Abstract
We herein report the first case of a pancreatic fistula extending into the thigh caused by the rupture of an intraductal papillary mucinous neoplasm (IPMN) of the pancreas. An 80-year-old man was suspected to have necrotizing fasciitis because of right femoral pain. Computed tomography showed fluid retention from the pancreatic head to the right iliopsoas muscle and an IPMN at the pancreatic head. The findings of endoscopic retrograde pancreatography led to the suspicion of a minor leak and a pancreatic stent was placed. The patient died due to an uncontrollable infection. A pathological autopsy showed a pancreatic fistula extending into the thigh that had been caused by the rupture of the IPMN.
Keywords: pancreatic fistula, intraductal papillary mucinous neoplasm
Introduction
Pancreatic fistula is a disease state that is characterized by the extrapancreatic leakage of pancreatic juice caused by pancreatic duct disruption, resulting from acute pancreatitis, chronic pancreatitis, trauma, and postoperative complications. Pancreatic fistulas can be caused by acute pancreatitis, an acute exacerbation of chronic pancreatitis, traumatic disease, postoperative complications, and pancreatic tumors. Apart from postoperative complications, the most common causes of pancreatic fistula are pancreatitis and trauma. As for the pathogenesis of pancreatic fistula, local acute inflammation occurring around the primary branch of the pancreatic duct and occlusion of the pancreatic duct by a protein plug are thought to disrupt the peripheral pancreatic ducts, causing the formation of a pancreatic pseudocyst, which ruptures, leading to the development of a pancreatic fistula (1,2). An external pancreatic fistula communicates with the body surface, and an internal pancreatic fistula communicates with other organs in the body cavity. Internal pancreatic fistulas form in the rough connective tissue with low resistance. The leakage of fluid into the abdominal cavity causes pancreatic ascites. Pancreatic pleural effusion results from the leakage of pancreatic fluid from the backside to the head of the pancreas into the thoracic cavity (2-4). The symptoms of a pancreatic fistula are diverse and depend on the site of the fistula. An appreciable number of patients have initial symptoms in the other organs. We herein describe our experience with a patient who had a pancreatic fistula that extended into the thigh. The fistula was caused by the rupture of an intraductal papillary mucinous neoplasm (IPMN) of the pancreatic head.
Case Report
The patient was an 80-year-old man who presented at the Department of Orthopedics in our hospital because of a fever of several days in duration and pain, swelling, and redness of the right thigh (Fig. 1). The patient had type 2 diabetes mellitus and chronic renal failure. The past history and family history were not relevant to the current disorder. As for the patient's life history, the patient had smoked 10 cigarettes per day for 10 years and consumed 33 g of alcohol per day for 40 years. Blood tests (on admission showed) an elevated C-reactive protein level, hypoalbuminemia, and high serum levels of lactic acid dehydrogenase, creatine phosphokinase, amylase, glucose, and creatinine (Table 1). Magnetic resonance imaging (MRI) was performed to determine the cause of inflammation of the right thigh (Fig. 2). Fat-suppressed T2-weighted images showed high signal intensity in the quadriceps femoris muscle, the gracilis muscle, and the semimembranosus muscle, suggesting the presence of necrotizing fasciitis. The patient was admitted to our hospital for further evaluation and treatment. A confirmatory incision was made in the right thigh for diagnostic purposes. There were no findings suggestive of necrotizing fasciitis. Blood tests on Day 5 (Table 2) showed that the serum amylase level (1,627 IU/L) and the serum lipase level (1,470 U/L) were markedly high. Although there was no abdominal pain, the possibility of acute pancreatitis could not be excluded. Abdominal computed tomography (Fig. 3) and magnetic resonance cholangiopancreatography (Fig. 4) were thus performed and showed cystic lesions in the pancreatic head. Fluid retention was seen in the region from the pancreatic head to the region around the right kidney and the right iliopsoas muscle, suggesting the presence of inflammatory changes caused by pancreatitis. IPMN of the pancreas associated with acute pancreatitis was diagnosed. The patient received ulinastatin and imipenem/cilastatin by intravenous infusion. However, the inflammation did not improve. Disseminated intravascular coagulation developed on the Day 11. The amylase levels in the exudate of the right thigh and the ascitic fluid were very high (21,802 IU/L and 10,037 IU/L, respectively). The cause of prolonged inflammation of the right thigh was suspected to be a pancreatic fistula that had been caused by the rupture of an IPMN of the pancreatic head. On Day 14, endoscopic retrograde cholangiopancreatography was performed in order to place a pancreatic stent (Fig. 5). Pancreatography revealed cystic dilatation of the pancreatic ducts at the pancreatic head (Fig. 4A, arrow). Although we could not find an obvious leak from the pancreatic ducts, the contrast medium washed out quickly and was suspected to have leaked into the retroperitoneal space. A 7-French, 12-cm pancreatic stent was therefore placed in the main pancreatic duct. We chose to perform internal drainage because of the risk of self-removal associated with external drainage. Subsequently, the patient's general condition deteriorated despite conservative therapy, and he died of septic shock on Day 23.
