Abstract
Maintenance of neural circuit activity requires appropriate regulation of excitatory and inhibitory synaptic transmission. Recently, glia have emerged as key partners in the modulation of neuronal excitability; however, the mechanisms by which glia regulate neuronal signaling are still being elucidated. Here, we describe an analysis of how Ca2+ signals within Drosophila astrocyte-like glia regulate excitability in the nervous system. We find that Drosophila astrocytes exhibit robust Ca2+ oscillatory activity manifested by fast, recurrent microdomain Ca2+ fluctuations within processes that infiltrate the synaptic neuropil. Unlike the enhanced neuronal activity and behavioral seizures that were previously observed during manipulations that trigger Ca2+ influx into Drosophila cortex glia, we find that acute induction of astrocyte Ca2+ influx leads to a rapid onset of behavioral paralysis and a suppression of neuronal activity. We observe that Ca2+ influx triggers rapid endocytosis of the GABA transporter (GAT) from astrocyte plasma membranes, suggesting that increased synaptic GABA levels contribute to the neuronal silencing and paralysis. We identify Rab11 as a novel regulator of GAT trafficking that is required for this form of activity regulation. Suppression of Rab11 function strongly offsets the reduction of neuronal activity caused by acute astrocyte Ca2+ influx, likely by inhibiting GAT endocytosis. Our data provide new insights into astrocyte Ca2+ signaling and indicate that distinct glial subtypes in the Drosophila brain can mediate opposing effects on neuronal excitability.
Keywords: astrocyte, Ca2+, Drosophila, GABA, GAT, Rab11
Significance Statement
Complex brain functions require precise control of neuronal activity, which is often disrupted in neurologic disorders such as epilepsy. Here, we show that Drosophila astrocytes exhibit endogenous spontaneous Ca2+ oscillatory activity. Acute elevation of astrocyte Ca2+ suppresses neuronal activity and causes rapid paralysis. This is accompanied by a rapid reduction in the levels of membrane GABA transporter (GAT), a transporter for the inhibitory neurotransmitter GABA. We identify Rab11 as a novel regulator of GAT trafficking that is required for this form of Ca2+-dependent astrocyte regulation of neuronal activity. Our study provides new insight into the acute regulation of neuronal activity by glia.
Introduction
The regulation of excitatory and inhibitory balance is critically important to neuronal circuit output. Mounting evidence indicates that glial cells are key regulators of neuronal activity. In particular, astrocytes have been suggested to modulate neuronal output via glia-neuron gap junctions (Nedergaard, 1994; Alvarez-Maubecin et al., 2000) or through the release of gliotransmitters, including glutamate, adenosine/ATP, and D-serine (Parpura et al., 1994; Henneberger et al., 2010; Harada et al., 2015; Ma et al., 2016). Despite being nonexcitable cells, astrocytes exhibit robust Ca2+ dynamics (Volterra et al., 2014; Bazargani and Attwell, 2016; Shigetomi et al., 2016). Early work in the field demonstrated that astrocytes could detect released neurotransmitters and initiate slow somatic Ca2+ waves secondary to Ca2+ release from internal stores (Cornell-Bell et al., 1990; Dani et al., 1992). However, a key role of this slower astrocytic Ca2+ oscillatory activity has fallen out of favor following the finding that animals lacking inositol trisphosphate receptor in astrocytes, which lack this type of glial calcium signaling, have relatively normal brain function (Petravicz et al., 2008; Agulhon et al., 2010). More recently, a form of faster, localized microdomain astrocyte Ca2+ oscillatory activity was discovered (Shigetomi et al., 2010). Such Ca2+ fluctuations are mainly contributed through influx of extracellular Ca2+ (Shigetomi et al., 2012; Rungta et al., 2016) and may represent a more important Ca2+ signaling pathway for astrocytes to regulate neural activity (Shigetomi et al., 2013).
Astrocytes also control neural activity through their function in neurotransmitter uptake. In the “tripartite synapse” model, astrocyte terminals remove neurotransmitters and terminate their action via neurotransmitter transporters (Eulenburg and Gomeza, 2010). Astrocyte uptake of GABA, the primary inhibitory neurotransmitter, via GABA transporters (GATs) is critical for proper regulation of the activity of neuronal circuits (Minelli et al., 1996). In humans, mutations in GAT that disrupt GABA uptake are associated with severe forms of epilepsy (Carvill et al., 2015). GAT has been shown to exhibit a very high turnover rate, with a third of the protein residing in a recycling pool (Wang and Quick, 2005), implying that regulation of GAT plasma membrane expression may serve as an acute mechanism to modulate neuronal activity via changes in the rate of GABA uptake. Several factors involved in GAT trafficking have been identified (Scimemi, 2014), but the mechanisms regulating fast turnover of GAT remain elusive.
Drosophila has several classes of glial cells (Freeman, 2015). Astrocyte-like glia (hereafter referred to as astrocytes) and cortex glia are the two main subtypes found intimately associated with neurons in the CNS (Kremer et al., 2017). It has recently been shown that Drosophila cortex glia exhibit near-membrane microdomain Ca2+ oscillatory activity (Melom and Littleton, 2013). Mutations in Zydeco, a glial-specific K+-dependent Na+/Ca2+ exchanger (NCKX), eliminate microdomain Ca2+ oscillations in cortex glia and lead to higher intracellular Ca2+ levels. This increase in cortex glial Ca2+ leads to enhanced seizure susceptibility, as does acute induction of Ca2+ influx through ectopic expression of TRP channels in cortex glia (Melom and Littleton, 2013). These observations have led to a model whereby increased Ca2+ influx into cortex glia leads to neuronal hyperexcitability. However, it is unknown whether Drosophila astrocytes also exhibit Ca2+ activity and whether astrocyte Ca2+ signals have a similar role in exciting neighboring neurons. In the present study, we report that Drosophila astrocytes exhibit spontaneous, microdomain Ca2+ transients, resembling those observed in their mammalian counterparts. Surprisingly, unlike cortex glia, acute Ca2+ influx in astrocytes causes behavioral paralysis and a rapid loss of neuronal activity. We find this suppression of neuronal activity is due in part to rapid GAT endocytosis from astrocyte membranes, leading to enhanced GABA levels in the synaptic cleft. We identify Rab11 as a key regulator of GAT trafficking downstream of astrocyte Ca2+ influx and find that reduction in GAT turnover via suppression of Rab11 function ameliorates the induced suppression of neuronal activity.
Materials and Methods
Drosophila stocks
All Drosophila stocks were maintained on standard media at room temperature (22°C). The following stocks were used: UAS-TrpA1 (BDSC 26263), Alrm-Gal4 (Doherty et al., 2009), UAS-Rab11-GFP (BDSC 8506), GAD1-Gal4 (Ng et al., 2002), UAS-Rab11-RNAi [#I, VDRC 22189 (Dietzl et al., 2007); #II, BDSC 27730 (Perkins et al., 2015)], UAS-Rab11N124I (Satoh et al., 2005), repo-Gal80 (Awasaki et al., 2008), and elav-Gal80 (Yang et al., 2009). To create a stable UAS-TrpA1-myc line, UAS > stop > TrpA1-myc flies (von Philipsborn et al., 2011) were crossed with βTub85D-FLP (BDSC 7196) to excise the transcriptional stop codon. For all experiments, flies of either sex were assayed.
