Abstract
Williams syndrome (WS) is a neurodevelopmental disorder associated with hypersociability and anxiety. However, little is known about how these salient aspects of the phenotype are related or their underlying physiology. We examined cortisol reactivity in WS because cortisol is responsive to psychosocial stress. Compared to typically developing adults, adults with WS had a significant cortisol decrease in response to a challenging cognitive battery. In contrast, cortisol levels in WS stayed stable in response to a solo musical performance, and baseline cortisol levels were significantly associated with musical skill. Results indicate that people with WS respond differentially to different socially-loaded situations. Implications for salience and arousal in cognitive and social situations are discussed.
Keywords: Williams syndrome, cortisol, stress, anxiety, music
Williams syndrome (WS), a neurodevelopmental disorder occurring in 1 in 7,500 births (Strømme, Bjørnstad, & Ramstad, 2002), is caused by the deletion of ~28 genes on chromosome 7q11.23 (Ewart et al., 1993). WS is increasingly studied by cognitive and affective neuroscientists because of its known genetic cause and its unique cognitive and behavioral characteristics. In particular, a hallmark of the WS phenotype is hypersociability (for a review, see Järvinen-Pasley et al., 2008). Based on their parents’ reports and clinical and laboratory observations, individuals with WS are described as friendly and outgoing (Gosch & Pankau, 1997; Jones et al., 2000). Children with WS will often interact with new children (Mervis et al., 2003) or adults (Dodd, Porter, Peters, & Rapee, 2010) without being prompted. Individuals with WS tend to rate photographs of happy faces as more approachable than typically developing individuals of similar chronological or mental age (e.g., Frigerio et al., 2006).
However, despite, or perhaps because of, their hypersociability, individuals with WS have difficulty forming and maintaining friendships (e.g., Dykens & Rosner, 1999; Gosch & Pankau, 1997). These difficulties may relate to challenges that people with WS have in modulating their behavior in response to peers, or to high levels of social worries such as fears of being teased or witnessing arguments (Dykens & Rosner, 1999; Dykens, 2003). Indeed, children and adults with WS endorsed items on a social threat scale such as “I'm afraid of what other kids will think of me” at the same rate as children with anxiety disorders (Dodd, Schniering, & Porter, 2009). Beyond these social concerns, people with WS often have generalized anxiety and nonsocial fears, and rates of anxiety disorders are much higher in WS compared to others with or without developmental disabilities (for a review see Dankner & Dykens, 2012).
Further insights into hypersociability and anxiety in WS can be gleaned from research approaches that use natural social interactions and physiological indices of arousal. For example, Doherty-Sneddon and colleagues (2009) found that compared to typical controls, 15 children with WS had decreased skin conductance levels (SCL), a measure of arousal, when answering math questions in front of an examiner. When thinking about math problems, those with WS also averted their gaze less often than controls, although both groups showed greater SCL when looking at the examiner than when looking away, and both modulated their gaze based on task difficulty (i.e., looking away more during more challenging versus easier math problems).
Despite the promise of such approaches, we know very little about the physiology associated with social behavior and anxiety in WS and how these two salient aspects of their phenotype may be linked. The hypothalamic-pituitary-adrenal axis (HPA), and its end product cortisol, is an appropriate point of study because the HPA is particularly responsive to psychosocial stressors (Dickerson & Kemeny, 2004). Additionally, cortisol output is determined by a feedback loop involving the amygdala, prefrontal cortex, and hippocampus (Dedovic, Duchesne, Andrews, Engert, & Pruessner, 2009), which are known to function atypically in WS (see Martens, Wilson, & Reutens, 2008 for a review).
In typically developing (TD) individuals, cortisol increases are particularly robust in situations of salient social evaluation (Dickerson & Kemeny, 2004). Such challenges may occur in contrived laboratory test settings (e.g., Trier Social Stress Test involving public speaking and mathematics; Kirschbaum, Pirke, & Hellhammer, 1993) or real-life competitions or performances (e.g., Rohleder, Beulen, Chen, Wolf, & Kirschbaum, 2007). Greater cortisol increases are seen, for example, when performing public speaking in front on an audience versus without an audience (Taylor et al., 2010); during a musical performance versus a rehearsal (Beck, Cesario, Yousefi, & Enamoto, 2000); during a dance competition versus dance training (Rohleder et al., 2007); and during a dance competition versus laboratory task (Rohleder et al., 2007).
Only two prior studies have examined cortisol levels in WS, and both found that similar to typical controls, the HPA in WS is indeed responsive to social situations and it also indexes anxiety (Lense, Tomarken, & Dykens, in press; Miodrag, Lense, & Dykens, 2012). Comparing diurnal cortisol in adults with WS across novel versus familiar settings, we found that cortisol levels in the novel setting were elevated only at times of higher social demands. Additionally, a greater cortisol awakening response was associated with anxiety and social problems. In a second study, cortisol levels in WS were found to decline each day in response to a mindfulness intervention, with the cortisol change predicted by the participants’ self-reported anxiety levels (Miodrag et al., 2012). However, cortisol reactivity to specific interpersonal stimuli has yet to be evaluated in WS. Thus, the first aim of this study was to examine cortisol levels and reactivity in people with WS in two different social situations. The second aim was to explore behavioral correlates of their HPA functioning.