Figure 1.
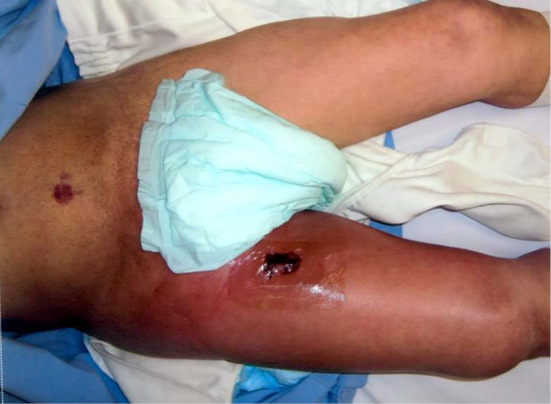
A picture of the right femoral region on admission. After a tentative incision was made, redness and swelling were observed in the right femoral region.
Table 1.
The Laboratory Data on Admission.
| WBC | 14,900 | /μL | LDH | 1,212 | IU/L |
| Seg | 95.6 | % | CPK | 1,840 | IU/L |
| Lym | 1.7 | % | Amy | 615 | IU/L |
| Eos | 0.2 | % | Glu | 223 | mg/dL |
| Mono | 2.4 | % | BUN | 77 | mg/dL |
| Baso | 0.1 | % | Cr | 3.39 | mg/dL |
| RBC | 3.76×106 | /μL | Na | 134 | mEq/L |
| Hb | 11.8 | g/dL | K | 3.4 | mEq/L |
| Ht | 33.3 | % | Cl | 100 | mEq/L |
| Plt | 19.0×104 | /μL | Ca | 4.2 | mg/dL |
| I-P | 2 | mg/dL | |||
| T-P | 5.3 | g/dL | CRP | 20.19 | mg/dL |
| Alb | 2.6 | g/dL | |||
| T-bil | 0.6 | mg/dL | HbA1c (NGSP) | 8.0 | % |
| AST | 49 | IU/L | |||
| ALT | 35 | IU/L | |||
| ALP | 229 | IU/L |
Figure 2.
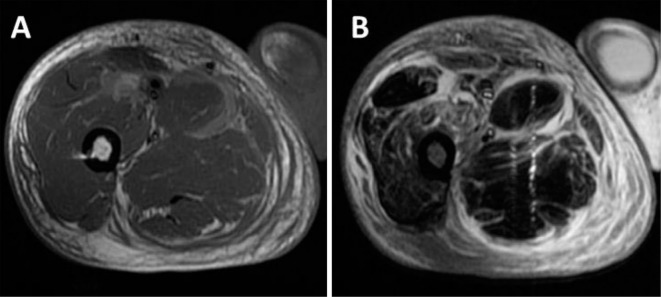
Magnetic resonance images. A: A T1-weighted image, showing moderate-to-high signal intensity around the muscle. B: A fat-suppressed T2-weighted image, showing high signal intensity in the quadriceps femoris muscle, the gracilis muscle, and the semimembranosus muscle.
