Abstract
OBJECTIVE:
Changes in the neonatal gut environment allow for the colonization of the mucin layer and lumen by anaerobic bacteria. The aim of the present study was to evaluate Bifidobacterium, Lactobacillus and Lactococcus colonization through the first year of life in a group of 12 Brazilian infants and to correlate these data with the levels of Escherichia coli. The presence of anaerobic members of the adult intestinal microbiota, including Eubacterium limosum and Faecalibacterium prausnitzii, was also evaluated.
METHODS:
Fecal samples were collected during the first year of life, and 16S rRNA from anaerobic and facultative bacteria was detected by real-time PCR.
RESULTS:
Bifidobacterium was present at the highest levels at all of the studied time points, followed by E. coli and Lactobacillus. E. limosum was rarely detected, and F. prausnitzii was detected only in the samples from the latest time points.
CONCLUSION:
These results are consistent with reports throughout the world on the community structure of the intestinal microbiota in infants fed a milk diet. Our findings also provide evidence for the influence of the environment on intestinal colonization due to the high abundance of E. coli. The presence of important anaerobic genera was observed in Brazilian infants living at a low socioeconomic level, a result that has already been well established for infants living in developed countries.
Keywords: Anaerobic Bacteria, Intestinal Microbiota, Brazilian Infants, Real-Time PCR
INTRODUCTION
Intestinal microbiota play an important role in immunity development 1, nutrition 2 and health 3. The intestinal environment is known to change during the first weeks of a child’s life 4. Soon after birth, the child’s gut has classically been described as initially dominated by a range of facultative bacteria, such as representatives of Enterobacteriaceae, Streptococcus, and Staphylococcus 5. Once the available oxygen is consumed, strict anaerobes, including species of Bifidobacterium, Bacteroides, and Clostridium, proliferate 6,7. At the end of the first year of life, the intestinal microbiota is mostly composed of anaerobic bacteria.
At the time of weaning, the populations of Bifidobacterium and Lactobacillus remain highly abundant in the intestinal microbiota, even after the introduction of solid foods to milk-fed infants 9,10. Indeed, some probiotic species from these genera are able to control the composition of the microbiota due to their production of lactate and acetate in the gut environment, and these products play beneficial roles in host health maintenance 11,12.
Faecalibacterium prausnitzii belongs to the C. leptum group of bacteria (Firmicutes) and is a highly active member of the adult intestinal microbial community that exhibits anti-inflammatory effects 13. Studies of intestinal microbiota based on DNA methodologies have reported a high abundance of F. prausnitzii in healthy adults 13, but this species is rarely present in the microbiota of newborns 14.
Our group described the microbial profiles of Brazilian newborns and infants after constructing a 16S rRNA library 8,15. Phylogenetic analysis based on 16S rRNA library construction has been widely used to characterize human fecal microbiota over the last two decades 16-18. However, 16S rRNA library construction may result in a less sensitive assessment of bacterial diversity 19,20, possibly due to bias involved in PCR-dependent methodologies 8.
Indeed, in our previous reports 8,15, we were unable to detect Bifidobacterium and some members of adult-like intestinal microbiota, such as Faecalibacterium prausnitzii 13 and Eubacterium limosum 21, even in older children. Lactobacillus was detected with low frequency and abundancy, and Bifidobacterium was only detected using qPCR methodology 8,15. qPCR has been widely applied for the quantification of bacterial DNA in different human samples, such as feces 22 and human milk 23, due its specificity and accuracy.
Brazilian newborns exhibited high relative abundances of Escherichia and Clostridium spp. in neonatal samples, whereas Staphylococcus and Bacteroides spp. were detected at low frequencies and abundances 15. In infants, the microbial community was composed of aerobic species of Bifidobacterium and Clostridium, with a high abundance of facultative anaerobe Escherichia 15. The pattern of colonization has been noted to differ from that observed for neonates living in developed countries 5,24.