In Study 1, we examined salivary cortisol levels in response to a battery of cognitive and behavioral tests in front of an experimenter. We were specifically interested in their performance on tests of long-term memory, as this is an area of debate within WS research (e.g., Brock, Brown, & Boucher, 2006; Jarrold, Baddeley, & Phillips, 2007; for a review, see Martens et al., 2008) and a robust literature in TD individuals reveals links between HPA activity and memory performance (e.g. Smeets, Otgaar, Candel, & Wolf, 2008; for a review, see Lupien et al., 2005). In TD individuals, an inverse U-function between cortisol levels and memory performance exists such that a moderate cortisol increase (particularly during memory consolidation) is associated with superior memory performance (Lupien et al., 2005; Smeets et al., 2008). To create a psychologically stressful environment for the laboratory tests, we aimed to capitalize on the desire to please others that is common in WS (Doyle, Bellugi, Korenberg, & Graham, 2004), as well as the tendency to become upset if not provided with reassurance (Papaeliou et al., 2011). The examiner instructed participants with what she wanted them to do, and that they needed to do their best, but then remained neutral throughout the testing and did not provide positive feedback. Additionally, between the initial learning phase of the memory task and the recall/recognition phase, we administered other neuropsychological tests that were not directly related to our hypotheses but that focused on areas of relative weakness for people with WS (e.g., visual-spatial functioning). Thus, we aimed to create a challenging, anxiety-provoking testing environment because it was difficult for the participants to do well at what the experimenter asked of them and they were not socially rewarded for what they accomplished. We compared cortisol levels in response to the testing situation between participants with WS and TD participants, as well as the relationships between cortisol and memory task performance in each group. Consistent with the literature in TD individuals, we hypothesized that participants in both the WS and TD groups who mounted moderate cortisol increases in the testing situation would perform better on the long-term memory task than those with nominal cortisol changes.
In contrast to Study 1, Study 2 created psychological stress by using tasks that people with WS often enjoy and that are areas of relative strength for them, specifically, musical performance. There is great variability in musical skill amongst people with WS, but regardless of skill, they are reported to engage in musical activities and emotionally respond to music at a level similar to or greater than typically developing controls (Lense & Dykens, 2011; Levitin, 2005). Study 2 therefore examined cortisol reactivity in attendees with WS at a music camp in response to a solo musical performance in front of a live audience of their peers and the camp music directors. Musical performance anxiety is relatively common among typically developing music students and professionals (Brugues, 2011) and has some overlap with social anxiety (Gorges, Alpers, & Pauli, 2007). Interestingly, among music students, increased musical skill is associated with greater musical performance anxiety, perhaps because of greater expectations from themselves and others (Osborne, Kenny, & Holsomback, 2005). Therefore, we hypothesized that participants with WS who were better musicians would have greater baseline cortisol levels (due to anticipation of the performance) and greater cortisol response to the performance.
Finally, in order to identify cortisol responses in individuals with WS across different types of stressful situations, we directly compared the cortisol response in participants with WS in Study 1 (memory test battery) versus Study 2 (musical performance). Importantly, participants with WS were enrolled in the music camp because of their considerable enjoyment and investment in music (conversely, no camper attended the program in order to take cognitive tests). Participants were also motivated to attend music camp for social reasons, to visit with others, and make new friends. We thus assumed that a musical performance in front of peers and music directors would be more salient to campers than cognitive tests. As such, we hypothesized that participants would manifest greater increases in cortisol in response to the musical performance (Study 2) than the memory test battery (Study 1).
Methods
Study 1: HPA Reactivity and Memory Participants
Study 1 included 19 adults with genetically confirmed WS and 13 TD control participants. According to a Cohen (1992) based power analysis, this sample size could detect medium-to-large effects. For example, a t-test between the two groups could detect an effect size of d = 0.71, falling between Cohen's guidelines for medium (d = 0.5) and large (d = 0.8) effects. Participants were recruited from a residential summer camp (WS) and the community (TD). Demographic and psychological information can be found in Table 1. There were no age or gender differences between the groups.
Table 1.
Demographics, Psychological Functioning, and Neuropsychological Test Scores (mean ± SD) in TD and WS groups in Study 1
| TD (n = 13) | WS (n = 19) | Difference | |
|---|---|---|---|
| Age (years) | 27.1 ± 3.6 | 27.8 ± 6.4 | t30 = .381, p = .706 |
| Sex (% male) | 38.5 | 52.6 | χ2 = .622, p = .430 |
| BDI-II | 2.8 ± 2.9 | 3.5 ± 4.1 | t30 = −.574, p = .570 |
| BAI | 3.2 ± 3.9 | 7.7 ±7.3 | t30 = 1.969, p = .058, d = 0.7 |
| K-BIT IQ | 115.4 ± 14.0 | 67.4 ± 13.8 | t30 = −9.585, p < .001, d = 3.5 |
| VMI [Age Equivalence (years)]a | 15.7 ± 3.3 | 5.6 ± 0.8 | U = 0.00, p < .001, d = 4.6 |
| Grooved Pegboard [time (s)]a,b | 122.2 ± 10.5 | 334.2 ± 37.9 | U = 0.00, p < .001, d = 7.0 |
BDI-II = Beck Depression Inventory-II
BAI = Beck Anxiety Inventory
VMI = Visual Motor Integration, 4th edition
Non-parametric statistics (Mann-Whitney U) used due to non-normality.
Due to concerns that allowing participants to complete the Grooved Pegboard would result in very different time durations for the neuropsychological battery for TD and WS participants, individuals were limited to 180 seconds per side on this task.
To assess baseline psychological functioning, parents of participants with WS completed the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) and Beck Anxiety Inventory (BAI; Beck & Steer, 1993) about their child, while TD participants completed the forms about themselves. Parents of individuals with WS have frequently been used as informants of their emotional functioning, including symptoms of anxiety and depression (e.g., Dodd et al., 2009; Dykens, Rosner, Ly, & Sagun, 2005; Lense et al., 2012). All participants had BDI-II scores in the minimal range and there was no difference in BDI-II scores between the WS and TD groups. While the majority of participants in both groups had BAI scores in the minimal range, there was a trend for WS participants to have higher anxiety levels than TD participants. None of the TD participants were on any medications. Eight of the WS participants used psychotropic or other medications with the potential to affect cortisol levels. However, cortisol values in participants using medications did not differ from those not using medications.
Both Study 1 and Study 2 were approved by the Institutional Review Board (IRB) of the university. Written informed consent was obtained from TD participants and parents of participants with WS. Individuals with WS provided written assent.