Table 2.
The Laboratory Data on Day 5.
| WBC | 19,700 | /μL | LDH | 726 | IU/L |
| Seg | 96.0 | % | CPK | 278 | IU/L |
| Lym | 1.4 | % | Amy | 1,627 | IU/L |
| Eos | 0.2 | % | Lipase | 1,470 | IU/L |
| Mono | 2.3 | % | Glu | 247 | mg/dL |
| Baso | 0.1 | % | BUN | 91 | mg/dL |
| RBC | 3.77×106 | /μL | Cr | 3.94 | mg/dL |
| Hb | 11.6 | g/dL | Na | 130 | mEq/L |
| Ht | 33.7 | % | K | 3.8 | mEq/L |
| Plt | 26.3×104 | /μL | Cl | 96 | mg/dL |
| Ca | 5.6 | mg/dL | |||
| T-P | 4.8 | g/dL | I-P | 6.0 | mg/dL |
| Alb | 1.9 | g/dL | CRP | 15.57 | mg/dL |
| T-bil | 1.1 | mg/dL | |||
| AST | 20 | IU/L | CEA | 4.5 | ng/mL |
| ALT | 21 | IU/L | CA19-9 | 21 | U/mL |
| ALP | 225 | IU/L |
Figure 3.
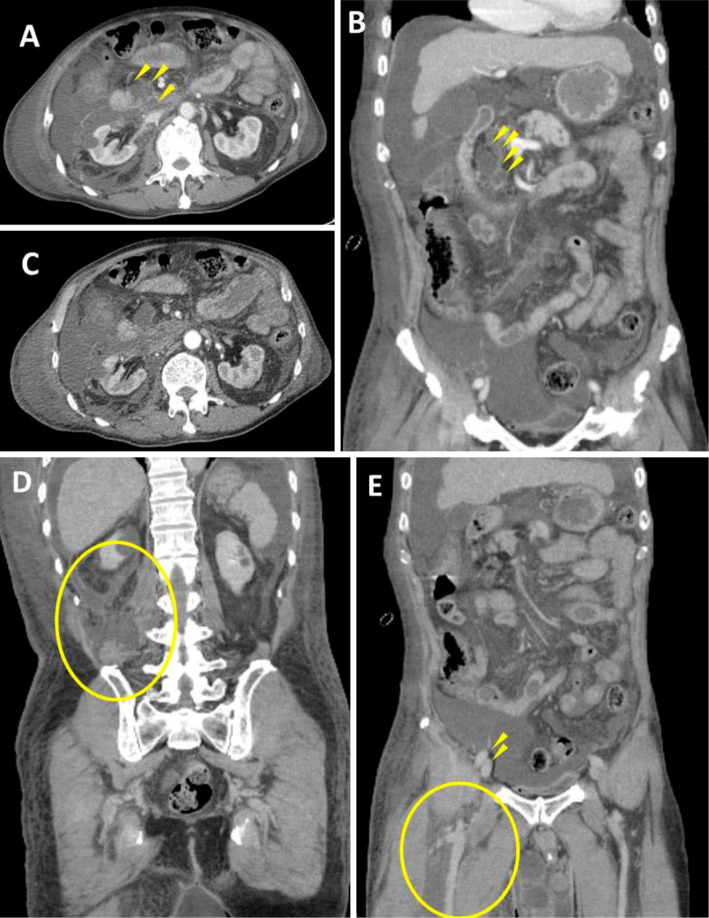
Abdominal contrast-enhanced computed tomographic scans. A, B: Atrophy and degeneration associated with a lack of parenchyma were observed in part of the uncinate process and the body of the pancreas. A good contrast effect was obtained in the pancreas. Cystic lesions were present in the uncinate process of the pancreas (arrowheads). C, D: Fluid retention extended from the duodenum to the right anterior pararenal space, the perirenal space, and the posterior pararenal space (circle). E: Fluid retention (circle) extended to the thigh over the ilium at the level of the femoral artery and vein (arrowheads).