Due to the importance of Bifidobacterium and Lactobacillus in the intestinal microbiota and because of the bias resulting from the methodology used 8, the aim of the present study was to use qPCR to investigate the process of Bifidobacterium and Lactobacillus colonization through the first year of life in a group of Brazilian infants living in low socio-economic conditions. We then correlated these data with the levels of Escherichia coli. The presence of anaerobic members of the adult-like intestinal microbial community (Eubacterium limosum and F. prausnitzii) was also evaluated to determine when these species are introduced into the intestinal microbiota of Brazilian children.
METHODS
Subjects and Study Design
Twelve infants, selected as previously described 15, were enrolled in this study. Briefly, all infants born by vaginal delivery at the University Hospital were followed monthly throughout the first 12 months of life. During the follow up period, information on breastfeeding, types of food consumed, eventual illnesses, antibiotic treatments, and social or economic disorders were gathered for each child during medical visits with a pediatrician. Fecal samples were collected at the hospital on the 2nd day after delivery (time point 1) and by the mother at home on the 7th (time point 2) and 30th days (time point 3) and at 3, 6 and 12 months of age (time points 4, 5 and 6, respectively). Among the 12 infants enrolled in this study, 8 of the mothers collected fecal samples at the 13th, 14th and 15th months of age, and those samples were stored at -80°C. The mothers were instructed to collect the fecal sample with a standardized spoon on the day of the medical appointment, immediately after elimination in a diaper, place it in a sterile plastic container, and store it in a freezer (-20°C) until the appointment a number of hours later. The samples were transported to the laboratory in an ice-filled polystyrene container.
Study population
All of the infants were exclusively breastfed (100%) for the first 30 days after delivery. During the remaining study period, a portion of the infants were exclusively breastfed until the end of the 5th month, whereas another portion were partially breastfed and supplemented with formula milk without prebiotic components (25% at the end of the 3rd month). All of the infants were eating solid food by the end of the 12th month of age 8. All of the infants lived in a low socioeconomic community and attended a day-care facility. Eight infants received oral antibiotics during the study period. These antibiotic treatments were prescribed for 7 or 10 days to treat respiratory infections, such as bronchiolitis, pneumonia, sinusitis and otitis 8.
DNA extraction
DNA was extracted from the stool samples using the QIAamp DNA Stool Mini-Kit (Qiagen, Canada) according to the manufacturer’s instructions. The extracted DNA was stored at -20°C until qPCR analysis.
Real-Time PCR Assays
Real-time PCR (qPCR) was performed on DNA isolated from fecal samples to detect and quantify the presence and abundance of the following anaerobic and facultative anaerobic bacteria: Bifidobacterium, Lactococcus, Lactobacillus, E. coli, E. limosum and F. prausnitzii. The reactions were performed using a 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). The Bifidobacterium animalis subsp. lactis HN019, Lactococcus lactis subsp. lactis and Lactobacillus acidophilus ATCC 4356 strains were cultured in Lactobacilli MRS Broth (Difco) at 37°C under anaerobic conditions, Escherichia coli ATCC 25922 were cultured in TSB (Difco) at 37°C under aerobic conditions. F. prausnitzii ATCC 27766 were cultured following ATCC® instructions. The genomic DNA from each bacterium was used to generate the standard curves and was extracted with the Wizard Genomic DNA Purification Kit (Promega) following the manufacturer’s instructions. The bacterial genomic DNA was used as a positive control for each corresponding reaction. Genomic DNA from Eubacterium limosum ATCC 8486 was obtained directly from the ATCC®. To quantify the copy numbers of the 16S rRNA gene in the fecal samples, standard curves for the relationship between 16S rRNA gene copy number and threshold cycle (Ct) values were created by analyzing 10-fold serial dilutions of species-specific bacterial strains. Only the results with a reaction efficiency ratio above 95% were considered. DNA of Bifidobacterium animalis subsp. lactis HN019 and Escherichia coli ATCC 25922 were used to construct the standard curves for the total bacteria experiments as described by Furret et al. 25. The results were expressed as log10 of the 16S rRNA copy number/g of feces. Technical triplicates were prepared for the amplification reactions for all of the bacterial strains. The total reaction volume of 20 µL contained 2 µL of DNA extracted from feces, as described earlier, and the remaining volume was composed of master mix (1x), primers and probes (Table 1). The amplification reactions for Bifidobacterium spp. and total bacteria were performed in a TaqMan® system using the following program: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. The amplification reactions for Lactococcus, Lactobacillus, E. limosum and F. prausnitzii were performed using the following program on a SYBR® Green I system: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. A melting step was added to evaluate and optimize the amplification specificity (95°C for 15 s, 60°C for 1 min, 95°C for 15 s and 60°C for 15 s).
