Abstract
Central nervous system graft-versus-host disease can present quite a diagnostic challenge. We herein present a case of histologically-confirmed chronic graft versus host disease (GVHD) involving the central nervous system that occurred at 19 months after peripheral blood stem cell transplantation. Cranial magnetic resonance imaging showed areas of confluent hyperintensity in the deep/subcortical white matter with multiple punctate and curvilinear gadolinium enhancements, suggesting the disruption of the blood-brain barrier. A brain biopsy revealed perivascular CD3-positive T cell infiltration around the small vessels. We propose that the detection of punctate-enhanced lesions by magnetic resonance imaging may be a useful finding that facilitates the early diagnosis of chronic GVHD involving the central nervous system.
Keywords: chronic graft-versus-host disease, hematopoietic cell transplantation, magnetic resonance imaging, contrast enhancement, brain biopsy, perivascular lymphocyte infiltration
Introduction
Hematopoietic cell transplantation (HCT), including bone marrow transplantation (BMT) and peripheral blood stem cell transplantation (PBSCT), is important in the treatment of hematologic malignancies and connective tissue diseases. As the number of patients receiving HCT has increased, reports on the association between neurological complications and HCT have also increased. Neurological complications after HCT may be caused by various factors, including the primary disease, post-transplant lymphoproliferative disease (PTLD), drug- or radiation-induced effects, and opportunistic infections due to immunosuppression. Chronic graft versus host disease (GVHD), which most often involves the skin, eyes, oral mucosa, lungs, gastrointestinal tract and liver, can also cause neurological complications. Although the National Institute of Health's working group on chronic GVHD acknowledges that the neuromuscular manifestation of chronic GVHD only involves the muscle, neuromuscular junctions, and peripheral nerves (1), the number of reported cases of chronic GVHD that involve the central nervous system (CNS-cGVHD) has increased in recent years. This paper reports a case of CNS-cGVHD that occurred at 19 months after PBSCT, which was performed to treat acute myeloid leukemia (AML). The correlations between the radiological and pathological characteristics are useful for diagnosing CNS-cGVHD. This paper discusses the present case in detail and refers to previous studies.
Case Report
A 46-year-old right-handed woman was admitted to the hospital with pancytopenia. She had received chemotherapy (cyclophosphamide, doxorubicin, and 5-fluorouracil) 8 years previously for a left breast carcinoma. Bone-marrow aspiration and biopsy revealed hypercellular marrow with an increased number of blasts (15.2%). The patient was therefore diagnosed with therapy-related myelodysplastic syndrome (t-MDS). She subsequently progressed to t-AML (French-American-British classification M0) and received idarubicin (IDR) and cytarabine (Ara-C) to induce remission. At five months after the initial diagnosis, the patient underwent allogeneic PBSCT (allo-PBSCT) from a one-allele mismatched related donor after preparation with cyclosporine A (CyA) and total body irradiation (TBI). Full engraftment was confirmed on day 16. The initial post-transplant course was complicated by acute oral, gastrointestinal tract, and skin GVHD that resolved after treatment with prednisolone (PSL), CyA, and methotrexate (MTX). At five months after PBSCT, the patient developed chronic pulmonary GVHD in the form of bronchiolitis obliterans organizing pneumonia (BOOP). She was treated with oral PSL, which was tapered after achieving a good result.