Generation of transgenic flies
To construct the plasmid pBID-UASc-myrGCaMP6s, GCaMP6s cDNA (Addgene #40753) was PCR amplified by designing primers with restriction enzyme cutting sites added on each end. Plasmid pBID-UASc-myrGCaMP5G (Melom and Littleton, 2013) was digested with NotI and XbaI and ligated with GCaMP6s using standard procedure. Cloning was verified by sequence analysis (Genewiz). To generate transgenic Drosophila, plasmids were injected into embryos of a strain with a third chromosome attP docking site (BDSC 8622) by Bestgene.
Behavioral assays
For testing the temperature sensitive behavioral phenotype of adult flies, 2-d-old flies were used. A total of 10-12 flies were transferred to each vial and placed in a water bath held at the indicated temperature. The number of flies with motor impairment was observed and manually recorded for up to 5 min, with an interval of 15 s in the first minute and 30 s in the following 4 min. Paralysis of adult flies was defined by complete loss of movement. For RNAi screening conducted at 30°C, Alrm > TrpA1 flies do not show 100% penetration of the paralysis phenotype. In this case, the number of flies that showed severe motor impairment and were unable to climb up the vial wall were recorded at each time point. For testing the behavior of headless flies, heads of adult flies were severed by fine scissors under CO2 anesthetization. These flies were then allowed to recover at room temperature in a closed Petri dish for 1 h before being transferred to Petri dishes preheated to the indicated temperature.
Immunofluorescence
For immunofluorescence, 3rd instar stage larvae were dissected in HL3.1 (70 mM NaCl, 5 mM KCl, 10 mM NaHCO3, 4 mM MgCl2, 5 mM trehalose, 115 mM sucrose, and 5 mM HEPES; pH 7.2; Feng et al., 2004) and fixed for 10 min either in Bouin’s fixative or 4% paraformaldehyde. Following washes with PBST (PBS containing 0.1% Triton X-100), larval fillets were blocked for 30 min with 5% normal goat serum in PBST, then incubated with primary antibody overnight at 4°C. Fixed larvae were then washed with PBST. Secondary antibody incubation was performed at room temperature for 2 h. Samples were washed and mounted in vectashield (Vector Laboratories). Antibodies for immunostaining and their dilution were as follows: rabbit anti-GAT, 1:2000 (Stork et al., 2014); mouse anti-Repo, 1:100, (DSHB 8D12); mouse anti-Brp, 1:100 (DSHB NC82); mouse anti-GFP, 1:500 (Thermo Fisher Scientific 33-2600); DyLight 649-conjugated anti-horseradish peroxidase, 1:500 (Jackson ImmunoResearch); Alexa Fluor 488-conjugated goat anti-mouse, fluorecein isothiocyanate-conjugated goat anti-rabbit; and Alexa Fluor 546-conjugated goat anti-mouse, 1:500 (Thermo Fisher Scientific). Images were acquired on a Zeiss LSM700 Laser Scanning confocal microscope with a 40× 1.3 NA, or a 100× 1.3 NA oil-immersion objective (CarlZeiss).
Western blot analysis
Adult flies were snap frozen in liquid nitrogen and five heads per sample were collected and homogenized in 50 µl of 1× Laemmli loading buffer (60 mM Tris, pH 6.8; 10% glycerol; 2% SDS; 1% β-mercaptoethanol; and 0.01% bromophenol blue), followed by centrifugation at 16,000 × g for 10 min to clear the homogenate. Supernatant was analyzed by SDS-PAGE (Bio-Rad 456-1086) and transferred to nitrocellulose membrane. Membranes were blocked with blocking buffer (2% bovine serum albumin, 2% nonfat dry milk, 0.02% Tween 20 in TBS) and then incubated with appropriate antibodies for immunoblotting. Primary antibody incubation were performed with gentle shaking at 4°C overnight followed by four 5-min washes using TBS containing 0.01% Tween 20, secondary antibody incubation for 1 h at room temperature, and four more 5-min washes using TBS containing 0.01% Tween 20. Then the membrane was briefly rinsed with TBS, and the blot was visualized using an Odyssey infrared scanner (Li-Cor). Antibodies for Western blotting: rabbit anti-GAT, 1:5000 (gift from Marc Freeman; Stork et al., 2014); mouse anti-Tubulin, 1:60,000 (Sigma-Aldrich, clone B5-1-2). Alexa Fluor 680-conjugated anti-rabbit and Alexa Fluor 800-conjugated anti-mouse, 1:10,000 (Invitrogen). All antibodies were diluted using blocking buffer.
Electrophysiology
Extracellular recordings were obtained from dissected 3rd instar larvae using a fire-polished patch electrode. To record central pattern generator (CPG) activity, the larval brain was left intact and care was taken during dissection to avoid damaging segmental nerves. Recordings were amplified using an AxoClamp 2B amplifier and digitized via an Molecular Devices Digidata 1550 (Molecular Devices). Clampfit 6.1 software was used to record and process electrophysiological data. Larvae were constantly perfused via a gravity system with HL3.1 solution containing 1.5 mM Ca2+. Saline was heated using an in-line solution heater (Harvard Apparatus), controlled by a Dual Channel Bipolar Temperature Controller (CL-200A). Additionally, the recording dish was temperature controlled using a Peltier device regulated by a fan-based heat sink and a Dual Channel Temperature Controller (Koolance, Warner Instruments).
Live imaging of astrocyte Ca2+ transients
UAS-myrGCaMP6s was expressed in astrocytes using the Alrm-Gal4 driver. L2 stage larvae were mounted on their ventral side in a small drop of water on a 25 × 60 mm coverslip. A single layer of scotch tape was applied on each side of the larva as a spacer to avoid damaging the animal when an 18 × 18 mm coverslip was placed on top. An upright PerkinElmer Ultraview Vox spinning disk confocal microscope equipped with a high-speed EM CCD camera, and a 43× 1.3 NA oil objective was used for image acquisition. Velocity software was used for data analysis. When imaging, the assembly was flipped so that the ventral side of the larvae faced the objective. Time series were acquired at a speed of ∼9 Hz using a single optical plane in the neuropil layer of the larval ventral nerve cord (VNC).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6. Data are presented as mean ± SEM in all figures. Statistical analysis is summarized in Table 1. Superscript letters following p values are used to refer to the corresponding comparisons described in the table.
Table 1:
Statistical table
| Data structure | Type of test | Observed power (α = 0.05) | |
|---|---|---|---|
| a (Fig. 2A) | Non-normal distribution | Mann-Whitney test | 1 |
| b (Fig. 3A) | Normal distribution | Student’s t test | 1 |
| c (Fig. 3B) | Non-normal distribution | Mann-Whitney test | 1 |
| d (Fig. 3D) | Normal distribution | Student’s t test | 0.0838271 |
| e (Fig. 3G) | Non-normal distribution | Wilcoxon signed-rank test | 0.2684 |
| f (Fig. 4B) | Normal distribution | Student’s t test | 0.0975257 |
| g (Fig. 4B) | Normal distribution | Student’s t test | 0.999942 |
Results
Drosophila astrocytes show microdomain Ca2+ transients
Expression of a genetically encoded membrane targeted myristoylated Ca2+ indicator (GECI) in cortex glia, a type of glia that associate with neuronal cell bodies in the Drosophila CNS, has revealed that these cells display robust near-membrane microdomain oscillatory activities (Melom and Littleton, 2013). To determine whether astrocytes, the other major glial cell type closely associated with neurons in the Drosophila CNS, show similar Ca2+ activity, we generated transgenic lines expressing a myristoylated form of GECI, myrGCaMP6s, under the control of an astrocyte-specific promoter, Alrm-Gal4 (Fig. 1A; Doherty et al., 2009). We found that astrocytes display robust spontaneous Ca2+ oscillatory activity in membrane microdomains of processes that invaded the synaptic neuropil, largely resembling the Ca2+ transients observed in cortex glia (Fig. 1B; Movie 1). We also monitored astrocyte Ca2+ activity using standard dissected preparations bathed in HL3.1 saline with up to 4 mM Ca2+. Even when the dissections were completed in <5 min, and extreme care was taken to avoid damage to the CNS, astrocyte microdomain Ca2+ transients were rarely observed. Because these spontaneous astrocyte Ca2+ events were very sensitive to injury, we conducted Ca2+ imaging experiments in live, undissected L2 stage larvae that were confined between cover slips (see Materials and Methods).