Table 1.
Primers and probes used in this study.
| Target | Primers and probes | Sequence (5ʹ – 3ʹ) | Conc. (nM) | Reference |
|---|---|---|---|---|
| Bifidobacterium spp. | F_Bifid 09c | CGG GTG AGT AAT GCG TGA CC | 300 | 25 |
| R_Bifid 06 | TGA TAG GAC GCG ACC CCA | 300 | ||
| P_Bifid | 6FAM-CTC CTG GAA ACG GGT G | 250 | ||
| Eubacterium limosum | LIMO-F | TGG ATC CTT CGG GTG ACA TT | 300 | 21 |
| LIMO-R | CTC ATT GGG TAC CGT CAT TC | 300 | ||
| Lactobacillus spp. | Lactobacillus F | AGC AGT AGG GAA TCT TCC A | 300 | 19 |
| Lactobacillus R | CAC CGC TAC ACA TGG AG | 300 | ||
| Lactococcus spp. | Llac05-F | AGC AGT AGG GAA TCT TCG GCA | 300 | 25 |
| Llac02-R | GGG TAG TTA CCG TCA CTT GAT GAG | 900 | ||
| Faecalibacterium prausnitzii | Fprau 07 | CCA TGA ATT GCC TTC AAA ACT GTT | 300 | 13 |
| Fprau 02 | GAG CCT CAG CGT CAG TTG GT | 300 | ||
| All bacteria | F_Bact 1369 | CGG TGA ATA CGT TCC CGG | 200 | 25 |
| R_Prok 1492 | TAC GGC TAC CTT GTT ACG ACT T | 200 | ||
| P_TM 1389F | 6FAM-CTT GTA CAC ACC GCC CGT C | 250 |
Ethical considerations
This research was approved by the Ethics Committee of the HU-USP (under registration number 574/05). All of the mothers enrolled in the research signed an informed consent form.