Figure 4.
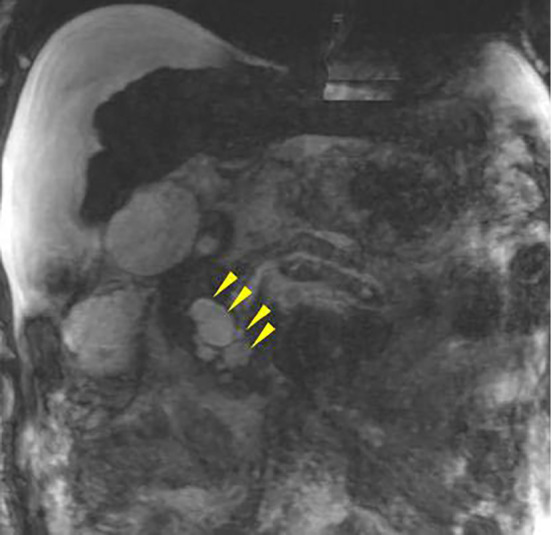
Magnetic resonance cholangiopancreatography. Cystic lesions were present in the uncinate process of the pancreas (arrowheads). This image was not clear because of an artifact and the presence of a large amount of ascites.
Figure 5.
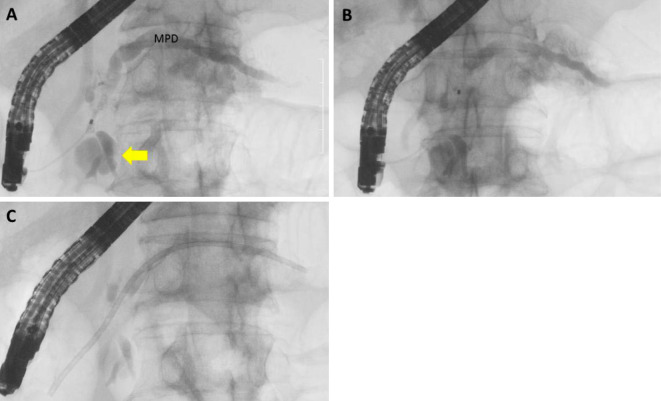
The endoscopic retrograde pancreatographic findings. A: Pancreatography showed the cystic dilation of a branch of the pancreatic duct at the head of the pancreas (arrow). The entire main pancreatic duct was dilated. Mild stricture was found in the main pancreatic duct at the head of the pancreas. B: Although we could not find an obvious leak from the pancreatic ducts, the contrast medium washed out quickly. C: Endoscopic pancreatic sphincterotomy was performed and a 7-French, 12-cm pancreatic stent was placed in the main pancreatic duct.