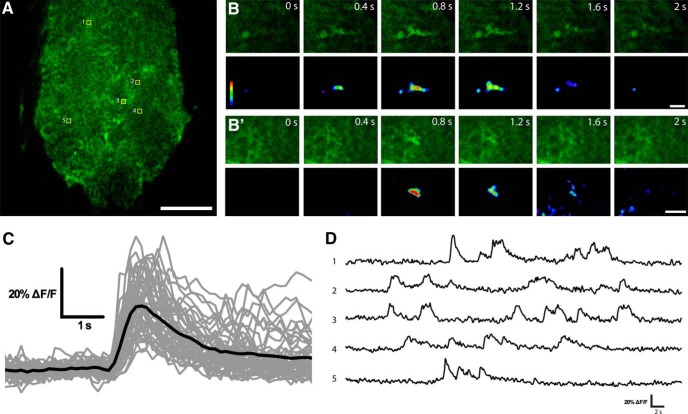
Figure 1.
Near-membrane Ca2+ activity in Drosophila astrocytes. A, Single confocal plane of larvae VNC showing the astrocyte-specific expression of myrGCaMP6s under the control of Alrm-Gal4. B, B’, Time-lapse image series of two microdomain astrocyte Ca2+ transients. C, Superimposition of astrocyte Ca2+ traces (in gray) and their average (black line; n = 46 individual traces). D, Sample traces of recurring Ca2+ transients in five different areas indicated in A. Scale bars 20 µm (A) and 5 µm (B and B’).
Drosophila astrocyte near-membrane Ca2+ activity detected with myrGCaMP6s. Ca2+ transients were recorded from the VNC of undissected 2nd instar larvae. Video speed, 2× real time. Scale bar, 20 µm.
Astrocyte near-membrane Ca2+ transients had an average duration of 3.69 ± 0.24 s, a half decay time of 1.00 ± 0.06 s, and a mean ΔF/Favg of 34.30 ± 1.70% (n = 46 individual Ca2+ oscillatory events). These events were similar to Ca2+ microdomains previously described in cortex glia, which had an average duration of 1.35 s, and a mean ΔF/Favg of 35% (Melom and Littleton, 2013). Recurrent Ca2+ transients within astrocyte processes were frequently observed in the same area (Fig. 1D), suggesting functional or structural subdomains where Ca2+ entry and removal are sequestered. Taken together, these results suggest that Drosophila astrocytes exhibit spontaneous Ca2+ oscillations that are similar to those previously described in cortex glia.
Acute astrocyte Ca2+ influx via ectopic TRPA1 activation leads to paralysis
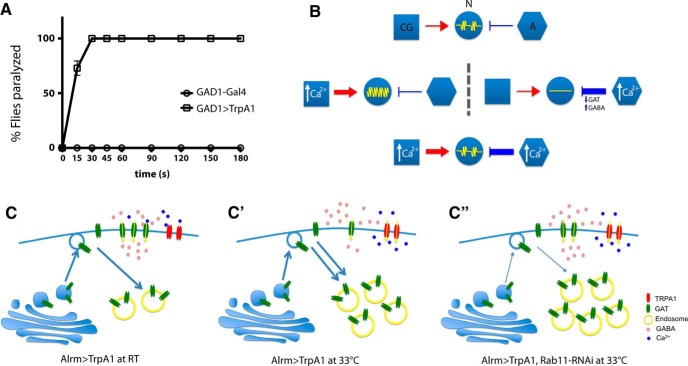
It has previously been shown that mutations in zyd, a Drosophila glial-specific K+-dependent Na+/Ca2+ exchanger (NCKX), led to elevated intracellular Ca2+ in cortex glia and enhanced seizure susceptibility, suggesting a role for glial Ca2+ signaling in regulating neuroexcitability (Melom and Littleton, 2013). We were interested in whether astrocyte Ca2+ signaling also regulates neural activity similar to that observed for cortex glia. To test this model, we used transgenic flies expressing TrpA1, a temperature-sensitive Ca2+ channel, under the control of a UAS promoter (UAS-TrpA1; Hamada et al., 2008). Prior studies expressing TrpA1 in cortex glia demonstrated this manipulation resulted in acute and robust seizure activity in neurons, with epileptic behavior observed in both larval and adult flies. When expressed using the astrocyte driver Alrm-Gal4, transgenic flies did not show any abnormality at room temperature (∼22°C). Strikingly, these animals became paralyzed within ∼30 s when acute Ca2+ influx was induced by shifting the temperature to 33°C, whereas control flies did not show any obvious behavioral defects (Fig. 2A; Movie 2). Similarly, acute astrocyte Ca2+ influx led to paralysis in 3rd instar stage Alrm > TrpA1 larvae but not in control animals (Movie 3). Pan-glial expression of Gal80, a Gal4 inhibitor, suppressed the paralysis in Alrm > TrpA1 flies, whereas pan-neuronal expression of Gal80 had no effect, indicating the observed paralysis was caused by acute Ca2+ influx into astrocytes rather than leaky expression in neurons (Fig. 2B).
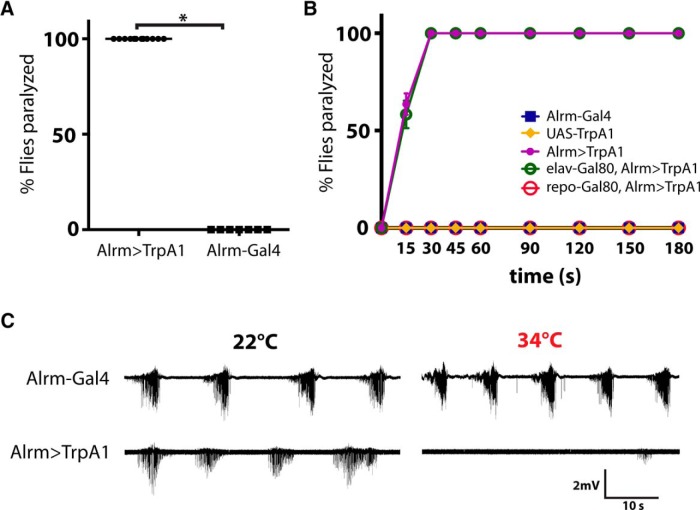
Figure 2.
Acute astrocyte Ca2+ influx through TrpA1 channels suppresses neuronal activity. A, All Alrm > TrpA1 flies became paralyzed within 30 s of exposure to 33°C, while control flies (Alrm-Gal4) show no behavioral defects. n > 80 flies per genotype; pa < 0.0001. B, Time course of paralysis in adult flies of the indicated genotypes on exposure to 33°C; repo-Gal80 suppresses the paralysis in Alrm > TrpA1 background, while elav-Gal80 has no effect. n > 70 flies for each genotype. C, Sample traces of CPG recording from larval NMJs. Postsynaptic potentials in Alrm > TrpA1 animals diminish during a temperature ramp from room temperature (22°C) to 34°C, while Alrm-Gal4 larvae maintain normal activity.