RESULTS
Inter-individual variation
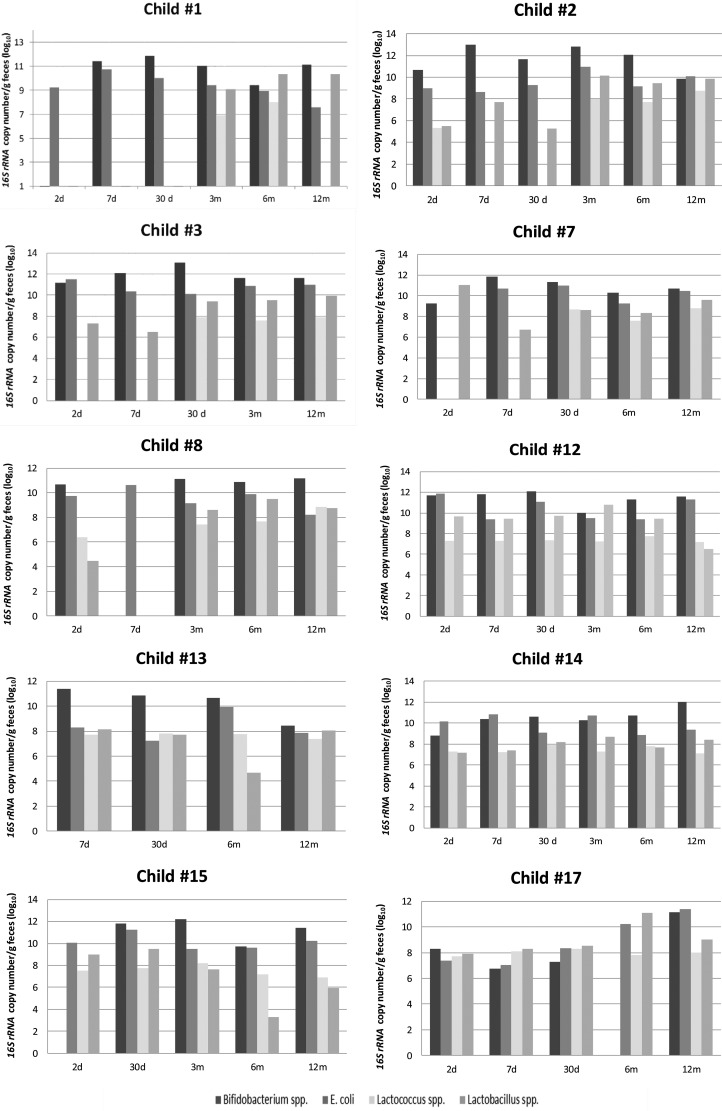
The 16S rRNA copy number of Bifidobacterium, E. coli, Lactobacillus and Lactococcus was quantified for each child enrolled in this study. Not all requested samples were delivered for children #6 and #16; thus, these children were excluded from the individual variation analysis. The individual results showed a distinct pattern of colonization in the initial days after birth (Figure 1), with a predominance of Bifidobacterium.
Figure 1.
Inter-individual quantification of anaerobic and facultative bacteria in the intestinal microbiota, expressed as log10 of 16S rRNA copy number/g of feces. Time points: 2 days; 7 days; 30 days; 3 months; 6 months; 12 months of age.
The bacterial load differed among the children, with variations of 3 log units (Figure 1), and 5 log units for children #7 and #17 in the first days of life. During the following months, the bacterial load of each studied genera increased, with some inter-individual variation.
Bifidobacterium was undetectable on the second day for children #1 and #15 and on the seventh day for child #8. A predominance of E. coli was observed on the second or seventh day for child #3, child #8, child #12 and child #14. The pediatrician reports for those six children indicated important external factors, such as the usage of antibiotics by mother during pregnancy (#1 and #12) or by the child at the 7th day (#3) or poor sanitary conditions (#8, #14 and #15).
Despite some intra-individual variations in the 16S rRNA copy number, after the 3rd month, the microbial pattern was similar for all children, with a predominance of Bifidobacterium followed by E. coli and Lactobacillus and the lowest counts of 16S rRNA copy number for Lactococcus until the end of the first year of age. Bifidobacterium was undetectable at the sixth month of age for child #17, and the pediatrician records indicated a respiratory infection and antibiotic prescription at that time.
F. prausnitzii was detected at some time points (Table 3). The abundance of this species had increased remarkably by the end of the first year of life, when it was detected in 45% of the infants. For the infants from whom fecal samples were collected after 12 months of age, the abundance of the 16S rRNA gene of F. prausnitzii was evaluated to determine its presence and abundance at these later time points. Of the 12 infants enrolled in this study, five of them were positive for F. prausnitzii during the first year, with values ranging from 5.7 log10 to 15.39 log10 copies/g of feces (Table 3). Of the eight infants from who samples were collected after the 13th month of age, 7 had higher detectable levels of F. prausnitzii (Table 3). E. limosum exhibited the lowest abundances and frequencies among the evaluated anaerobic bacteria. The few samples in which this species was detected were collected from child #8 at time point 1 (4.83 log10 copies/g of feces), from child #15 at time point 2 (4.0 log10 copies/ g of feces) and from child #6 at time point 4 (7.5 log10 copies/g of feces). After 12 months of age, none of the children had detectable E. limosum.