Procedure
All participants were tested individually by an examiner in a quiet room. For all but three participants with WS, examiners were new and unfamiliar to participants. Due to the diurnal change in cortisol, all participants were tested between 10 a.m.−5 p.m., with nothing to eat or drink except water in the hour prior to sample collection. After providing consent/assent, participants completed a baseline salivary cortisol sample by chewing on a Salivette (Sarstedt, Nümbrecht, Germany). Participants then completed the learning and immediate memory tests for the Faces and Words tasks (~5 minutes total; order of tasks counterbalanced among participants; see below for task information). After each immediate memory task, participants were told that they should try to remember the materials because they would be asked about them again later. During the retention interval, participants completed three neuropsychological tests: the Kaufman Brief Intelligence Test, second edition (KBIT-2; Kaufman & Kaufman, 2004); Grooved Pegboard (e.g., Heaton, Grant, & Matthews, 1992); and the Beery-Buktenica Developmental Test of Visual-Motor Integration, fourth edition (VMI; Beery, 1997). (Results and group differences on these tests can be found in Table 1.) Following these neuropsychological tests, participants completed the delayed memory test for the Faces and Words stimuli. They then provided a second salivary cortisol sample. The total elapsed time between cortisol samples was approximately 45 minutes. Thus, the second sample corresponded to cortisol levels approximately 20 minutes after completion of the initial learning and immediate memory tests, i.e., during the neuro-psychological test battery, because of the ~20 minute time delay between cortisol release and its availability in saliva (Kirschbaum, Wüst, Faig, & Hellhammer, 1992). This timing allowed us to index whether or not the neuropsychological test battery created a stressful experience (i.e., cortisol increase). Previous studies in TD individuals have indicated that post-learning stressors (e.g., cold pressor test; Cahill, Gorski, & Le, 2003; Smeets et al., 2008) precipitate a cortisol increase and modulate memory consolidation. Throughout the entire experiment, the examiner remained affectively neutral and did not engage in tangential conversation with the participants, redirecting them to the test materials as needed. Participants were told to try their best but were not given any praise or criticism for their performance.
Memory Stimuli and Testing
Faces
Twenty-four unique faces were selected from the Radboud Faces Database, a free database of Caucasian faces displaying prototypical neutral and emotional facial expressions (Langner et al., 2010). Twelve faces were used for the target learning stimuli, and 12 faces were used as distractors during the memory tests. An oval of the person's face was cut out to avoid any extra cues to identity such as hair color and style or head size. Ovals were sized to be 17.8 cm long by 11.4 cm wide. The faces were evenly divided between male and female and neutral, happy, and angry expressions. In the learning phase, participants were shown each of the 12 target faces for two seconds and instructed to study each face carefully to remember it. Directly following the learning phase, they were shown all 24 of the faces in a fixed order and responded whether or not it was a new face or one that they had seen in the learning phase. They were not given any feedback on their performance. In the delayed memory phase, they were again shown all 24 faces and responded whether or not it was one of the original faces from the learning phase.
Words
Three Deese-Roediger-McDermott (DRM)-style word lists of 12 words each were created. In a DRM word list, all the words are associated with each other and with a non-presented critical word, which is often falsely recalled during memory testing (Deese, 1959; Roediger & McDermott, 1995). The word lists in the present experiment were based on critical words of happy, anger, and music. Word lists were based on previously published word lists (Bauer, Olheiser, Altarriba, & Landi, 2009; Roediger & McDermott, 1995) and were matched for syllable lengths. In the learning phase, participants were read a list of words and instructed to repeat each word after the examiner to be sure they were attending to the stimuli. They then recalled as many words as they could from the list in any order. This was repeated with each word list (order of word lists counterbalanced among participants). In the delayed memory phase, participants were instructed to recall as many words as they could in any order.
Saliva Collection and Data Analysis
Saliva was collected using Salivettes (Sarstedt) and frozen at −80°C until assay. Samples were split and assayed in duplicate using the Alpco enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Alpco Diagnostics, Salem, NH). Intra-assay and inter-assay variability were 6.6 and 3.0%, respectively.
For the faces delayed memory test, performance was quantified via a’, a criterion-free measure of discriminability. a’ is a more accurate reflection of performance than simple number correct because it also takes into account correct rejections and false alarms. Two measures of delayed memory were computed for the words delayed memory test: total number correctly recalled and total number falsely recalled. To limit the number of analyses and control for Type I error, performance across all valences were collapsed, with follow-up analyses planned for each valence when there were significant findings for overall performance. The a’ values for faces and number of correct words recalled were compared between the WS and TD groups using Student's t-test. Due to non-normality, number of false words recalled was compared between groups using the Mann-Whitney nonparametric test.
One TD cortisol value was considered a potential outlier since it was greater than three standard deviations above the mean cortisol level, so analyses were conducted both including and excluding this participant. Participants were considered cortisol responders if they had at least 10% increase in cortisol values from baseline (Granger et al., 2006). χ2 tests were used to compare the percentage of cortisol responders in the WS and TD groups. To examine cortisol change, we computed a delta cortisol score for each participant by subtracting the baseline cortisol value from the post-testing cortisol value. We then computed Pearson correlations between baseline cortisol (natural log transformed for normality) and delta cortisol with memory performance for the WS and TD groups.
Results
The TD group outperformed the WS group on the delayed faces and words memory tests (mean faces a’: TD 0.66 ± 0.12 vs. WS 0.56 ± 0.09, t30 = −2.688, p = .012, Cohen's d = 0.94; words: TD 12.85 ± 4.74 vs. WS 4.32 ± 4.24, t30 = −5.327, p < .001, d = 1.9). There was no significant difference between the groups on the number of falsely recalled words (TD 3.31 ± 2.29 vs. WS 6.47 ± 7.26, Mann-Whitney U = 97.0, p = .323).