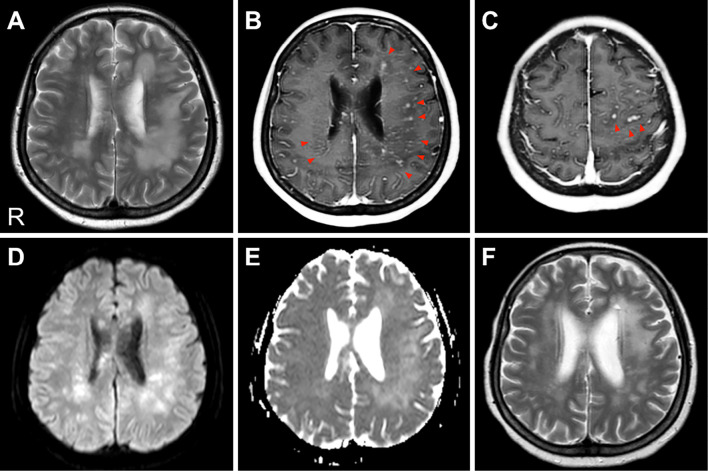
At 19 months after PBSCT, the patient developed headache, night sweats, difficulty writing due to shaking hands, and memory loss. Her blood test results were normal. Cerebrospinal fluid (CSF) studies revealed pleocytosis (33.3×106/L), protein elevation (85 mg/dL), and low CSF glucose (45 mg/dL), while the patient was negative for oligoclonal IgG bands and malignant cells. Bacterial and fungal cultures, a CSF polymerase chain reaction (PCR) for the JC virus, and tests for Toxoplasma gondii, Cytomegalovirus, Herpes simplex, and Varicella zoster virus were all negative. Magnetic resonance imaging (MRI) revealed areas of confluent and asymmetrical hyperintensity on T2-weighted images (T2WI) and fluid-attenuated inversion recovery (FLAIR) in the bilateral cerebral white matter (Fig. 1A). Gadolinium-enhanced MRI of the brain revealed multiple punctate and curvilinear lesions spreading from the deep cerebral region to the subcortical white matter, the pons, and the cerebellar vermis (Fig. 1B and C). Furthermore, both diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) maps revealed diffuse areas of high-intensity (Fig. 1D and E). MR angiography (MRA) and susceptibility-weighted imaging (SWI) revealed normal findings.
Figure 1.
Cranial MRI. (A) A T2-weighted image (TR/TE=3,000.0/80.0 ms). High-intensity plaque lesions are evident in the bilateral cerebral white matter, predominantly on the left side. (B, C) A gadolinium-enhanced T1-weighted image (TR/TE=6.0/2.3 ms). These lesions are enhanced with gadolinium, especially in the perivascular sites (red arrows). No gadolinium enhancement of the meninges was detected. (D, E) A diffusion-weighted imaging (DWI) and an apparent diffusion coefficient (ADC) map (b=1,000 s/mm2, TR/TE=5,000.0/65.0 ms). Both DWI and the ADC map show increased values in the involved areas. (F) A T2-weighted image (TR/TE=3,000.0/100.0 ms) after steroid pulse therapy. The high-intensity lesions of the white matter were significantly reduced. R: right
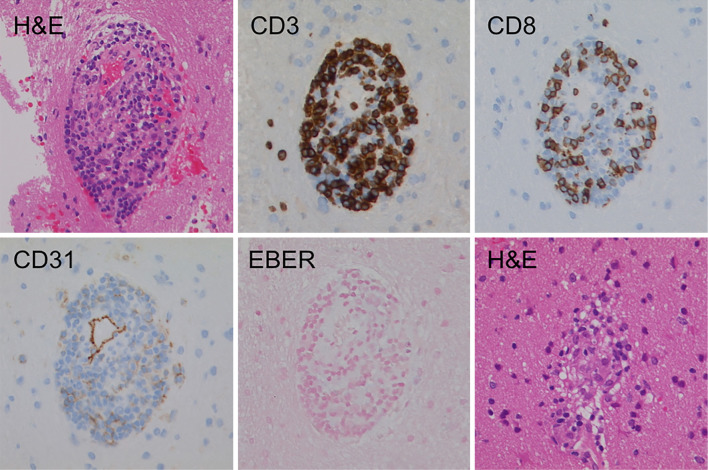
Fourteen days after MRI, an open brain biopsy was performed to collect a piece of brain from the patient's left frontal lobe white matter. The biopsy revealed the perivascular infiltration of small, mature lymphocytes in the brain parenchyma, without any signs of inflammatory cell infiltration into the vessel walls. These infiltrates were mostly CD3- and CD8-positive on immunostaining. The formation of granulomas was observed in some portions of the examined brain tissue. In situ hybridization for Epstein-Barr Virus (EBV)-encoded small RNAs (EBERs) was negative (Fig. 2). Furthermore, no findings indicated the recurrence of the primary disease. In view of these facts, we diagnosed the patient as having CNS-cGVHD.