Table 3.
Quantification of Faecalibacterium prausnitzii in feces of infants.
| Child | Age | copy number (log10) |
|---|---|---|
| 1 | 12 months | ND** |
| 13 months | 7.59 | |
| 2 | 12 months | 7.24 |
| 13 months | 10.92 | |
| 3 | 12 months | 9.24 |
| 13 months | NA* | |
| 6 | 12 months | ND** |
| 13 months | 10.29 | |
| 7 | 12 months | ND** |
| 13 months | ND** | |
| 14 months | 6.31 | |
| 15 months | 7.64 | |
| 8 | 12 months | 9.73 |
| 13 months | NA* | |
| 12 | 2 days | 5.70 |
| 12 months | 8.44 | |
| 13 months | 9.66 | |
| 14 months | ND** | |
| 13 | 12 months | ND** |
| 16 months | 9.90 | |
| 14 | 7 days | 6.77 |
| 12 months | 6.56 | |
| 13 months | NA* | |
| 15 | 4 months | 7.54 |
| 6 months | 6.44 | |
| 12 months | ND** | |
| 15 months | ND** | |
| 16 | 12 months | NA* |
| 17 | 12 months | ND** |
| 15 months | 7.08 |
NA – not available
ND – not detected
Time point variation
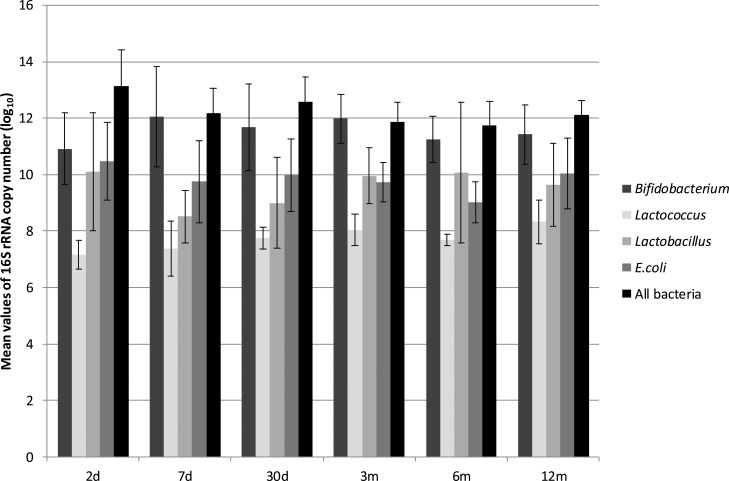
The quantification of the total bacteria at each time point revealed the highest values on the second day of life, with levels that were one or two log units higher than the average values (Figure 2, Table 2). At time point 1 (2 days of age) and throughout the first year, the mean values of total bacteria at each time point did not change significantly, and the differences between time points were on the order of 1 log unit (Figure 2, Table 2).
Figure 2.
Quantification of anaerobic and facultative bacteria in the intestinal microbiota of infants, expressed as log10 of 16S rRNA copy number/g of feces. Time points: 2 days; 7 days; 30 days; 3 months; 6 months; 12 months of age.
Table 2.