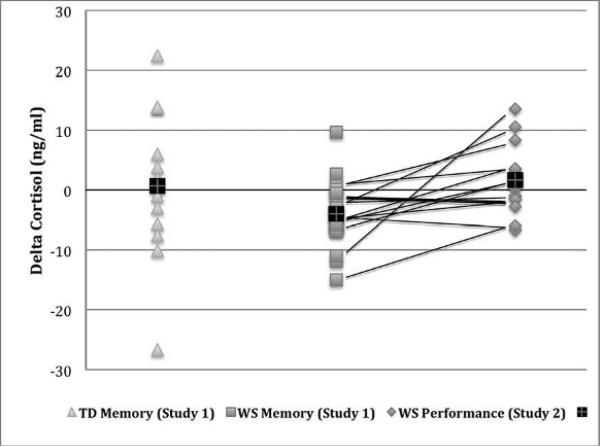
Cortisol values (using the natural log) can be found in Table 2 and individual cortisol change scores (raw delta cortisol) by group can be found in Figure 1 (left and center). Examination of the delta cortisol values revealed a different pattern of results in the TD and WS groups. Using a 10% increase from baseline as a criterion for change (Granger et al., 2006), 6 of 13 TD and 2 of 19 WS participants were considered cortisol responders (χ21 = 5.23, p = .022, ϕ = .40). The same pattern of results was observed when the potential TD outlier was excluded (6 of 12 TD responders vs. 2 of 19 WS responders: χ21 = 5.985, p = .014, ϕ =.44). There were no associations between baseline cortisol and memory performance in either the WS or TD group. In the TD group, there was a marginal positive relationship between cortisol change and delayed memory performance for words (r = 0.561, p = .046 including potential outlier participant; r = 0.405, p = .192 omitting potential outlier participant) but not faces. There were no associations between delta cortisol and memory performance in the WS group.
Table 2.
Average ln Transformed Cortisol Values (ng/mL) (Mean ± SD) for Study 1 (Memory) and Study 2 (Performance)
| TD Memory (n = 13) | WS Memory (n = 19) | WS Performance (n = 13) | |
|---|---|---|---|
| Baseline | 3.1 ± 0.7 | 2.9 ± 0.4 | 2.6 ± 0.5 |
| Post-task | 3.2 ± 0.5 | 2.7 ± 0.3 | 2.7 ± 0.6 |
Figure 1.
Individual raw cortisol change scores across the two studies. Scores above zero reflect an increase in cortisol from baseline to post-testing, while scores below zero reflect a cortisol decrease. Black crosses represent average change scores in each group. Significantly more TD than WS participants were cortisol responders (change score > 10%) in Study 1 (Memory; ϕ = .40). Among WS participants in both studies, there was a significantly greater cortisol decrease in Study 1 (Memory) than Study 2 (Musical Performance; Cohen's d = 0.67). Black lines connect individuals’ change scores across the two studies.
Study 2: HPA Reactivity and Musical Performance Participants
A subset of 13 participants with WS from Study 1 participated in Study 2. There was no difference in age, sex, BDI-II, BAI, musical skill, and musical background between those who participated in Study 2 and those who did not. All individuals on psychotropic medications from Study 1 also participated in Study 2 and used the same medications. Basic demographic, psychological functioning, and musical background and skill (see Measures section below) information can be found in Table 3.
Table 3.
Demographic Data (Mean ± SD) for Individuals with WS in Study 2 (n = 13)
| Age | 29.47 ± 6.35 |
| Sex (% male) | 61.5% |
| BDI-II | 2.77 ± 2.89 |
| BAI | 8.85 ± 7.07 |
| Pre-performance anxiety | 3.77 ± 2.55 |
| Musical Skill (%) | 65.65 ± 25.10 |
| Age began Music Lessons (years) | 10.20 ± 3.74 |
| Time spent playing music currently (hours) | 1.62 ± 1.67 |
| Time spent listening to music currently (hours) | 3.23 ± 2.89 |
Procedure
Study 2 occurred during a musical performance activity on the first evening at a residential summer music camp. Participants were instructed in advance to prepare a solo musical performance of their choice (any song, vocal or instrumental) of approximately three minutes. During the performance prior to their own, participants waited offstage with an unfamiliar researcher who observed the participant for behavioral signs of anxiety (see below). The researcher was affectively neutral and did not provide reassurance or praise the participant. At this time, the participant provided a baseline saliva sample. The participant then performed their musical solo in front of an audience of their peers at the camp, as well as the camp staff and music directors. The audience thus consisted of familiar and unfamiliar individuals, since the performance was when the participants were first meeting each other and the staff. Twenty minutes following their performance, they provided a post-performance saliva sample.
Measures
Musical background
Parents of all participants completed a musical background questionnaire with information about the participant's musical training and interest.
Musical skill
Three trained musical judges (music educators and/or semi-professional musicians) rated each participant's musical performance using Likert scales adapted from the music education literature (e.g., Holahan & Saunders, 1997). The scales assessed specific elements of musical performance (e.g., tempo, intonation, rhythmic accuracy, interpretation, etc.). Total scores were created by summing across all elements and converting to a percentage. The intraclass correlation among the three judges was 0.77 (absolute agreement).
Anxiety
For each participant, a trained researcher rated the presence (definite, somewhat, or absent) of 10 signs of performance anxiety (e.g., physically tense; shallow breathing; trembling (e.g., Kenny & Osborne, 2006; Osborne et al., 2005), as well as an overall rating of their anxiety levels on a one to five scale. A total summary score was computed to reflect the performance anxiety level of each participant.
Salivary cortisol
Saliva collection and assay was the same as in Study 1.
Data Analysis
Cortisol values and anxiety scores were natural log transformed for normality. Cortisol change (delta cortisol) was computed by subtracting baseline cortisol from post-performance cortisol. Baseline cortisol and delta cortisol variables were then correlated with musical skill, performance anxiety, and BAI.