Astrocyte Ca2+ influx leads to suppression of neuronal activity in adult flies. Acute paralysis was induced in Alrm > TrpA1 flies when transferred to preheated water bath held at 33°C; control flies did not show obvious behavioral deficit. Video speed is real time.
Acute astrocyte Ca2+ influx leads to paralysis in Drosophila larvae. Third instar Alrm > TrpA1 larvae rapidly lose voluntary muscle contraction and became paralyzed following transfer to a preheated Petri dish held at 34°C. Control larvae show enhanced locomotion activity. Video speed is real time.
We next recorded CPG activity from the neuromuscular junction (NMJ) of 3rd instar stage larvae. Both control and Alrm > TrpA1 animals demonstrated normal spaced bursting activity that underlies larval locomotion at room temperature (Fig. 2C). However, when the temperature was ramped from ∼22°C to ∼34°C, neuronal activity in the Alrm > TrpA1 animals quickly diminished, whereas controls were unaffected (Fig. 2C). In contrast to cortex glia, our data indicate that acute astrocyte Ca2+ influx suppresses neuronal activity and leads to paralysis.
Acute astrocyte Ca2+ influx-induced paralysis does not require central brain function
In Drosophila, astrocytes extend fine processes and associate with synapses in the neuropil of both the central brain and the ventral ganglion (Muthukumar et al., 2014). The ventral ganglion is sufficient for conveying many motor functions. Headless flies with intact ventral ganglion survive for many hours and maintain posture, walk, and can learn to avoid electric shock (Booker and Quinn, 1981). It is possible that acute Ca2+ influx into astrocytes could suppress central brain function and cause motor impairment through descending neural circuit(s). Alternatively, the observed paralysis could be the consequence of direct interactions between astrocytes and local neurons in the ventral ganglion. To determine whether central brain function is necessary for conveying the astrocyte Ca2+ influx-induced paralysis, we severed the heads of adult flies and preformed behavioral tests on headless flies. Headless flies expressing TrpA1 in astrocytes rapidly became paralyzed when the temperature was shifted to 33°C (Movie 4). Therefore, acute Ca2+ influx in astrocytes within the ventral ganglion is sufficient to trigger paralysis; central brain function is dispensable for this process.
A candidate screen for modifiers of astrocyte Ca2+ influx-induced paralysis reveals a key role for Rab11 in GAT trafficking
To understand the mechanism of acute astrocyte Ca2+ influx-induced suppression of neuronal activity, we conducted a candidate RNAi screen to identify modifiers of the paralysis phenotype of Alrm > TrpA1 flies. Candidate UAS-RNAi constructs were expressed in astrocytes by crossing with Alrm > TrpA1 flies, and the progeny were subjected to behavioral analysis in vials placed in a heated water bath. The screen was conducted at a lower temperature of 30°C, at which ∼80% of the Alrm > TrpA1 became paralyzed, so that both suppressors and enhancers of motor impairment could be identified. We assembled a collection of ∼140 RNAi lines for proteins that are (1) involved in vesicle transport, (2) enriched in astrocytes, or (3) involved in regulation of Ca2+ signaling. Using this collection, we identified 13 RNAi lines that either enhanced or suppressed the motor impairments of Alrm > TrpA1 flies (Table 2).
Table 2:
A screen for modifiers of the TS paralysis phenotype of Alrm > TrpA1 flies
| Gene product | CG no. | Effect | Function | RNAi stock no. |
|---|---|---|---|---|
| Esyt2 | CG6643 | Enhance | ER-PM tethering | v28418, v28419 |
| SCAMP | CG9195 | Enhance | SV trafficking | v9130 |
| Nep4 | CG4058 | Enhance | Metalloendopeptidase | v16669, v100189 |
| Syt4 | CG10047 | Suppress | Vesicle trafficking | v33317, BL26730 |
| Syx1A | CG31136 | Suppress | SNARE; Vesicle trafficking | v33113 |
| Syt12 | CG10617 | Suppress | Vesicle trafficking | v47503 |
| α-SNAP | CG6625 | Suppress | SNARE disassembly | v101341, BL29587 |
| Rab11 | CG5771 | Suppress | Endosome trafficking | v22198, BL27730 |
Among the RNAi lines that suppressed the paralysis phenotype of Alrm > TrpA1 flies, Rab11-knockdown displayed the strongest effect (Fig. 3A; Alrm > TrpA1: 87.92 ± 2.043%; Alrm > TrpA1, Rab11-RNAi: 31.12 ± 4.813%; n > 100 flies for both groups; p b < 0.0001). We verified the RNAi knockdown result using a dominant-negative mutant form of Rab11, Rab11N124I (Satoh et al., 2005). Overexpression of UAS-Rab11N124I in astrocytes resulted in a comparable suppression of the paralysis in Alrm > TrpA1 flies (Fig. 3B; Alrm > TrpA1: 80.56 ± 7.425%; Alrm > TrpA1, Rab11-DN: 3.75 ± 2.192%; n > 45 flies for both groups; p c = 0.0095). Rab11 is a member of the Rab family of small-GTPases, and it is crucial for recycling endosome function and post-Golgi transport (Welz et al., 2014). It is possible that the reduced susceptibility to paralysis after suppression of Rab11 function in astrocytes is a result of impaired TrpA1 transport to astrocyte membranes and hence reduced Ca2+ influx on temperature shifts. However, following expression of a UAS-TrpA1-myc transgene, we did not observe alteration in TrpA1-Myc localization (Fig. 3C) or intensity with rab11 knockdown (Fig. 3D; control: 1 ± 0.0903, Rab11-RNAi: 0.9323 ± 0.0764, n = 8 single optical sections for each group, p d = 0.5761).
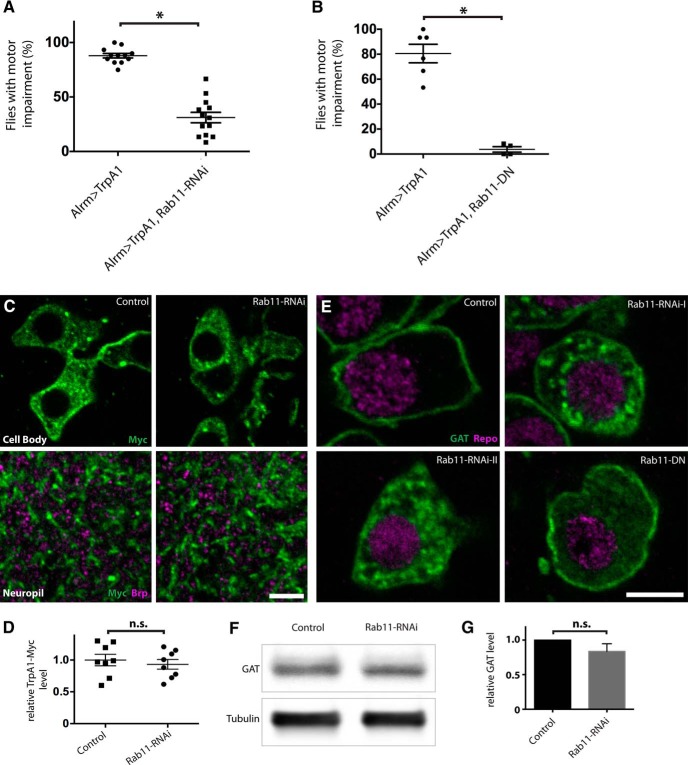
Figure 3.