Minimum, maximum and mean 16S rRNA copy number of bacteria in feces of infants. Values expressed as log10.
| Bifidobacterium | Lactobacillus | Lactococcus | Escherichia coli | All bacteria | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| min. values | max values | average (SD) | min. values | max values | average (SD) | min. values | max values | average (SD) | min. values | max values | average (SD) | min. values | max values | average (SD) | |
| 2 d | ND | 11.18 | 10.9 (±1.27) | ND | 11.04 | 10.10 (±2.1) | ND | 7.74 | 7.16 (±0.52) | 8.53 | 11.14 | 10.48 (±1.37) | 9.76 | 14.12 | 13.13 (±1.28) |
| 7 d | ND | 12.98 | 12.06 (±1.78) | ND | 9.45 | 8.51 (±0.92) | ND | 8.08 | 7.38 (±0.97) | 6.33 | 10.16 | 9.75 (±1.46) | 10.37 | 12.56 | 12.19 (±0.88) |
| 1 mo | 10.09 | 12.08 | 11.68 (±1.54) | ND | 9.74 | 9.00 (±1.6) | ND | 7.95 | 7.75 (±0.39) | 6.51 | 10.52 | 9.98 (±1.28) | 10.76 | 13.25 | 12.57 (±0.88) |
| 3 mo | 10.00 | 12.81 | 11.98 (±0.87) | ND | 10.79 | 9.96 (±0.99) | 7.22 | 8.67 | 8.03 (±0.56) | 8.45 | 10.28 | 9.72 (±0.70) | 10.53 | 12.38 | 11.85 (±0.72) |
| 6 mo | ND | 12.06 | 11.25 (±0.82) | ND | 8.03 | 10.08 (±2.49) | ND | 12.06 | 7.69 (±0.20) | 8.18 | 10.18 | 9.02 (±0.72) | 9.87 | 12.54 | 11.74 (±0.85) |
| 12 mo | 8.43 | 12.03 | 11.42 (±1.05) | 5.95 | 10.34 | 9.64 (±1.47) | ND | 8.83 | 8.33 (±0.77) | 7.53 | 10.69 | 10.04 (±1.26) | 11.45 | 12.55 | 12.10 (±0.51) |
ND – not detected
Bifidobacterium was not detected at some time points for a few children, as mentioned previously (Figure 1). Of the anaerobic genera evaluated, Bifidobacterium was present in the greatest numbers at all of the time points tested, with maximum values after time point 3 (3 months of age). The mean values of the abundance of Bifidobacterium were similar at time points 3, 4 and 5 (Table 2). The abundance of Bifidobacterium did not appear to be associated with variations in diet (proportion of breast milk or formula milk).
E. coli was found in all of the children and at all time points (Table 2), and the 16S rRNA copy numbers were the second most abundant among the study subjects. The maximum 16S rRNA values were detected at time point 1 (Figure 2), and then the mean values ranged from 9.75 log10 copies/g of feces on the 7th day to 10.04 log10 copies/g of feces at the 12th month, with a minimum value at time point 4 (Table 2). At time points 3 and 4, the mean values for Lactobacillus were higher than those for Escherichia; however, this was seen in all of the children.
Lactobacillus spp. and Lactococcus spp. were not detected in some children, (Figure 1, Table 2), with mean abundances that were between 1 and 3 log units lower than the abundances of Bifidobacterium (Figure 2). The maximum abundance of Lactobacillus was observed at time point 1 (Table 2), and the minimum abundance was observed at time point 2 (Figure 2). The mean abundance values of Lactococcus at each time point did not change significantly over the course of the study, and the differences among them were on the order of 1 log unit (Figure 2, Table 2).
DISCUSSION
The results of quantification of the total bacteria 16S rRNA copy number in the feces of the children enrolled in this study were similar to those reported in the literature 22,26, and some inter-individual variation was observed throughout the observation period. The data revealed that the bacteria population in the gut was highest at the time the delivery. However, the Bifidobacterium counts increased and the E. coli counts decreased at the subsequent time point. These data are consistent with reports throughout the world that the intestinal ecosystem shifts toward an anaerobic environment after birth, and the levels of anaerobic bacteria increase 6.