Figure 2.
The immunohistological characteristics of brain tissue. A brain biopsy showing perivascular inflammation, which was predominantly located within the brain parenchyma. The inflammatory cells were mainly T lymphocytes with a cytotoxic T-cell immunophenotype, expressing CD3 and CD8. No EBV-encoded small RNA (EBER) transcripts were detected byin situ hybridization. Hematoxylin and Eosin staining demonstrated non-caseating granulomas containing lymphocytes, histiocytes, giant cells, and rare eosinophils. Original magnification, 100×.
Treatment with three cycles of methylprednisolone pulse therapy (1,000 mg/day for 3 days) was initiated, which led to a dramatic improvement in the patient's cognitive and neuropsychiatric symptoms. Her brain MRI showed the shrinkage of the white matter lesions and the disappearance of the gadolinium-enhanced lesions (Fig. 1F). She was subsequently placed on maintenance therapy with oral PSL and tacrolimus. Three months after the onset of symptoms, we confirmed the disappearance of almost all of her neurological symptoms.
Discussion
The CNS can be the target organ of GVHD. Approximately 30 years previously, Rouah et al. first described the local infiltration of lymphocytes into the CNS in a study of the autopsied brains of GVHD patients (2). Similar findings have been reported in recent years using animal models of chronic GVHD (3,4). Openshaw and Grauer et al. proposed the following six criteria for diagnosing CNS-cGVHD: the occurrence with chronic GVHD affecting other organs, neurological signs of CNS involvement without other explanation, corresponding brain MRI abnormalities, abnormal CSF studies (pleocytosis, elevated protein or immunoglobulin G, oligoclonal bands), a pathological brain biopsy or post-mortem examination, and a response to immunosuppressive therapy (5,6). The patient in the present case met all of these criteria. The exact pathophysiology of chronic GVHD remains uncertain, and it is often difficult to distinguish chronic GVHD from other neurological complications. This may be one reason why only a few cases of CNS-cGVHD have been reported to date, despite the increasing number of transplantations that are being performed.
A total of 24 cases of CNS-cGVHD after HCT have been reported to date. The mean age at the onset of neurological complications was 32 years (range: 9-63 years), the male-to-female ratio was 1:0.7, and the median interval between the time of transplant and the onset of symptoms was 18 months (range: 2-220 months) (7-22). Although many of these patients suffered from chronic GVHD of other organs as well, some had chronic GVHD that only involved the CNS (7-10). The neurological symptoms were diverse, and included motor paralysis, ataxia, and convulsions. Some patients presented with only psychiatric symptoms and cognitive dysfunction (9,11,12,14), whereas others presented with optic neuritis and myelitis, similar to the optic neuritis and myelitis induced by multiple sclerosis (8,13).
Only 13 biopsy- or autopsy-confirmed cases have been reported (Table). The histological features can be categorized into three types: cerebral vasculitis, encephalitis, and demyelination. The most common feature is the infiltration of CD3-positive T cell-dominant inflammatory cells in the perivascular space or within the vessel wall, whereas only scattered infiltrates were observed in brain parenchyma. Most of these inflammatory cells were CD8-positive cytotoxic T cells. The infiltration of CD68-positive monocytes/microglia (10) and human leucocyte antigen (HLA)-DR-positive microglia has also been reported (15). The use of a short tandem repeat (STR) analysis (14) or a sex chromosome analysis (10) to demonstrate donor-derived lymphocyte infiltration has also been reported to be useful for diagnosing chronic GVHD.