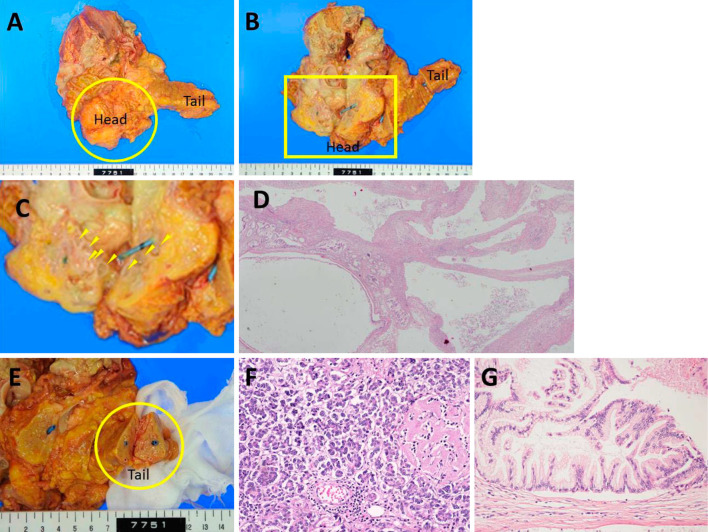
A pathological autopsy was performed. Macroscopically, an elastic soft mass (6×8×5 cm) was found to extend from the pancreatic head and surround Gerota's fascia of the right kidney. The cut surface of the mass showed cystic lesions of various sizes at the pancreatic head and discharged milky-white viscous fluid (Fig. 6A-D). The structures of the body and tail of the pancreas were relatively well preserved (Fig. 6E and F). A small region of diffuse fat necrosis was evident. Histologically, atypical papillary proliferation was observed in some of the cystic lesions at the pancreatic head, indicating the presence of an intraductal papillary mucinous adenoma (IPMA) of the pancreas (Fig. 6G). Some of these cystic lesions were contiguous with the connective tissue around the pancreas, suggesting that the partial rupture of the IPMA caused the pancreatic fistula. Accessory lesions, including intraperitoneal fat necrosis, extensive fat necrosis in Gerota's fascia of the right kidney, extensive necrosis of the right retroperitoneum, necrosis that was predominantly located around the subcutaneous tissue of the right thigh and femoral muscle were observed, and 4,000 mL of transparent yellow ascites was present. These findings demonstrated that rupture of the IPMN of the pancreatic head had caused the pancreatic fistula extending into the right thigh.
Figure 6.
The pathological findings. A: An elastic soft mass (6×8×5 cm, circle) extended from the pancreatic head and surrounded Gerota’s fascia of the right kidney. B: The cut surface of the pancreatic head showed the presence of cystic lesions of various sizes, with the leakage of milky-white viscous fluid (arrowhead). The rupture point of the IPMN could not be located due to the presence of inflammation, necrosis, and fibrosis. A pancreatic stent had been placed in the main pancreatic duct. C: An enlarged view of the square area of B. D (×20): The histological findings of pancreatic head showed the presence of cystic lesions of various sizes. E: Macroscopic findings showed that the structures of the tail (circle) of the pancreas were relatively well preserved. F (×200): The histological findings showed that the acinar structures of the tail of the pancreas were relatively well preserved; small regions of fat necrosis were sporadically found and hyalinization of the islets of Langerhans had occurred due to diabetes mellitus. No fibrosis was found in the tail of the pancreas. The macroscopic and histological findings showed that there was no obvious chronic pancreatitis. G (×200): Atypical papillary proliferation was found in some of the cystic lesions at the pancreatic head, indicating the presence of an intraductal papillary mucinous adenoma (IPMA) of the pancreas.
Discussion
We searched the PubMed/Medline and Embase databases using the terms “IPMN” and “rupture” as keywords and found 2 previously reported cases in which the leakage of pancreatic juice into the peritoneal cavity was caused by the rupture of an IPMN. The characteristics of the 3 patients are shown in Table 3 (5,6). All of the patients were men. The IPMN was located in the head of the pancreas in 1 patient, the body and tail of the pancreas in 1 patient, and the tail of the pancreas in 1 patient. The tumor diameters were 20.0 cm, 8.5 cm, and 8.0 cm, respectively. The pancreatic duct diameters were 14.1 mm, unknown, and 7.2 mm. Right femoral pain was the initial symptom in 1 patient; the other 2 patients had no symptoms. Distal pancreatectomy was performed in 2 of the 3 patients. The pathological diagnosis was adenoma in 1 patient, a borderline lesion in 1 patient, and cancer in 1 patient. Two patients were alive at the time of the reports, and 1 patient had died. No previous studies have reported that the leakage of pancreatic juice due to the rupture of an IPMN had effects on organs other than those located around the pancreas. To our knowledge, this is the first report of the rupture of an IPMN causing a pancreatic fistula extending into the right thigh.