Results
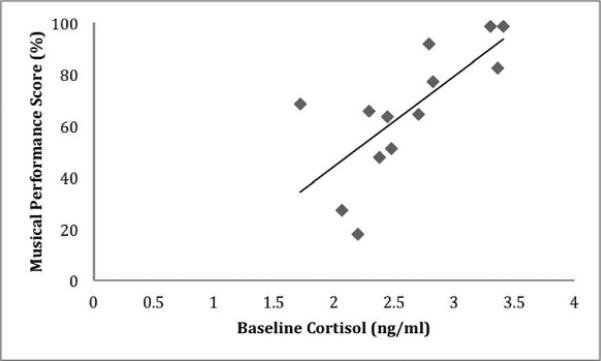
There was a wide variety in musical skill with performance scores ranging from 17.88 to 98.61%. Greater musical skill was associated with starting music lessons at a younger age (r = −.633, p = .049), exposure to multiple types of music lessons (r = .693, p = .009), and amount of time currently spent playing music (r = .756, p = .003), but not amount of time currently spent listening to music (r = −.064, p = .836). Cortisol values (ln transformed) and individual change scores can be found in Table 2 and Figure 1 (right), respectively. On average, there was no significant cortisol change in response to the musical performance (t12 = −0.453, p = 0.659, d = 0.07). However, there was much heterogeneity, with 5 of 13 participants exhibiting >10% cortisol increase from baseline to post-performance. Baseline cortisol was significantly positively correlated with musical skill (r = .725, p = .005; see Figure 2) but not with music listening nor anxiety. Higher baseline cortisol levels might reflect increased arousal levels in anticipation of their performance. Delta cortisol was not associated with musical skill, music listening, or anxiety levels.
Figure 2.
Association between baseline cortisol levels (ln transformed; ng/mL) and musical performance score (%). Individuals with higher musical skill had higher baseline cortisol levels (r = .725, p = .005).
Comparison between Study 1 and Study 2
Analyses
For the 13 participants who provided cortisol samples in both studies, we conducted a matched-pair t-test comparing their cortisol change values between the two studies.
Results
The raw cortisol change scores across the two studies for individual participants are depicted in Figure 1 (center, right). There was significantly greater cortisol decrease in Study 1 (memory battery; −4.65 ± 4.69 ng mL) versus Study 2 (musical performance; 0.84 ± 6.61 ng/mL) (t12 = −2.428, p = .032, d = .67).
Discussion
The HPA axis is particularly responsive to socio-evaluative stressors (Dickerson & Kemeny, 2004). Numerous studies in TD individuals have demonstrated this relationship across a variety of laboratory and natural settings, including public speaking (e.g., Bosch et al., 2009), musical performance (Beck et al., 2000; Fredrikson & Gunnarsson, 1992), ballroom dance competitions (Rohleder et al., 2007), and martial arts (Parmigiani et al., 2009). Across settings, absolute cortisol levels or cortisol reactivity is typically greater during a real versus simulated competition, performance versus rehearsal, and in the presence versus absence of an audience (regardless of the audience's supportiveness; Taylor et al., 2010), thus highlighting the role of social evaluation in the cortisol response.
This study revealed that adults with WS exhibit different patterns of cortisol reactivity in different types of socially-loaded situations. In a situation based around challenging laboratory/academic tasks in front of one examiner, individuals with WS had a marked cortisol decrease. This cortisol decrease was not seen in TD controls, and also contrasted with stable cortisol levels in response to a solo musical performance in front of an audience. Cortisol levels and reactivity in WS did not relate to their performance on a cognitive task, but did relate to their performance on a musical performance task.
In Study 1, the results of our TD participants mirrored those from other laboratories (e.g., Merz, Wolf, & Hennig, 2010; Schwabe, Bohringer, Chatterjee, & Schachinger, 2008; Smeets et al., 2008). Approximately half of the TD participants showed a cortisol increase in response to the memory testing and a cortisol increase was marginally associated with greater verbal memory. Though there were no associations with memory for faces, this may have been related to the difficulty of the faces task.
In contrast to controls, participants with WS did not mount a cortisol increase to a standard laboratory-testing situation and there were no associations between their cortisol response and memory performance. Indeed, the opposite unexpectedly occurred. Their cortisol decreased from baseline to post-testing, suggesting a marked lack of arousal to these cognitive tasks in front of an examiner. It may be that the socio-evaluation of the cognitive task performance was not salient enough in WS participants, as the TD literature indicates that the socio-evaluative nature of the cognitive task appears to be more important for precipitating a cortisol response than the cognitive task itself (Dickerson & Kemeny, 2004). It is unknown if a standard laboratory stress paradigm such as the Trier Social Stress Test (Kirschbaum et al., 1993) would have provided a stronger manipulation of the stress response in the WS group.
In contrast to Study 1, average cortisol levels in Study 2 stayed stable in response to the solo musical performances of individuals with WS. However, nearly 40% of individuals mounted a cortisol response. Though we did not see any associations between cortisol reactivity and performance factors, this may have been due to our use of other-ratings but not self-ratings of their performance. Previous studies have found that cortisol reactivity to a musical performance is associated with one's satisfaction with their own performance (Beck, Gottfried, Hall, Cisler, & Bozeman, 2006). Instead, we found that musical skill during their performance as rated by others was strongly associated with their baseline cortisol values. In contrast, time spent simply listening to music was not associated with their baseline cortisol values. It is plausible that for individuals with greater musical skill (associated with greater musical training and time spent playing music themselves), a “judged” musical performance is more salient than for individuals without musical training or with less skill (even if they spend time listening to music). Since participants knew about the performance in advance, there could have been greater anticipatory anxiety and arousal about the musical performance in those with more skill, leading to increased baseline cortisol. Nevertheless, when comparing the results across the two studies, the different pattern of cortisol responses suggests that people with WS respond differentially to different types of socially-loaded situations.
Interestingly, parents of individuals with WS frequently report that their children are not motivated to try to succeed at a task that is at all difficult for them (Mervis & John, 2010). Indeed, compared to those with Down syndrome, individuals with WS exhibit less mastery motivation (individual goal-directed behavior and pleasure in success) on both observational tasks and parent report, despite having higher cognitive and adaptive skills (Rowe, 2008). Though speculative, such findings, coupled with the differential cortisol responses in the two different study settings here, suggest that people with WS might benefit from strategies aimed at increasing the salience of challenging tasks. Although teachers and other professionals routinely enhance learner arousal or motivation, perhaps those with WS could additionally benefit from the well-established ability of music to induce different states of arousal (e.g., Grape, Sandgren, Hansson, Ericson, & Theorell, 2003; Krumhansl, 1997; Nakahara, Furuya, Francis, & Kinoshita, 2010). Anecdotally, people with WS show better attention during musical than non-musical tasks (Don, Schellenberg, & Rourke, 1999; Martens, Jungers, & Steele, 2011), and children with WS with musical training show better memory for information learned via singing then speaking (Martens et al., 2011). Even so, further studies are needed to determine if people with WS are particularly responsive to music as a way to modulate arousal, learning and memory.