Suppression of Rab11 function ameliorates astrocyte Ca2+ influx-induced behavioral defects and impairs GAT trafficking. A, Quantification of paralyzed flies when exposed to 30°C, showing rab11-knockdown suppressed the paralysis in Alrm > TrpA1 flies. n > 100 flies per genotype; pb < 0.00001. B, Quantification of paralyzed flies when Alrm > TrpA1 and Alrm > TrpA1, Rab11-DN flies were transferred to 30°C. n > 45 flies per genotype; pc = 0.0095. C, Single optical section showing astrocyte cell body and neuropil in the VNCs of Alrm > TrpA1-myc (control) and Alrm > TrpA1-myc, Rab11-RNAi (Rab11-RNAi) larvae stained with antibodies against Myc (green) and Brp (magenta). Note the apposition between synapses and astrocyte processes in the neuropil. D, Quantification of relative TrpA1-Myc level, normalized to control. n = 8 individual optical planes for each groups; p d = 0.5761. E, Optical sections showing GAT localization in astrocytes of larval VNCs stained with antibodies against GAT (green) and Repo (magenta) in control and when Rab11-RNAi or Rab11-DN is expressed in astrocytes. F, Western blotting of adult heads showing the effect of Rab11-RNAi expression in astrocytes on GAT level. G, Quantification of F. n = 6 experiments; p e = 0.3125. Scale bars in C and E, 5 µm.
One of the major functions of astrocytes is to uptake neurotransmitters following synaptic activity (Eulenburg and Gomeza, 2010). Drosophila astrocytes express GAT, the transporter responsible for synaptic clearance of the major inhibitory neurotransmitter GABA (Stork et al., 2014). It has been shown that membrane GAT levels are highly dynamic, and changes in GAT level can regulate neuronal excitability (Wang and Quick, 2005; Muthukumar et al., 2014). If GAT function or surface levels were suppressed by Ca2+ influx into astrocytes, one might predict an acute behavioral paralysis due to high synaptic GABA levels and a general suppression of neuronal excitability. We tested whether suppression of Rab11 alters the transport or localization of GAT. Indeed, Rab11-knockdown in astrocytes led to accumulation of large amounts of GAT-containing vesicles in the cytoplasm, although GAT is normally predominantly localized to the plasma membrane (Fig. 3E). Overexpression of Rab11N124I caused a similar defect in GAT localization (Fig. 3E). We also assayed whether suppression of Rab11 function altered the overall level of GAT. Compared with controls, rab11-knockdown animals have similar levels of overall GAT (Fig. 3F,G; control: 1, Rab11-RNAi: 0.8368 ± 0.1102, n = 6 experiments, p e = 0.3125). These data suggest that Rab11 is a novel regulator of GAT trafficking and likely ameliorates the acute astrocyte Ca2+ influx-induced paralysis of Alrm > TrpA1 flies by indirectly regulating GABA uptake.
Acute astrocyte Ca2+ influx leads to reduced membrane GAT
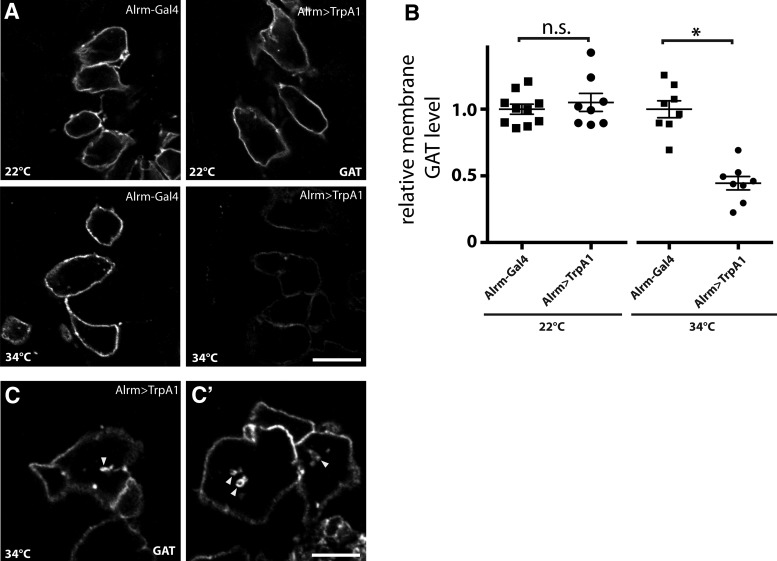
At room temperature, Alrm > TrpA1 larvae display normal GAT expression and localization to astrocyte plasma membrane processes (Fig. 4A,B; Alrm-Gal4: 1 ± 0.03759, Alrm > TrpA1: 1.051 ± 0.06748, n = 8-10 single optical sections for each group, p f = 0.4974). However, after subjecting animals to 33°C, Alrm > TrpA1 larvae showed a severe reduction of surface GAT expression compared with controls (Fig. 4A,B; Alrm-Gal4: 1 ± 0.06333, Alrm > TrpA1: 0.4451 ± 0.05023, n = 8 single optical sections for each group, p g < 0.0001). Large, GAT-containing membranous structures were frequently observed in the cytoplasm following acute Ca2+ influx in astrocytes, indicating Ca2+ elevation may drive GAT endocytosis (Fig. 4C).
Figure 4.
Astrocyte Ca2+ influx leads to reduced membrane GAT. A, Confocal images of larval VNC showing astrocyte membrane GAT level in control and Alrm > TrpA1 animals at room temperature (22°C) or 34°C. B, Quantification of relative GAT level shown in A, normalized to Alrm-Gal4 animals at each temperature. n = 8-10 individual optical planes for all groups; p f = 0.4974 and p g < 0.0001, respectively. C, C’, Confocal images with longer exposure showing GAT-containing vesicles (arrowheads) forming in the cytoplasm of Alrm > TrpA1 larvae exposed to 34°C. Scale bars in A and C, 5 µm.
These results suggest a Ca2+-dependent endocytotic pathway dynamically regulates membrane GAT levels. We hypothesize that excessive synaptic GABA due to the reduction in astrocyte surface GAT levels triggers the Ca2+ influx-induced paralysis. To test whether increased GABAergic signaling could lead to rapid paralysis, we expressed TrpA1 using a GABAergic neuron driver, GAD1-Gal4 (Ng et al., 2002). These flies became paralyzed within ∼30 s after being transferred to 30°C, indicating excessive GABA synaptic levels result in a strong suppression of neuronal activity and rapid behavioral paralysis in Drosophila (Fig. 5A).
Figure 5.
Excessive GABA leads to suppression of neuronal activity. A, Quantification of paralyzed flies on exposure to 30°C. Ectopic activation of GABAergic neurons via GAD1-Gal4-induced expression of TrpA1 leads to acute suppression of neuronal activity and paralysis. B, Distinct impact of cortex glia and astrocyte Ca2+ signals on neuronal activity. Ca2+ influx in cortex glia leads to increased neural activity and seizure-like behavior, whereas enhanced astrocyte Ca2+ signal causes suppression of neuronal activity and paralysis. Together, they constitute a Ca2+-dependent glial mechanism to fine-tune neuronal function. C-C”, Model of how astrocyte Ca2+ signaling regulates neuronal activity. Astrocyte Ca2+ influx leads to acute endocytosis of membrane GAT, reduced GABA uptake, and suppression of neuronal activity. Inhibition of Rab11 function reduces the removal of membrane GAT and sustains GABA uptake, hence ameliorating the paralysis caused by astrocyte Ca2+ influx.