Table.
The Characteristics of the Patients with Chronic GVHD of the Central Nervous System after HCT.
| Reference No. |
Age/ Sex |
Primary disease |
Type of HCT |
Post-HCT period (months) |
Other cGVHD |
Radiological findings |
Pathological findings | Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 7 | 32/M | CML | BMT | 18 | none | ND | perivascular monocytosis | ND | dead |
| 11 | 9/M | AA | BMT | 8 | none | CA | perivascular CD3+ cell infiltration | ND | dead |
| 13/F | ALL | BMT | 3 | lung GIT | abnormal signaling of cerebrum | perivascular CD3+ cell infiltration | ND | dead | |
| 12 | 43/M | CML | BMT | 18 | skin liver | WM lesions | brain edema, hematoma, multifocal distribution of inflammatory infiltrations of blood vessel walls and perivascular areas | CPM PSL | improved |
| 14 | 18/F | AML | BMT | 2 | skin | leukoencepha lopathy; CA | angiitis | MP | Improved |
| 21 | 44/F | ATLL | allo- BMT | 18 | skin | infiltrating lesion in WM with CE | parenchymal perivascular CD3+ cell infiltration | MP | Improved |
| 58/F | Ph+ B-ALL | allo- HCT | 15 | skin GIT | patchy WMHs | leptomeningeal perivascular CD3+ cell infiltration | MP | Improved | |
| 16 | 41/M | FL | allo- HCT | 18 | skin mouth eyes | multiple round lesions with CE | perivascular CD3+ cell infiltration, noncaseating granulomas | Dex CyA | Improved |
| 15 | 56/M | NHL | allo- PBSCT | 39 | pharynx eyes skin liver | multiple T2 WMHs | perivascular CD3+ cell infiltration, activation of microglia | MP PSL MMF | worsened |
| 10 | 35/M | CML | allo- BMT | 45 | skin liver | multiple T2 WMHs | microangiitis | PSL CPM MTX | improved |
| 28/F | AML | allo- BMT | 37 | none | multiple T2 WMHs with perivascular CE | angiitis | MP PSL CPM | improved | |
| 20/M | SCID | allo- BMT | 220 | none | multiple T2 WMHs; CE at meninges | angiitis | Dex MP CPM | improved | |
| 33/M | CLL | allo- BMT | 24 | skin liver GIT | CA | Parenchyma, leptomeningeal angiitis | MP CPM | improved | |
| This case | 46/F | AML | allo- PBSCT | 19 | lung | multiple T2 WMHs with perivascular CE | perivascular CD3+ cell infiltration, noncaseating granulomas | MP PSL FK506 | improved |
M: male, F: female, AA: aplastic anemia, ATLL: adult-onset T-cell lymphoma, AML: acute myeloid leukemia, B-ALL: B-cell acute lymphoblastic leukemia, BMT: bone marrow transplantation, CA: cerebral atrophy, CDVP: combination therapy of cisplatin, dacarbazine, vinblastine, and prednisolone, CE: contrast enhancement, CHOP: combination therapy of cyclophosphamide, doxorubicin, vincristine, and prednisolone, CLL: chronic lymphocytic leukemia, CML: chronic myelocytic leukemia, CPM: cyclophosphamide, CyA: cyclosporin A, Dex: dexamethasone, FK506: tacrolimus, FL: follicular lymphoma, GIT: gastrointestinal tract, HCT: hematopoietic cell transplantation, HIAs: hyperintense areas, MMF: mycophenolate mofetil, MP: methylprednisolone, ND: not described, NHL: Non-Hodgkin lymphoma, WM: white matter, WMH: white matter hyperintensity, PBSCT: peripheral blood stem cell transplantation, PSL: prednisolone, SCID: severe combined immunodeficiency disease, T2: T2-weighted imaging