There are several limitations to the current study. First, the relatively small sample sizes of the TD group in Study 1 and the WS group in Study 2 may have limited our ability to detect significant relationships. Nevertheless, the sample size was consistent with other neuropsychological and neuroimaging studies in WS (e.g., Riby & Hancock, 2009; Thornton-Wells et al., 2010), and studies of HPA activity in TD individuals in natural settings (Beck et al., 2006; Rohleder et al., 2007). Second, we only collected one baseline and one post-activity cortisol sample, so were not able to assess recovery of cortisol responses. As more time elapses, cortisol levels may rebound following cognitive testing situations, or decline from musical performances. We also did not formally measure behavioral signs of anxiety in Study 1 and we do not have information on exact time since eating or awakening, which could affect cortisol levels. Finally, the audience/observers between the two studies differed in regards to number of people and familiarity. However, the presence of a supportive versus unsupportive audience does not affect the stress response, which is instead more dependent upon the evaluative component of the performance (Taylor et al., 2010).
In conclusion, the current study demonstrated a unique pattern of cortisol activity in WS in different types of social situations. Findings underscore the need to use naturalistic, real-life settings in conjunction with laboratory-based studies when studying constructs such as social anxiety and sociability. These results, together with previous studies of cortisol in WS (Lense et al., in press; Miodrag et al., 2012), suggest that the HPA axis in people with WS is indeed responsive to social situations and anxiety. In contrast to TD individuals, those with WS exhibited a marked cortisol decline in response to a cognitive test situation. However, they exhibited stable cortisol levels in response to a solo musical performance. In particular, musical training and performance abilities were associated with higher baseline cortisol. Thus, future studies can utilize neuroendocrine measures to further elucidate the relationship between sociability and anxiety, two salient characteristics of the WS phenotype.
Acknowledgments
The authors thank the participants and their families for taking part in the study. They also thank Carol Holt for performing the cortisol assays. Study data were managed using Research Electronic Data Capture (REDCap) tools hosted at Vanderbilt (Harris et al., 2009). This work was supported in part by a National Science Foundation Graduate Research Fellowship and a grant from NICHD (P30 HD015052-30). The project was also supported by the National Center for Research Resources, Grant UL1 RR024975-01, which is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06.
Contributor Information
Miriam D. Lense, Department of Psychology and Human Development, 230 Appleton Place, Vanderbilt University, Nashville, TN, USA; Miriam.lense@vanderbilt.edu
Elisabeth M. Dykens, Vanderbilt University.
References
- Bauer LM, Olheiser EL, Altaribba J, Landi N. Word type effects in false recall: Concrete, abstract, and emotion word critical lures. The American Journal of Psychology. 2009;122(4):469–481. [PubMed] [Google Scholar]
- Beck R, Cesario T, Yousefi A, Enamoto H. Choral singing, performance perception, and immune system changes in salivary immunoglobulin A and cortisol. Music Perception. 2000;18(1):87–106. [Google Scholar]
- Beck RJ, Gottfried TL, Hall DJ, Cisler CA, Bozeman KW. Supporting the health of college solo singers: The relationship of positive emotions and stress to changes in salivary IgA and cortisol during singing. Journal for Learning Through the Arts. 2006;2(1):1–17. [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory Manual. Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Beery KE. The Beery-Buktenica developmental test of visual-motor integration. 4th ed. Modern Curriculum Press; New Jersey: 1997. [Google Scholar]
- Bosch JA, de Geus EJC, Carroll D, Goedhart AD, Anane LA, Veldhuizen van Zanten JJ, Edwards KM. A general enhancement of autonomic and cortisol responses during social evaluative threat. Psychosomatic Medicine. 2009;71(8):877–885. doi: 10.1097/PSY.0b013e3181baef05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J, Brown GDA, Boucher J. Free recall in Williams syndrome: Is there a dissociation between short- and long-term memory? Cortex. 2006;42(3):366–375. doi: 10.1016/s0010-9452(08)70363-0. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning and Memory. 2003;10(4):270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Dankner N, Dykens EM. Anxiety in intellectual disabilities: Challenges and next steps. International Review of Research in Developmental Disabilities. 2012;42:57–84. [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. NeuroImage. 2009;47(3):864–871. doi: 10.1016/j.neuroimage.2009.05.074. doi:10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Deese J. On the prediction of occurrence of particular verbal intrusions in immediate recall. Journal of Experimental Psychology. 1959;58(1):17–22. doi: 10.1037/h0046671. [DOI] [PubMed] [Google Scholar]
- de Veld DMJ, Riksen-Walraven JM, de Weerth C. The relation between emotion regulation strategies and physiological stress responses in middle childhood. Psychoneuroendocrinology. 2012;37:1309–1319. doi: 10.1016/j.psyneuen.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dodd HF, Porter MA, Peters GL, Rapee RM. Social approach in pre-school children with Williams syndrome: The role of the face. Journal of Intellectual Disability Research. 2010;54(3):194–203. doi: 10.1111/j.1365-2788.2009.01241.x. [DOI] [PubMed] [Google Scholar]
- Dodd HF, Schniering CA, Porter MA. Beyond behaviour: Is social anxiety low in Williams syndrome? Journal of Autism and Developmental Disorders. 2009;39:1673–1681. doi: 10.1007/s10803-009-0806-4. [DOI] [PubMed] [Google Scholar]
- Doherty-Sneddon G, Riby DM, Calderwood L, Ainsworth L. Stuck on you: Face-to-face arousal and gaze aversion in Williams syndrome. Cognitive Neuropsychiatry. 2009;14(6):510–523. doi: 10.1080/13546800903043336. [DOI] [PubMed] [Google Scholar]