Discussion
In the present study, we investigated Ca2+ influx in Drosophila astrocytes and the role of astrocyte Ca2+ signals in regulating neuronal activity. We found that similar to cortex glia (Melom and Littleton, 2013), Drosophila astrocytes exhibit spontaneous Ca2+ transients. However, in contrast to cortex glia, acute astrocyte Ca2+ influx leads to suppression of neuronal activity. Elevated cytoplasmic Ca2+ in astrocytes is associated with fast endocytosis of GAT and a reduction in surface expression, which is likely to disrupt GABA uptake and induce rapid paralysis. Suppression of Rab11 led to a disruption in GAT distribution and ameliorated the paralysis caused by astrocyte Ca2+ influx, revealing a novel mechanism of Rab11-dependent control of membrane GAT and neuronal activity.
Drosophila astrocytes demonstrate spontaneous microdomain Ca2+ activity
Current data indicate that astrocytes display diverse forms of intracellular Ca2+ oscillations through distinct cellular mechanisms (Bazargani and Attwell, 2016). Changes in astrocyte Ca2+ levels could be a response to neuronal activity. In isolated rat retinas, light flashing causes an increase in Ca2+ in retinal glial cells (Newman, 2005). In the cortex of anesthetized mice, astrocytes were also found to elevate intracellular Ca2+ in response to whisker stimulation (Wang et al., 2006). However, the Ca2+ oscillatory activities that we observe in Drosophila astrocytes appear to more closely resemble another form of fast, microdomain Ca2+ fluctuation, which has not yet been demonstrated to correlate with neuronal activity. In a rodent neuron-astrocyte coculture system, Ca2+ oscillations in astrocytes appeared to be mediated by spontaneous opening of TRPA1 channels independent of neuronal spiking (Shigetomi et al., 2012). Another study using acute mouse hippocampal brain slices also showed that pharmacological blockage of neuronal activity did not affect either the frequency or the amplitude of localized Ca2+ transients at fine processes of CA1 astrocytes (Rungta et al., 2016).
It is interesting that Ca2+ oscillations in Drosophila astrocytes bear remarkable similarity with those observed in cortex glia (Melom and Littleton, 2013), despite the distinction in the morphology between these two types of glial cells. The similar patterns of Ca2+ oscillations may suggest a common cellular mechanism for their origin. Unlike astrocytes, cortex glia cells are segregated from the synaptic neuropil and, instead, enwrap neuronal cell bodies in the cortex region of the CNS with their thin processes (Ito et al., 1995). It is unclear whether cortex glial might directly sense neuronal activity as a trigger for Ca2+ oscillations due to their lack of direct contact with chemical synapses. With the caveat that we could not use pharmacological methods to directly test the origin of astrocyte Ca2+ transients due to their sensitivity to injury, these microdomain Ca2+ fluctuations are likely a result of spontaneous glial channel opening rather than being directly triggered by neuronal activity.
Distinct functions of glial Ca2+ signals on neuronal activity
We find that acute Ca2+ influx into astrocytes leads to suppression of neuronal activity, an effect opposite to the enhancement of neuronal firing following elevated cortex glia Ca2+ signaling (Melom and Littleton, 2013). Although the exact mechanism is unclear, increased cortex glial Ca2+ level may trigger excessive neuronal activity through their ability to influence the environment around neuronal cell bodies, or through gliotransmission that activates surface receptors on neuronal cell bodies. Based on our results, astrocyte Ca2+ influx appears to balance cortex glia excitation through inhibition of neuronal activity, and together they constitute a glial mechanism to fine-tune the balance between excitatory and inhibitory signals to maintain normal neuronal excitability (Fig. 5B).
Perturbations in glial Ca2+ activity have been implicated in several forms of neurologic disorders (Nedergaard et al., 2010; Shigetomi et al., 2016). For example, it was found that both the basal astrocyte Ca2+ level and the frequency of somatic Ca2+ activity increase in a mouse model of Alzheimer’s disease, which might contribute to alterations in brain function (Kuchibhotla et al., 2009). Although we did not find evidence that the transient induction of astrocyte Ca2+ influx used in our study resulted in long-lasting pathology (data not shown), it will be interesting to explore the role of chronic increases in glial Ca2+ signals in the pathogenesis of neurologic diseases using the Drosophila model.
In the Drosophila CNS, astrocytes invade the entire neuropil with their meshwork of fine processes, and each astrocyte establishes unique and stereotypical territory (Muthukumar et al., 2014; Stork et al., 2014). Although much is known about the distinct origin and functions of astrocytes in different mammalian brain regions (Bayraktar et al., 2014), the functional heterogeneity and/or redundancy of astrocytes in Drosophila is still largely unexplored. Here, as a first step, we show that astrocytes in the ventral ganglion are sufficient to convey the suppression of neuronal activity caused by acute Ca2+ influx.
A mechanism regulating astrocyte membrane GAT and neuroactivity
Modulation of GABA uptake is an important glial mechanism to actively participate in the regulation of neuronal activity. It has been previously shown that global GAT levels in Drosophila astrocytes are developmentally regulated and sensitive to GABAergic neuronal output during synaptogenesis (Muthukumar et al., 2014). Suppression of metabotropic GABAB receptor signaling reduces GAT levels and suppresses seizure induction in hyperexcitable Drosophila seizure mutants, likely through dampening the rate of GABA uptake (Muthukumar et al., 2014).
Our study suggests the existence of a new mechanism for astrocytes to acutely regulate neuronal activity through fast modulation of GAT turnover. Compared with other known membrane proteins, GAT has one of the highest turnover rates (Tanner and Lienhard, 1987; Harwood and Pellarin, 1997; Menne et al., 2002; Wang and Quick, 2005). Plasma membrane GAT levels are dynamically regulated via a balance between fast endocytosis and exocytosis in primary rat cortical neuron cultures (Wang and Quick, 2005). Pharmacological activation of protein kinase C (PKC) can trigger enhanced endocytosis of GAT, leading to reduced membrane expression and reduction in GABA uptake (Wang and Quick, 2005). Here, our results suggest that astrocyte Ca2+ influx induces a rapid decrease in membrane GAT levels via endocytosis, which correlates with acute suppression of neural activity, representing a mechanism for glial cells to actively modulate neuronal function.
Our data reveal Rab11 as a novel regulator of GAT trafficking in Drosophila astrocytes. The accumulation of GAT-containing vesicles in rab11-knowdown animals likely represents a strong suppression of post-Golgi transport of GAT to the plasma membrane. However, it is important to note that suppression of Rab11 function did not totally abolish GAT localization to astrocyte membranes (Fig. 3D). Consistently, rab11-knowdown flies do not have strong locomotion defects like GAT-deficient animals (Stork et al., 2014). Indeed, it has been shown that GAT is delivered to the plasma membrane via multiple pathways (Reiterer et al., 2008). Since rab11-knockdown did not alter total GAT levels (Fig. 3E,F), constitutive membrane GAT in these animals seems sufficient to support baseline GABA uptake. Although primarily considered a regulator of recycling endosomes, Rab11 has been shown to promote endocytosis through indirect mechanisms. In the Drosophila eye, suppression of Rab11 function decreases the rate of endocytosis and eliminates the formation of endocytosis-dependent multivesicular bodies (Satoh et al., 2005). Ectopic expression of a dominant negative form of Rab11 also disrupts endocytosis in cultured Drosophila Garland cells (Satoh et al., 2005). Consistent with these observations, our data support a model in which suppression of Rab11 function blocks GAT endocytosis triggered by astrocyte Ca2+ influx, leading to sustained GABA uptake and a suppression of the paralysis and loss of neural activity normally observed following excessive astrocyte Ca2+ influx (Fig. 5C).
Taken together, our study reveals an important function of glial Ca2+ signaling in regulating neuronal activity. Acute modulation of the rate of neurotransmitter uptake through Ca2+-dependent glial signaling provides a robust mechanism for astrocytes to actively modulate the function of neural circuits.