Brain MRI often revealed multiple hyperintense lesions, showing signs of healing, that were predominantly located in the white matter, particularly in common sites of lacunar infarction (12). There is also a report of a patient with chronic GVHD who presented only brain atrophy at an earlier stage of the disease (14). After hematopoietic cell transplantation, leukoencephalopathy can also be caused by many immunosuppressants, radiation therapy, and opportunistic infections. The magnetic resonance appearance of these forms of toxic leukoencephalopathy involves symmetric hyperintense lesions in the white matter on T2WI and FLAIR, which are associated with the foci of restricted diffusion within these areas. Typically, there is no associated contrast enhancement (23). The perivascular spaces (Virchow-Robin spaces) are not normally enhanced with gadolinium (24). In the present case, punctate and curvilinear gadolinium enhancement (PCGE) was found along the path of the perforating medullary arteries. Similar findings were also reported by Sostak et al (10). PCGE may be seen when the blood-brain barrier (BBB) of the small vessels is disrupted, either by the direct injury of the endothelial cells or by angiocentric infiltrates composed of various combinations of T lymphocytes, B lymphocytes, and histiocytes. A brain biopsy should be performed to determine the phenotypes of the infiltrating cells and assess EBV reactivation to distinguish between posterior reversible encephalopathy syndrome (PRES), primary angiitis of the CNS (PACNS), demyelinating disease, immune reconstitution inflammatory syndrome (IRIS), lymphomatoid granulomatosis (LYG), and chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) (25). The pathogenesis of CNS-cGVHD may be similar to these diseases, as is indicated by their similar radiological findings. In recent years, integrins have also been implicated in the pathogenesis of intestinal GVHD. Anti-alpha4 integrin therapy has now been approved by the Food and Drug Administration (FDA) for the treatment of multiple sclerosis and inflammatory bowel disease. Drugs that block the infiltration of leukocytes via the BBB could also be effective for CNS-cGVHD.
The post-transplantation survival periods are expected to increase with advancements in transplantation technology. It is important to bear in mind that patients receiving HLA-mismatched transplantation, patients with a history of calcineurin inhibitor-induced immune suppression, and patients receiving TBI, whole-brain irradiation, or intrathecal injections are at risk for developing late-onset neurological complications. As a result, neuropsychological tests must occasionally be performed to closely monitor the higher brain function and neurological symptoms of these patients (26). Chronic GVHD is particularly difficult to diagnose because it can develop over years and sometimes only involves the CNS. MRI is useful for detecting distinct lesions that are localized to the CNS. In other words, MRI gives us a clue as to whether a brain biopsy is required for a patient who is suspected of having chronic GVHD; this makes MRI the key to facilitating the early and definitive diagnosis of chronic GVHD and to delivering effective treatment in a timely manner.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
This work was supported by JSPS KAKENHI Grant number JP 26460901.