- Don AJ, Schellenberg EG, Rourke BP. Music and language skills of children with Williams syndrome. Child Neuropsychology. 1999;5(3):154–170. [Google Scholar]
- Doyle TF, Bellugi U, Korenberg JR, Graham J. “Everybody in the world is my friend” hypersociability in young children with Williams syndrome. American Journal of Medical Genetics. Part A. 2004;124A(3):263–273. doi: 10.1002/ajmg.a.20416. [DOI] [PubMed] [Google Scholar]
- Dykens EM. Anxiety, fears, and phobias in persons with Williams syndrome. Developmental Neuropsychology. 2003;23(1–2):291–316. doi: 10.1080/87565641.2003.9651896. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Rosner BA. Refining behavioral phenotypes: Personality-motivation in Williams and Prader-Willi syndromes. American Journal of Mental Retardation. 1999;104(2):158–169. doi: 10.1352/0895-8017(1999)104<0158:RBPPIW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Rosner BA, Ly T, Sagun J. Music and anxiety in Williams syndrome: A harmonious or discordant relationship. American Journal on Mental Retardation. 2005;110(5):346–458. doi: 10.1352/0895-8017(2005)110[346:MAAIWS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ewart AK, Morris CA, Ensing GJ, Loker J, Moore C, Leppert M, Keating M. A human vascular disorder, supravalvular aortic stenosis, maps to chromosome 7. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3226–3230. doi: 10.1073/pnas.90.8.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farran EK, Jarrold C. Visuospatial cognition in Williams syndrome: Reviewing and accounting for the strengths and weaknesses in performance. Developmental Neuro-psychology. 2003;23(1–2):173–200. doi: 10.1080/87565641.2003.9651891. [DOI] [PubMed] [Google Scholar]
- Fredrikson M, Gunnarsson R. Psychobiology of stage fright: The effect of public performance on neuroendocrine, cardiovascular and subjective reactions. Biological Psychology. 1992;33(1):51–61. doi: 10.1016/0301-0511(92)90005-f. [DOI] [PubMed] [Google Scholar]
- Frigerio E, Burt DM, Gagliardi C, Cioffi G, Martelli S, Perrett DI, Borgatti R. Is everybody always my friend? Perception of approachability in Williams syndrome. Neuropsychologia. 2006;44(2):254–259. doi: 10.1016/j.neuropsychologia.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Gorges S, Alpers GW, Pauli P. Musical performance anxiety as a form of social anxiety? International Symposium on Performance Science. 2007;1:67–72. [Google Scholar]
- Gosch A, Pankau R. Personality characteristics and behaviour problems in individuals of different ages with Williams syndrome. Developmental Medicine and Child Neurology. 1997;39(8):527–533. doi: 10.1111/j.1469-8749.1997.tb07481.x. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Blair C, El-Sheikh M, Mize J, Lisonbee JA, Handwerger K. Integrating the measurement of salivary alpha-amylase into studies of child health, development, and social relationships. Journal of Social and Personal Relationships. 2006;23(2):267–290. [Google Scholar]
- Grape C, Sandgren M, Hansson L-O, Ericson M, Theorell T. Does singing promote well-being?: An empirical study of professional and amateur singers during a singing lesson. Integrative Physiological and Behavioral Science. 2003;38(1):65–74. doi: 10.1007/BF02734261. [DOI] [PubMed] [Google Scholar]
- Haas BW, Hoeft F, Searcy YM, Mills D, Bellugi U, Reiss A. Individual differences in social behavior predict amygdala response to fearful facial expressions in Williams syndrome. Neuropsychologia. 2010;48(5):1283–1288. doi: 10.1016/j.neuropsychologia.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Mills D, Yam A, Hoeft F, Bellugi U, Reiss A. Genetic influences on sociability: heightened amygdala reactivity and event-related responses to positive social stimuli in Williams syndrome. The Journal of Neuroscience. 2009;29(4):1132–1139. doi: 10.1523/JNEUROSCI.5324-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan Battery. Psychological Assessment Resources; Odessa, FL: 1992. [Google Scholar]
- Holahan JM, Saunders TC. Criteria-specific rating scales in the evaluation of high school instrumental performance. Journal of Research in Music Education. 1997;45(2):259–272. [Google Scholar]
- Jarrold C, Baddeley AD, Phillips C. Long-term memory for verbal and visual information in Down syndrome and Williams syndrome: Performance on the Doors and People test. Cortex. 2007;43(2):233–247. doi: 10.1016/s0010-9452(08)70478-7. [DOI] [PubMed] [Google Scholar]
- Järvinen-Pasley A, Bellugi U, Reilly J, Mills DL, Galaburda A, Reiss AL, Korenberg JR. Defining the social phenotype in Williams Syndrome: A model for linking gene, the brain, and behavior. Development and Psychopathology. 2008;20(1):1–35. doi: 10.1017/S0954579408000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Bellugi U, Lai Z, Chiles M, Reilly J, Lincoln A, Adolphs R. II. Hypersociability in Williams Syndrome. Journal of Cognitive Neuroscience. 2000;12(Suppl 1):30–46. doi: 10.1162/089892900561968. [DOI] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N. Kaufman Brief Intelligence Test. 2nd ed. American Guidance Service; Circle Pines, MN: 2004. [Google Scholar]
- Kenny DT, Osborne MS. Music performance anxiety: New insights from young musicians. Advances in Cognitive Psychology. 2006;2(2):103–112. [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier Social Stress Test”–A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsycho-biology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wüst S, Faig HG, Hellhammer DH. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. Journal of Clinical Endocrinology & Metabolism. 1992;75(6):1526–1530. doi: 10.1210/jcem.75.6.1464659. [DOI] [PubMed] [Google Scholar]
- Krumhansl CL. An exploratory study of musical emotions and psychophysiology. Canadian Journal of Experimental Psychology. 1997;51(4):336–353. doi: 10.1037/1196-1961.51.4.336. [DOI] [PubMed] [Google Scholar]
- Langner O, Dotsch R, Bijlstra G, Wigboldus DHJ, Hawk ST, van Knippenberg A. Presentation and validation of the Radboud Faces Database. Cognition and Emotion. 2010;24(8):1377–1388. [Google Scholar]