Ca2+ influx in adult ventral ganglion astrocytes causes paralysis. Headless adult Alrm > TrpA1 flies show paralysis when transfer to a preheated Petri dish held at 33°C. The video started 30 s after the flies were transferred to the Petri dish. Video speed is real time.
Acknowledgments
Acknowledgements: We thank Marc Freeman, Tzumin Lee, Yuh Nung Jan, Akiko Satoh, the Developmental Studies Hybridoma Bank at the university of Iowa, the Bloomington Drosophila Stock Center at Indiana University, the Vienne Drosophila Resource Center, and the Drosophila stock center at National Institute of Genetics of Japan for providing reagents and flies strains.
Synthesis
Reviewing Editor: Maiken Nedergaard, University of Rochester Medical Center
Decisions are customarily a result of the Reviewing Editor and the peer reviewers coming together and discussing their recommendations until a consensus is reached. When revisions are invited, a fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision will be listed below. The following reviewer(s) agreed to reveal their identity: Leonard Khiroug
Synthesis of Reviews:
Your manuscript was well-received by both reviewers. The reviewers have raised several important questions that will need to be addressed. In addition, I will ask for a comparison of spontaneous and Ca2+ increases in transgenic flies expressing TrpA1 under the control of the temperature sensitive promoter. I worry that the calcium increases are pathological high and/or long-lasting and it will important to also address this point in the discussion.
Reviewer #1 Comments:
The article under review is well-written and advances the field of glial-neuronal communication in at least two ways: first, it provides a nice example of network fine-tuning via opposite effects of two sub-populations of glial cells (cortical glia and astrocyte-like glia) on neuronal activity; second, it provides novel mechanistic details on the link between astrocytic Ca2+ signaling and neuronal network silencing (via Rab11-mediated internalization of GAT resulting in increased extracellular GABA and neuronal inhibition).
There are no major concerns.
Minor concerns and suggestions are listed below:
1) Regarding the power analysis for the statistics shown in Fig.3F: is the group size (n=6) and the alpha value (0.2684, Table 1) sufficient to justify the conclusion that the overall GAT level is NOT changed by Rab11-RNAi? Please elaborate.
2) The use of the term "astrocyte-like glia" seems more appropriate than "astrocytes", however it is also too long for repeated use in the text; to address this, I suggest adding a sentence like "...Drosophila astrocyte-like glia (also referred to as "astrocytes" throughout this paper), ..."
3) In line 107, please add details on how exactly the behavior was recorded (was it a manual count/score? any automation of behavior analysis?)
4) In line 187, please explain in which manner did injury affect the spontaneous Ca2+ events (e.g., were they more frequent upon injury? did the time course of individual events change? etc)
5) While in general the article is very well written, I would like to suggest a couple of changes: first, on a number of occasions (e.g., lines 212, 240, 252, 304 and 544) the word "paralyze" is used in active voice, which does not make sense because flies "get paralyzed" or "become paralyzed" rather than "paralyze someone"; second, there are some mismatches between plural versus singular nouns (e.g., lines 4, 21, 64, etc).
6) Please add some quantification of TrpA1 membrane localization (similar to Fig.4B), to support the qualitative evidence shown in Fig.3C and the conclusion about lack of TrpA1 translocation.
7) I would recommend editing movies 2, 3 and 4 by adding a mark that indicates the start (and duration) of the temperature ramp from RT to 30-34C.
Reviewer #2 Comments:
In the paper entitled "Astrocyte Ca2+ Influx Negatively Regulates Neuronal Activity", Authors are suggesting that Ca influx in Drosophila astrocytes negatively regulates neural activity via GAT internalization through Rab11, caused transient paralysis.
The purpose of this study is potentially important in this research field. The manuscript is well written enough, however some of the points need to be addressed.
Suggestions are details as follows;
Major points
1 Authors mentioned astrocytic Ca2+ elevation trigger GAT internalization. I wonder the spatial distribution between Ca2+ hot spot and GABAergic terminal. In Fig3 and 4 authors showed that GAT internalization occurred through the whole cell. Does it happen through the down regulation of Rab11 activity in 30 sec? What is the mechanism that induced Rab11 inactivation through the local Ca2+ elevation.
2 If so, how does local Ca2+ elevation trigger the down regulation of Rab11
3 GAT expression in Fig3 and 4 seemed to be different. Fig 3 with Rab11 inhibition seemed to have GAT expression in cell soma but seem to have weak staining or less expression in Fig4. Author should mention for the reason.
4 Author should quantitatively measure the membrane expression of GAT or expression of Rab11 with the activation of astrocyte.
5 If possible, author have better to take correlation between Ca2+ level and GAT internalization. For instance, see if they have any correlation with the frequency of Ca2+ transients and GAT staining at membrane.
Minor points
1. Authors lack the Figure number
2. In Fig1, author should put the outline of astrocyte to make recognize cell contour.
3. Authors have better to compare the Ca2+ in Alrm-Gal4 transgenic Drosophila at different temperature to confirm their activation.
4. Author should show better staining for neuropils in Fig3 to observe their co-localization with astrocyte.
5. Misspelling "myrisotoylated (177)"
6. Misspelling "TrapA1 (line252)"
7. Misspelling "RNA11-RNAi (Fig5.C")"
References
- Agulhon C, Fiacco TA, McCarthy KD (2010) Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327:1250–1254. 10.1126/science.1184821 [DOI] [PubMed] [Google Scholar]
- Alvarez-Maubecin V, Garcia-Hernandez F, Williams JT, Van Bockstaele EJ (2000) Functional coupling between neurons and glia. J Neurosci 20:4091–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T, Lai SL, Ito K, Lee T (2008) Organization and postembryonic development of glial cells in the adult central brain of Drosophila . J Neurosci 28:13742–13753. 10.1523/JNEUROSCI.4844-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH (2014) Astrocyte development and heterogeneity. Cold Spring Harb Perspect Biol 7:a020362. 10.1101/cshperspect.a020362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazargani N, Attwell D (2016) Astrocyte calcium signaling: the third wave. Nat Neurosci 19:182–189. 10.1038/nn.4201 [DOI] [PubMed] [Google Scholar]
- Booker R, Quinn WG (1981) Conditioning of leg position in normal and mutant Drosophila . Proc Natl Acad Sci USA 78:3940–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvill GL, McMahon JM, Schneider A, Zemel M, Myers CT, Saykally J, Nguyen J, Robbiano A, Zara F, Specchio N, Mecarelli O, Smith RL, Leventer RJ, Moller RS, Nikanorova M, Dimova P, Jordanova A, Petrou S, EuroEPINOMICS Rare Epilepsy Syndrome Myoclonic-Astatic Epilepsy & Dravet working group, Helbig I, et al. (2015) Mutations in the GABA transporter SLC6A1 cause epilepsy with myoclonic-atonic seizures. Am J Hum Genet 96:808–815. 10.1016/j.ajhg.2015.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ (1990) Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247:470–473. [DOI] [PubMed] [Google Scholar]
- Dani JW, Chernjavsky A, Smith SJ (1992) Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 8:429–440. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448:151–156. 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- Doherty J, Logan MA, Taşdemir OE, Freeman MR (2009) Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci 29:4768–4781. 10.1523/JNEUROSCI.5951-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulenburg V, Gomeza J (2010) Neurotransmitter transporters expressed in glial cells as regulators of synapse function. Brain Res Rev 63:103–112. 10.1016/j.brainresrev.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Feng Y, Ueda A, Wu CF (2004) A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J Neurogenet 18:377–402. 10.1080/01677060490894522 [DOI] [PubMed] [Google Scholar]