Table 3.
Case Reports of IPMN Rupture.
| Reference No. | Age/Sex | Site | Max Size of Cyst (cm) | Diameter of Pancreatic Duct (mm) | Symptoms | Operation | Final Pathological Findings | Adjuvant Therapy | Outcome | Survival (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 75 /M | Tail | 20 | 14.1 | None | DP | B | None | cure | >48 |
| 6 | 55 /M | Body /Tail | 8.5 | NS | None | DP | IV | SC | cure | >3 |
| Present case | 80 /M | Head | 8 | 7.2 | Rt- Femoral pain | None | A | None | death | DWD |
NS: not stated, DP: distal pancreatectomy, B: borderline (moderate dysplasia), IV: invasive carcinoma, A: adenoma, SC: systemic chemotherapy, DWD: dead with disease
Pancreatic fistulas are often associated with symptoms that suggest the involvement of other organs, which makes them difficult to diagnose. Our patient had thigh pain and elevated levels of pancreatic enzymes, but no abdominal pain. A considerable amount of time was therefore required to reach a diagnosis. Some patients with pancreatic pleural effusion have only respiratory symptoms caused by pleural effusion, which may lead to a delayed diagnosis (7). Maule et al. described a patient who had a fistula extending from the retroperitoneum to the inguinal region that was caused by a pancreatic pseudocyst. Inguinal hernia-like swelling was caused by the retention of pancreatic juice. The patient had only inguinal pain and elevated levels of pancreatic enzymes, but no abdominal pain (8). Other case reports have documented a patient with a urethral obstruction caused by a pancreatic fistula that developed after pancreatitis (2), and a patient with dyspnea and bloody sputum caused by a pancreatic fistula that extended to the bronchi (9).
In our patient, the amylase levels in the ascitic fluid and the exudate from the thigh were high. We had observed the IPMN of pancreas head and the background pancreas showed no evidence of chronic pancreatitis (a possible cause of a pancreatic pseudocyst). At autopsy, the cystic lesions in the pancreatic head were found to be contiguous with the connective tissue around the pancreas. These findings suggested that the rupture of part of the cystic component of the IPMN caused the pancreatic fistula. The leaked pancreatic juice might have passed through the retroperitoneum and moved along the right kidney and the iliopsoas muscle to arrive at the thigh (Fig. 7). We thought that there were two main reasons why the pancreatic fistula extended into the thigh. First, the iliopsoas muscle originates along the lateral surfaces of the vertebral bodies of T12 and L1-L3 and the iliac fossa of the pelvis, and crosses the hip joint, inserting into the lesser trochanter of the femur. Secondly, the force of gravity and vibration might have caused the leaked pancreatic juice to descend from pancreatic head towards the thigh because the patient had walked all day before the appearance of the symptom. As for the cause of IPMN rupture, pancreatography showed no distinct evidence of a filling defect, which would have suggested a protein plug. However, pancreatic duct stenosis was present at the head of the pancreas, and the flow of pancreatic juice was interrupted by a protein plug and mucus, which most likely increased the internal pressure of the IPMN, leading to its rupture (2-4). A patient in whom a rupture of the main pancreatic duct was caused by pancreatic duct obstruction due to mucus produced by IPMN (4) and a patient in whom a pancreatobiliary fistula was caused by IPMN have also been reported (10). In our patient, a pancreatic fistula was suspected, and a pancreatic stent was placed on Day 14 in order to prevent the leakage of pancreatic juice into the fistula. Studies showing that the use of a pancreatic stent was effective for the management of pancreatic fistulas associated with surgery or trauma have been sporadically reported (11-14). In our patient, the amylase level in the exudates from the open wound decreased from 21,802 IU/L to 435 IU/L after the placement of the pancreatic stent, suggesting that the pancreatic stent suppressed the leakage of pancreatic juice into the fistula (Fig. 8). However, considerable time was required to diagnose the pancreatic fistula, which resulted in the deterioration of the patient's general condition and death.
