thicker needle or to perform a rapid on-site evaluation to assess the quantity of specimens. Although the diagnostic accuracy of EUS-FNA for pancreatic lesions is considered to be high and histopathological evaluation has been demonstrated to be a highly sensitive method for diagnosing primary pancreatic cancer or metastases from colorectal cancer, few reports have described the diagnosis of patients using the combination of EUS-FNA and immunohistochemical staining (Table 2). Thus far, only 7 reports, involving 14 cases (including the present case), have been described (4,7-11). Among these reports, immunohistochemical staining of EUS-FNA specimens was performed in 8 cases (CK20 and CK7 staining in 4 cases; CK20, CK7, and CDX2 staining in 4 cases), and a comparison between the EUS-FNA specimens and the resected pancreas specimens was only possible in 3 cases. Moreover, in these reports, there were only 2 cases (aside from the present case) that involved metachronous metastases to both the lungs and the pancreas. However, the 2 cases did not include any description of the histopathological findings of the resected pulmonary specimens.
Table 2.
Clinical Features, Treatments, and Follow-up of Patients with Pancreatic Metastases from Colorectal Cancer Diagnosed by EUS-FNA.
| Reference | No. | Age (years) | Sex | Primary site | Metachronous pulmonary metasatsis | Interval (months) | Site | Size (mm) | EUS-FNA | Surgery | Follow-up (months) | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytological diagnosis | Immunohistochemical findings | ||||||||||||
| 7 | 2 | 54 | M | Colon | - | 65 | NA | 70 | ADC | CK20+/CK7- | - | 16 | Death |
| 47 | M | Colon | - | NA | NA | 37 | ADC | CK20+/CK7- | - | NA | Unknown | ||
| 8 | 1 | 74 | M | Left colon | - | 24 | Body | 21 | ADC | - | DP | 6 | Alive |
| 4 | 2 | 67 | M | Rectal | + | 72 | Head/Body | 17/16 | ADC | CK20+/CK7- | MSPP | 16 | Alive |
| 58 | M | Rectal | + | 84 | Body | NA | ADC | CK20+/CK7- | DP | 6 | Alive | ||
| 9 | 4 | 62 | M3 | Colon (n = 4) | - | 65* | Head | 27* | ADC (n = 2) | - | - | NA | Death (n = 3) |
| F1 | (12-96) | (20-70) | ADC (n = 2) | CK20+/CK7-/CDX2+ | - | NA | Unknown (n = 1) | ||||||
| 10 | 1 | 67 | F | Right colon | - | 72 | Head | 40 | ADC | CK20+/CK7-/CDX2+ | - | NA | NA |
| 11 | 3 | 65 | F | Colon | - | 37 | NA | 25 | ADC | NA | PD | 12 | Death |
| 74 | F | Colon | - | 25 | NA | 12 | ADC | NA | EL | 13 | Death | ||
| 78 | F | Colon | - | 39 | NA | 12 | ADC | NA | - | 5 | Death | ||
| Present study | 1 | 77 | F | Rectal | + | 132 | Tail | 30 | ADC | CK20+/CK7-/CDX2+ | DP | 17 | Alive |
ADC: adenocarcinoma, CDX: caudal-related homeodomain protein, CK: cytokeratin, DP: distal pancreatectomy, EL: exploratory laparotomy, MSPP: middle-segmentpreserving pancreatectomy, NA: not applicable, PD: pancreaticoduodenectomy
* median (range)
In present case, although we initially considered the pancreatic lesion to be primary pancreatic cancer, the EUS-FNA histopathological findings showed that the pancreatic lesion had metastasized from rectal cancer. Our retrospective evaluation of the pulmonary resected specimen revealed the histopathological and immunohistochemical findings were the same as those of the rectal cancer. Thus, we could reach a final, definitive diagnosis of metachronous metastases to the lungs and pancreas from rectal cancer.
In conclusion, we described a rare case of primary rectal adenocarcinoma with metachronous pulmonary and pancreatic metastases 8 and 11 years after the resection of rectal cancer. EUS-FNA made it possible to obtain a definitive diagnosis before pancreatic resection and enabled the comparison of the pancreatic lesion with the previously resected cancer specimens from other sites. In cases involving patients with a past history of colorectal cancer, it is important to not only perform EUS-FNA but also evaluate the findings, including the immunohistochemical staining results to distinguish metastasis to the pancreas, even when imaging examinations show findings that are typical of primary pancreatic cancer.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors are indebted to Dr. Edward F. Barroga, Associate Professor and Senior Medical Editor of the Department of International Medical Communications of Tokyo Medical University for editing and reviewing the manuscript.