- Freeman MR (2015) Drosophila Central Nervous System Glia. Cold Spring Harb Perspect Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA (2008) An internal thermal sensor controlling temperature preference in Drosophila. Nature 454:217–220. 10.1038/nature07001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, Kamiya T, Tsuboi T (2015) Gliotransmitter release from astrocytes: functional, developmental, and pathological implications in the brain. Front Neurosci 9:499. 10.3389/fnins.2015.00499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood HJ Jr, Pellarin LD (1997) Kinetics of low-density lipoprotein receptor activity in Hep-G2 cells: derivation and validation of a Briggs-Haldane-based kinetic model for evaluating receptor-mediated endocytotic processes in which receptors recycle. Biochem J 323:649–659. 10.1042/bj3230649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA (2010) Long-term potentiation depends on release of D-serine from astrocytes. Nature 463:232–236. 10.1038/nature08673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Urban J, Technau GM (1995) Distribution, classification, and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Roux’s Archive in Developmental Biology 204:284–307. 10.1007/BF02179499 [DOI] [PubMed] [Google Scholar]
- Kremer MC, Jung C, Batelli S, Rubin GM, Gaul U (2017) The glia of the adult Drosophila nervous system. Glia 65:606–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ (2009) Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323:1211–1215. 10.1126/science.1169096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Stork T, Bergles DE, Freeman MR (2016) Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature 539:428–432. 10.1038/nature20145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melom JE, Littleton JT (2013) Mutation of a NCKX eliminates glial microdomain calcium oscillations and enhances seizure susceptibility. J Neurosci 33:1169–1178. 10.1523/JNEUROSCI.3920-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menne C, Møller Sørensen T, Siersma V, von Essen M, Ødum N, Geisler C (2002) Endo- and exocytic rate constants for spontaneous and protein kinase C-activated T cell receptor cycling. Eur J Immunol 32:616–626. [DOI] [PubMed] [Google Scholar]
- Minelli A, DeBiasi S, Brecha NC, Zuccarello LV, Conti F (1996) GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J Neurosci 16:6255–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumar AK, Stork T, Freeman MR (2014) Activity-dependent regulation of astrocyte GAT levels during synaptogenesis. Nat Neurosci 17:1340–1350. 10.1038/nn.3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M (1994) Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263:1768–1771. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Rodríguez JJ, Verkhratsky A (2010) Glial calcium and diseases of the nervous system. Cell Calcium 47:140–149. 10.1016/j.ceca.2009.11.010 [DOI] [PubMed] [Google Scholar]
- Newman EA (2005) Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J Neurosci 25:5502–5510. 10.1523/JNEUROSCI.1354-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P, Miesenböck G (2002) Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 36:463–474. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG (1994) Glutamate-mediated astrocyte-neuron signalling. Nature 369:744–747. 10.1038/369744a0 [DOI] [PubMed] [Google Scholar]
- Perkins LA, Holderbaum L, Tao R, Hu Y, Sopko R, McCall K, Yang-Zhou D, Flockhart I, Binari R, Shim HS, Miller A, Housden A, Foos M, Randkelv S, Kelley C, Namgyal P, Villalta C, Liu LP, Jiang X, Huan-Huan Q, et al. (2015) The transgenic RNAi project at Harvard Medical School: resources and validation. Genetics 201:843–852. 10.1534/genetics.115.180208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petravicz J, Fiacco TA, McCarthy KD (2008) Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci 28:4967–4973. 10.1523/JNEUROSCI.5572-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiterer V, Maier S, Sitte HH, Kriz A, Rüegg MA, Hauri HP, Freissmuth M, Farhan H (2008) Sec24- and ARFGAP1-dependent trafficking of GABA transporter-1 is a prerequisite for correct axonal targeting. J Neurosci 28:12453–12464. 10.1523/JNEUROSCI.3451-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungta RL, Bernier LP, Dissing-Olesen L, Groten CJ, LeDue JM, Ko R, Drissler S, MacVicar BA (2016) Ca2+ transients in astrocyte fine processes occur via Ca2+ influx in the adult mouse hippocampus. Glia 64:2093–2103. [DOI] [PubMed] [Google Scholar]
- Satoh AK, O'Tousa JE, Ozaki K, Ready DF (2005) Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development 132:1487–1497. 10.1242/dev.01704 [DOI] [PubMed] [Google Scholar]
- Scimemi A (2014) Structure, function, and plasticity of GABA transporters. Front Cell Neurosci 8:161. 10.3389/fncel.2014.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Jackson-Weaver O, Huckstepp RT, O'Dell TJ, Khakh BS (2013) TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J Neurosci 33:10143–10153. 10.1523/JNEUROSCI.5779-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Kracun S, Sofroniew MV, Khakh BS (2010) A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat Neurosci 13:759–766. 10.1038/nn.2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Patel S, Khakh BS (2016) Probing the complexities of astrocyte calcium signaling. Trends Cell Biol 26:300–312. 10.1016/j.tcb.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS (2012) TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci 15:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork T, Sheehan A, Tasdemir-Yilmaz OE, Freeman MR (2014) Neuron-glia interactions through the heartless FGF receptor signaling pathway mediate morphogenesis of Drosophila astrocytes. Neuron 83:388–403. 10.1016/j.neuron.2014.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner LI, Lienhard GE (1987) Insulin elicits a redistribution of transferrin receptors in 3T3-L1 adipocytes through an increase in the rate constant for receptor externalization. J Biol Chem 262:8975–8980. [PubMed] [Google Scholar]
- Volterra A, Liaudet N, Savtchouk I (2014) Astrocyte Ca2+ signalling: an unexpected complexity. Nat Rev Neurosci 15:327–335. 10.1038/nrn3725 [DOI] [PubMed] [Google Scholar]
- von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ (2011) Neuronal control of Drosophila courtship song. Neuron 69:509–522. 10.1016/j.neuron.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Wang D, Quick MW (2005) Trafficking of the plasma membrane gamma-aminobutyric acid transporter GAT1. Size and rates of an acutely recycling pool. J Biol Chem 280:18703–18709. [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M (2006) Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci 9:816–823. 10.1038/nn1703 [DOI] [PubMed] [Google Scholar]
- Welz T, Wellbourne-Wood J, Kerkhoff E (2014) Orchestration of cell surface proteins by Rab11. Trends Cell Biol 24:407–415. 10.1016/j.tcb.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Yang CH, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, Jan YN (2009) Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron 61:519–526. 10.1016/j.neuron.2008.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Drosophila astrocyte near-membrane Ca2+ activity detected with myrGCaMP6s. Ca2+ transients were recorded from the VNC of undissected 2nd instar larvae. Video speed, 2× real time. Scale bar, 20 µm.
Astrocyte Ca2+ influx leads to suppression of neuronal activity in adult flies. Acute paralysis was induced in Alrm > TrpA1 flies when transferred to preheated water bath held at 33°C; control flies did not show obvious behavioral deficit. Video speed is real time.
Acute astrocyte Ca2+ influx leads to paralysis in Drosophila larvae. Third instar Alrm > TrpA1 larvae rapidly lose voluntary muscle contraction and became paralyzed following transfer to a preheated Petri dish held at 34°C. Control larvae show enhanced locomotion activity. Video speed is real time.
Ca2+ influx in adult ventral ganglion astrocytes causes paralysis. Headless adult Alrm > TrpA1 flies show paralysis when transfer to a preheated Petri dish held at 33°C. The video started 30 s after the flies were transferred to the Petri dish. Video speed is real time.