Figure 7.
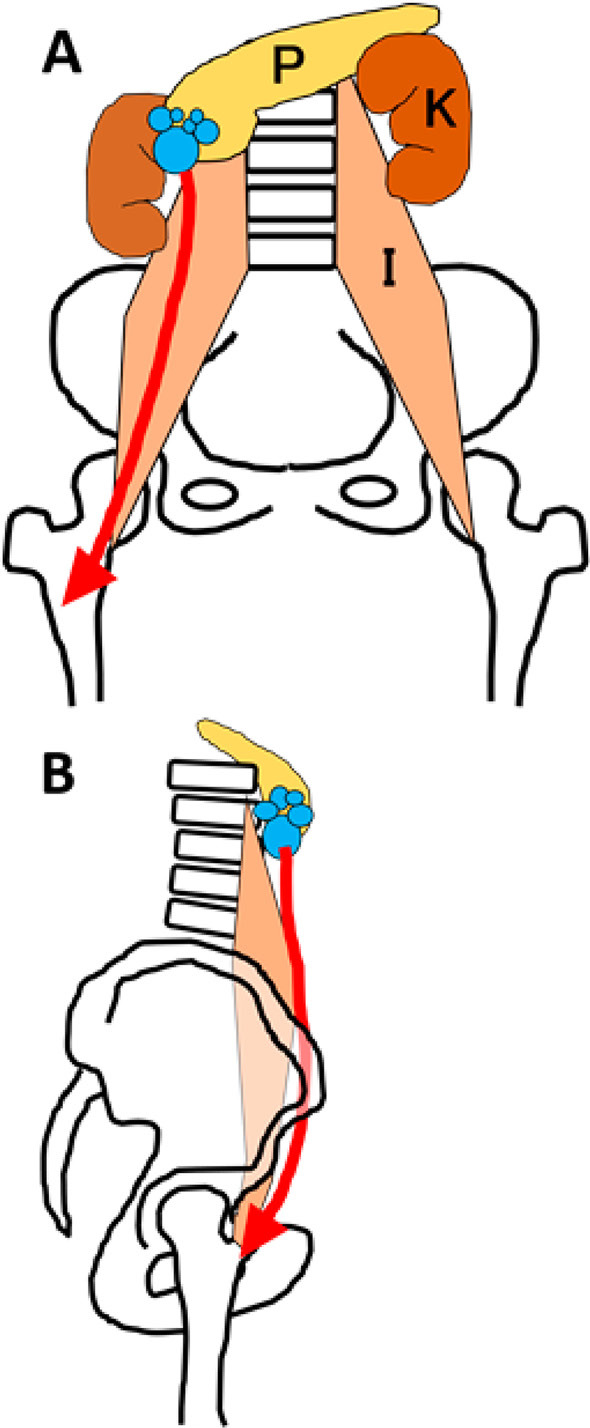
A schematic illustration of the pancreatic fistula extending into the right thigh. The leaked pancreatic juice might have passed through the retroperitoneum and moved along the right kidney and the iliopsoas muscle to arrive at the thigh. A: Frontal view. B: Lateral view. (P: pancreas, K: kidney, I: iliopsoas)
Figure 8.
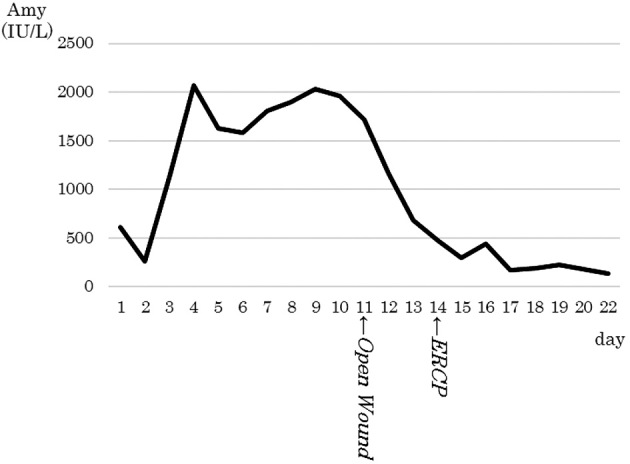
The time course of the serum amylase level. The serum amylase level increased after admission due to the worsening of the patient’s pancreatitis, and decreased after the opening of the incised wound in the femoral region.