References
- 1.Z'graggen K, Fernández-del CC, Rattner DW, Sigala H, Warshaw AL. Metastases to the pancreas and their surgical extirpation. Arch Surg 133: 413-417, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Stankard CE, Karl RC. The treatment of isolated pancreatic metastases from renal cell carcinoma: a surgical review. Am J Gastroenterol 87: 1658-1660, 1992. [PubMed] [Google Scholar]
- 3.Inagaki H, Nakao A, Ando N, et al. A case of solitary metastatic pancreatic cancer from rectal carcinoma: a case report. Hepatogastroenterology 45: 2413-2417, 1998. [PubMed] [Google Scholar]
- 4.Tanemura A, Mizuno S, Okura Y, et al. Margin-negative limited resection of metastatic pancreatic tumors from rectal cancer preoperatively diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsies: report of two cases. Surg Today 44: 366-372, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Lee CW, Wu RC, Hsu JT, et al. Isolated pancreatic metastasis from rectal cancer: a case report and review of literature. World J Surg Oncol 8: 26, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy S, Wolfgang CL. The role of surgery in the management of isolated metastases to the pancreas. Lancet Oncol 10: 287-293, 2009. [DOI] [PubMed] [Google Scholar]
- 7.DeWitt J, Jowell P, Leblanc J, et al. EUS-guided FNA of pancreatic metastases: a multicenter experience. Gastrointest Endosc 61: 689-696, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Stoltz A, Barnoud R, Plok V, Ducerf C, Baulieux J, Mabrut JY. A pancreatic metastasis from a colon cancer. Clin Res Hepatol Gastroenterol 35: 586-589, 2011. [DOI] [PubMed] [Google Scholar]
- 9.El Hajj II, LeBlanc JK, Sherman S, et al. Endoscopic ultrasound-guided biopsy of pancreatic metastases: a large single-center experience. Pancreas 42: 524-530, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Carbonari AP, Assef MS, Nakao FS, da Silva AS, do Valle LC, Chaves DM. Pancreatic metastasis from colon carcinoma diagnosed by endoscopic ultrasound fine needle aspiration. Endosc Ultrasound 2: 109-111, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ardengh JC, Lopes CV, Kemp R, Venco F, de Lima-Filho ER, dos Santos JS. Accuracy of endoscopic ultrasound-guided fine-needle aspiration in the suspicion of pancreatic metastases. BMC Gastroenterol 13: 63, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 3: 74-85, 1950. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura E, Shimizu M, Itoh T, Manabe T. Secondary tumors of the pancreas: clinicopathological study of 103 autopsy cases of Japanese patients. Pathol Int 51: 686-690, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary tumors of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch 444: 527-535, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Sperti C, Moletta L, Patanè G. Metastatic tumors to the pancreas: the role of surgery. World J Gastrointest Oncol 6: 381-392, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachmann J, Michalski CW, Bergmann F, Büchler MW, Kleeff J, Friess H. Metastasis of rectal adenocarcinoma to the pancreas. Two case reports and a review of the literature. JOP 8: 214-222, 2007. [PubMed] [Google Scholar]
- 17.Baierlein SA, Wistop A, Looser C, Bussmann C, von Flüe M, Peterli R. Primary pancreatic neoplasia or metastasis from colon carcinoma? Acta Gastroenterol Belg 71: 401-408, 2008. [PubMed] [Google Scholar]
- 18.Zerbi A, Ortolano E, Balzano G, Borri A, Beneduce AA, Di Carlo V. Pancreatic metastasis from renal cell carcinoma: which patients benefit from surgical resection? Ann Surg Oncol 15: 1161-1168, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Adler H, Redmond CE, Heneghan HM, et al. Pancreatectomy for metastatic disease: a systematic review. Eur J Surg Oncol 40: 379-386, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Palmowski M, Hacke N, Satzl S, et al. Metastasis to the pancreas: characterization by morphology and contrast enhancement features on CT and MRI. Pancreatology 8: 199-203, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Smith AL, Odronic SI, Springer BS, Reynolds JP. Solid tumor metastases to the pancreas diagnosed by FNA: A single-institution experience and review of the literature. Cancer Cytopathol 123: 347-355, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Hijioka S, Matsuo K, Mizuno N, et al. Role of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in diagnosing metastasis to the pancreas: A tertiary center experience. Pancreatology 11: 390-398, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert CM, Monaco SE, Cooper ST, Khalbuss WE. Endoscopic ultrasound-guided fine-needle aspiration of metastases to the pancreas: A study of 25 cases. Cytojournal 8: 7, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol 13: 962-972, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Matros E, Bailey G, Clancy T, et al. Cytokeratin 20 expression identifies a subtype of pancreatic adenocarcinoma with decreased overall survival. Cancer 106: 693-702, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Xiao W, Hong H, Awadallah A, Zhou L, Xin W. Utilization of CDX2 expression in diagnosing pancreatic ductal adenocarcinoma and predicting prognosis. PLoS One 9: e86853, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uehara H, Ikezawa K, Kawada N, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. J Gastroenterol Hepatol 26: 1256-1261, 2011. [DOI] [PubMed] [Google Scholar]