- Lense MD, Dykens EM. Musical interests and abilities in individuals with developmental disabilities. International Review of Research in Developmental Disabilities. 2011;41:265–312. [Google Scholar]
- Lense MD, Tomarken AT, Dykens EM. Diurnal cortisol profile in Williams syndrome in novel and familiar settings. American Journal on Intellectual and Developmental Disabilities. 2013;118:201–210. doi: 10.1352/1944-7558-118.3.201. [DOI] [PubMed] [Google Scholar]
- Levitin DJ. Musical behavior in a neurogenetic developmental disorder: Evidence from Williams Syndrome. Annals of the New York Academy of Sciences. 2005;1060:325–334. doi: 10.1196/annals.1360.027. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30(3):225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Martens MA, Jungers MK, Steele AL. Effect of musical experience on verbal memory in Williams syndrome: evidence from a novel word learning task. Neuropsychologia. 2011;49(11):3093–3102. doi: 10.1016/j.neuropsychologia.2011.07.016. doi:10.1016/j.neuropsychologia.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Martens MA, Wilson SJ, Reutens DC. Research Review: Williams syndrome: a critical review of the cognitive, behavioral, and neuroanatomical phenotype. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2008;49(6):576–608. doi: 10.1111/j.1469-7610.2008.01887.x. [DOI] [PubMed] [Google Scholar]
- Mervis CB, John AE. Cognitive and behavioral characteristics of children with Williams syndrome: implications for intervention approaches. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 2010;154C(2):229–248. doi: 10.1002/ajmg.c.30263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Morris CA, Klein-Tasman BP, Bertrand J, Kwitny S, Appelbaum LG, Rice CE. Attentional characteristics of infants and toddlers with Williams syndrome during triadic interactions. Developmental Neuropsychology. 2003;23(1–2):243–268. doi: 10.1080/87565641.2003.9651894. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Wolf OT, Hennig J. Stress impairs retrieval of socially relevant information. Behavioral Neuroscience. 2010;124(2):288–293. doi: 10.1037/a0018942. [DOI] [PubMed] [Google Scholar]
- Miodrag N, Lense MD, Dykens EM. A pilot study of a mindfulness intervention for individuals with Williams syndrome: Physiological outcomes. Mindfulness. 2013;4(2):137–147. doi 10.1007/s12671-012-0178-2. [Google Scholar]
- Nakahara H, Furuya S, Francis PR, Kinoshita H. Psycho-physiological responses to expressive piano performance. International Journal of Psychophysiology. 2010;75(3):268–276. doi: 10.1016/j.ijpsycho.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Osborne MS, Kenny DT, Holsomback R. Assessment of music performance anxiety in late childhood: A validation study of the Music Performance Anxiety Inventory for Adolescents (MPAI-A). International Journal of Stress Management. 2005;12(4):312–330. [Google Scholar]
- Papaeliou C, Polemikos N, Fryssira E, Kodakos A, Kaila M, Yiota X, Vrettopoulou M. Behavioral profile and maternal stress in Greek young children with Williams syndrome. Child: Care, Health and Development. 2011;38(6):844–853. doi: 10.1111/j.1365-2214.2011.01306.x. doi:10.1111/j.1365-2214.2011.01306.x. [DOI] [PubMed] [Google Scholar]
- Parmigiani S, Dadomo H, Bartolomucci A, Brain PF, Carbucicchio A, Costantino C, Volpi R. Personality traits and endocrine response as possible asymmetry factors of agonistic outcome in karate athletes. Aggressive Behavior. 2009;35(4):324–333. doi: 10.1002/ab.20306. [DOI] [PubMed] [Google Scholar]
- Riby D, Hancock PJB. Looking at movies and cartoons: Eye-tracking evidence from Williams syndrome and autism. Journal of Intellectual Disability Research. 2009;53(2):169–181. doi: 10.1111/j.1365-2788.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: Remembering words not presented in lists. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21(4):803–814. [Google Scholar]
- Rohleder N, Beulen SE, Chen E, Wolf JM, Kirschbaum C. Stress on the dance floor: the cortisol stress response to social-evaluative threat in competitive ballroom dancers. Personality and Social Psychology Bulletin. 2007;33(1):69–84. doi: 10.1177/0146167206293986. [DOI] [PubMed] [Google Scholar]
- Rowe ML. Mastery motivation in young children with Williams syndrome and Down syndrome. University of Louisville; 2007. Unpublished doctoral dissertation. [Google Scholar]
- Schwabe L, Bohringer A, Chatterjee M, Schachinger H. Effects of pre-learning stress on memory for neutral, positive and negative words: Different roles of cortisol and autonomic arousal. Neurobiology of Learning and Memory. 2008;90(1):44–53. doi: 10.1016/j.nlm.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Smeets T, Otgaar H, Candel I, Wolf OT. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology. 2008;33(10):1378–1386. doi: 10.1016/j.psyneuen.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Strømme P, Bjørnstad PG, Ramstad K. Prevalence estimation of Williams syndrome. Journal of Child Neurology. 2002;17:269–271. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Seeman TE, Eisenberger NI, Kozanian TA, Moore AN, Moons WG. Effects of a supportive or an unsupportive audience on biological and psychological responses to stress. Journal of Personality and Social Psychology. 2010;98(1):47–56. doi: 10.1037/a0016563. [DOI] [PubMed] [Google Scholar]
- Thornton-Wells TA, Cannistraci CJ, Anderson AW, Kim C-Y, Eapen M, Gore JC, Blake R, et al. Auditory attraction: activation of visual cortex by music and sound in Williams syndrome. American Journal on Intellectual and Developmental Disabilities. 2010;115(2):172–189. doi: 10.1352/1944-7588-115.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Phillips ML, Brammer MJ, Skerrett D, Lagopoulos J, Rennie C, Gordon E. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. NeuroImage. 2001;14(5):1070–1079. doi: 10.1006/nimg.2001.0904. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research. Brain Research Reviews. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]