References
- 1.Jagasia MH, Greinix HT, Arora M, et al. . National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 21: 389-401, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rouah E, Gruber R, Shearer W, Armstrong D, Hawkins EP. Graft-versus-host disease in the central nervous system. A real entity? Am J Clin Pathol 89: 543-546, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Hartrampf S, Dudakov JA, Johnson LK, et al. . The central nervous system is a target of acute graft versus host disease in mice. Blood 121: 1906-1910, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaliyaperumal S, Watkins B, Sharma P, et al. . CD8-predominant T-cell CNS infiltration accompanies GVHD in primates and is improved with immunoprophylaxis. Blood 123: 1967-1969, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Openshaw H. Neurological manifestations of chronic graft versus host disease. In: Chronic Graft versus Host Disease. Vogelsang GB, Pavletic SZ, Eds. Cambridge University Press, New York, 2009: 243-251. [Google Scholar]
- 6.Grauer O, Wolff D, Bertz H, et al. . Neurological manifestations of chronic graft-versus-host disease after allogeneic haematopoietic stem cell transplantation: report from the Consensus Conference on Clinical Practice in chronic graft-versus-host disease. Brain 133: 2852-2865, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Marosi C, Budka H, Grimm G, et al. . Fatal encephalitis in a patient with chronic graft-versus-host disease. Bone Marrow Transplant 6: 53-57, 1990. [PubMed] [Google Scholar]
- 8.Provenzale JM, Graham ML. Reversible leukoencephalopathy associated with graft-versus-host disease: MR findings. AJNR Am J Neuroradiol 17: 1290-1294, 1996. [PMC free article] [PubMed] [Google Scholar]
- 9.Shortt J, Hutton E, Faragher M, Spencer A. Central nervous system graft-versus-host disease post allogeneic stem cell transplant. Br J Haematol 132: 245-247, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Sostak P, Padovan CS, Eigenbrod S, et al. . Cerebral angiitis in four patients with chronic GVHD. Bone Marrow Transplant 45: 1181-1188, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki Y, Sako K, Ohara Y, et al. . Subacute panencephalitis associated with chronic graftversus-host disease. Acta Neuropathol 85: 566-572, 1993. [DOI] [PubMed] [Google Scholar]
- 12.Padovan CS, Bise K, Hahn J, et al. . Angiitis of the central nervous system after allogeneic bone marrow transplantation? Stroke 30: 1651-1656, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo Y, Kamezaki K, Takeishi S, et al. . Encephalomyelitis mimicking multiple sclerosis associated with chronic graft-versus-host disease after allogeneic bone marrow transplantation. Intern Med 48: 1453-1456, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Ma M, Barnes G, Pulliam J, Jezek D, Baumann RJ, Berger JR. CNS angiitis in graft vs host disease. Neurology 59: 1994-1997, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Saad AG, Alyea EP 3rd, Wen PY, Degirolami U, Kesari S. Graft-versus-host disease of the CNS after allogeneic bone marrow transplantation. J Clin Oncol 27: e147-e149, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Kew AK, Macaulay R, Burrell S, Rubin S, Dow G, Couban S. Central nervous system graft-versus-host disease presenting with granulomatous encephalitis. Bone Marrow Transplant 40: 183-184, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Majhail NS, Rizzo JD, Lee SJ, et al. . Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone Marrow Transplant 47: 337-341, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azuno Y, Yaga K, Kaneko T, Kaku K, Oka Y. Chronic graft-versus-host disease and seizure. Blood 91: 2626-2628, 1998. [PubMed] [Google Scholar]
- 19.Solaro C, Murialdo A, Giunti D, Mancardi G, Uccelli A. Central and peripheral nervous system complications following allogeneic bone marrow transplantation. Eur J Neurol 8: 77-80, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Campbell JN, Morris PP. Cerebral vasculitis in graft-versus-host disease: a case report. AJNR Am J Neuroradiol 26: 654-656, 2005. [PMC free article] [PubMed] [Google Scholar]
- 21.Kamble RT, Chang CC, Sanchez S, Carrum G. Central nervous system graft-versus-host disease: report of two cases and literature review. Bone Marrow Transplant 39: 49-52, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Harvey CM, Gottipati R, Schwarz S, et al. . Acute disseminated encephalomyelitis following allo-SCT: central nervous system manifestation of GVHD. Bone Marrow Transplant 49: 854-856, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Rimkus Cde M, Andrade CS, Leite Cda C, et al. . Toxic leukoencephalopathies, including drug, medication, environmental, and radiation-induced encephalopathic syndromes. Semin Ultrasound CT MR 35: 97-117, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics 27: 1071-1086, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Taieb G, Duran-Peña A, deChamfleur NM, et al. . Punctate and curvilinear gadolinium enhancing lesions in the brain: a practical approach. Neuroradiology 58: 221-235, 2016. [DOI] [PubMed] [Google Scholar]
- 26.Majhail NS, Rizzo JD, Lee SJ, et al. . Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone Marrow Transplant 47: 337-341, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]