We described our experience with a patient who had a pancreatic fistula extending into the thigh that was caused by rupture of an IPMN of the pancreas. Pancreatic fistulas occasionally present with symptoms related to other organs, causing difficulty in diagnosis. The possibility of a pancreatic fistula should be considered when patients with nonpancreatic symptoms present with elevated levels of pancreatic enzymes in their serum or pancreatic fluid.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Semba D, Wada Y, Ishihara Y, Kaji T, Kuroda A, Morioka Y. Massive pancreatic pleural effusion: pathogenesis of pancreatic duct disruption. Gastroenterol 99: 528-532, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi S, Homma H, Akiyama T, et al. A case of intraductal papillary mucinous neoplasm with internal pancreatic fistula causing left ureteral obstruction. Jpn J Gastroenterol 104: 1236-1244, 2007. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 3.Kuroda A, Semba D, Nagai H, Atomi Y, Morioka Y. Pancreatic pleural effusions and ascites. Tan to Sui 7: 407-415, 1986(in Japanese). [Google Scholar]
- 4.Hong TM, Lee RC, Chiang JH, Chang CY. Intraductal papillary mucinous tumor of the pancreas: computerized tomography and magnetic resonance imaging features. Kaohsiung J Med Sci 19: 55-61, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberger LH, Stein LH, Witkiewicz AK, Kennedy EP, Yeo CJ. Intraductal Papillary Mucinous Neoplasm (IPMN) with extra-pancreatic mucin: a case series and review of the literature. J Gastrointest Surg 16: 762-770, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SE, Jang JY, Yang H, Kim SW. Intraductul papillary mucinous carcinoma with atypical manifestations: report of two cases. World J Gastroenterol 13: 1622-1625, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tay C, Chang S. Diagnosis and management of pancreaticopleural fistula. Singapore Med J 54: 190-194, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Maule W, Reber H. Diagnosis and management of pancreatic pseudocysts, pancreatic ascites, and pancreatic fistulas. In: The Pancreas, Biology, Pathology and Diseases. 2nd ed Raven Press, New York, 1993: 741-750. [Google Scholar]
- 9.Yasuda T, Ueda T, Fujino Y, et al. Pancreaticobronchial fistula associated with chronic pancreatitis: report of a case. Surg Today 37: 338-341, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Okada K, Furuuchi T, Tamada T, et al. Pancreatobiliary fistula associated with an Intraductal Papillary-Mucinous Pancreatic Neoplasm manifesting as obstructive jaundice: Report of a case. Surg Today 38: 371-376, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Kaida S, Takarabe S, Ichikawa H, Kishikawa H, Nishida J, Morishita T. Two cases of pancreatic ductal injury successfully treated with endoscopic pancreatic stenting. JAEM 31: 647-650, 2011. (in Japanese, Abstract in English). [Google Scholar]
- 12.Abe T, Nagai T, Murakami K, et al. Pancreatic injury successfully treated with endoscoic stenting for major pancreatic duct disruption. Intern Med 48: 1889-1892, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Bhasin DK, Rana S, Rao C, et al. Endoscopic management of pancreatic injury due to abdominal trauma. JOP 13: 187-192, 2012. [PubMed] [Google Scholar]
- 14.Wang Q, He XR, Tian JH, Yang KH. Pancreatic duct stents at pancreaticoduodenectomy: a meta-analysis. Dig Surg 30: 415-424, 2013. [DOI] [PubMed] [Google Scholar]