Bifidobacterium is an important genus of gut microbiota, and some species play beneficial roles in maintaining host health 12. Breastmilk is both an important source of Bifidobacterium for infants 29 and also a source of carbohydrates, which promote Bifidobacterium colonization, even in mixed feeding diets 10. The infants enrolled in this study were breastfed during their early life, and even during the introduction of formula milk, the mother’s milk was also present in their diet, characterizing the diet as mixed feeding 8. Bifidobacterium was not detected at some time points for a few children. Interestingly, the pediatrician reports indicated the use of antibiotics by some of these children before sample collection or the prescription of antibiotic treatment to the mothers for urinary tract infection during the last trimester of pregnancy. The ability of antibiotic treatment to disturb the microbiota composition, including a decrease in Bifidobacterium abundance, has been reported previously 10. Although our sample size is limited, our results suggested that such changes may also occur in children.
However, Bifidobacterium was the predominant species detected in this group of infants at twelve months of age, corroborating the known benefits of breastfeeding as a source of Bifidobacterium and its maintenance in the gut mucosa 9,10. This genus has been described throughout the world as the predominant bacterial group detected in the feces of infants 26-28. Our data showed a lower 16S rRNA copy number of Bifidobacterium after six months of age, potentially because of the introduction of new genera of bacteria via solid foods 30.
Lactobacillus colonization at birth has been attributed to the maternal vaginal flora 31 and, possibly, to the presence of Lactobacillus in the womb environment 32. These findings may explain the observation that the maximum abundance of Lactobacillus occurred on the second day of life in Brazilian infants, followed by a decrease at the seventh day and an increase after one month of age. These data suggest that known environmental changes 6 cause a decrease in the initial levels of the maternal microbiota and that an infant’s microbiota has begun to become established by this time; the increase in the abundance of Lactobacillus after the first month of life highlights the fact that breast milk is an important natural promoter of this bacterial genus.
The inter-individual analysis showed that, in the first days of life, microbiota colonization is affected by individual exposure to environmental factors; in subsequent months, the pattern of anaerobic and facultative genera colonization appears to be mediated by the milk diet, with a predominance of Bifidobacterium and, with lower abundance, Lactobacillus, supporting global knowledge about the role of dietary milk in infant intestinal colonization 9,10.
Because our previous study detected Escherichia in high abundance in this group of children based on library construction 8, we quantified Escherichia at different time points. At some time points, particularly at the second and 12th months, the copy numbers of Escherichia 16S rRNA were the second highest, consistent with our previous results 8,15. However, due to the presence of Bifidobacterium and Lactobacillus and their protective properties 11 in these childreńs feces, lower values of Escherichia than those observed in the present study were expected. In a study of healthy children in Africa 33, a high proportion of Escherichia was detected in the intestinal microbiota in children ranging between zero and 11 months of age. The environmental forces controlling the establishment of the fecal microbiota may favor the maintenance of E. coli in this community 8,15, as well as in other developing countries 33.
F. prausnitzii is a well-established member of the adult intestinal microbiota with anti-inflammatory properties 13. A few papers have described the presence of this species in children’s feces in other populations 28,33,34, but this adult-like bacterium has not been detected in Brazilian infants’ feces. In the present study, this bacterial species was only observed in the fecal microbiota of two newborns on the second and seventh days of life, suggesting maternal transmission. These results suggest that the intestinal environment is unfavorable for its maintenance. After the sixth month of life, a gradual increase in the colonization quantity and frequency was observed. Interestingly, the majority of the fecal samples collected from children after one year (at 13-15 months of age) had greater abundances of F. prausnitzii. These findings are in accordance with those published by Hopkins et al. 28 and Pop et al. 33, in which the abundance of this species increased at the end of the first year of life. These data suggest that until the sixth month of life, the intestinal environment is unfavorable for the establishment of F. prausnitzii and that with the introduction of solid food and the development of a more stable environment, this genus becomes an important intestinal colonizer. Lin et al. 34 reported the presence of F. prausnitzii in older children from both the USA and Bangladesh, with a higher abundance in American children. However, the role of environmental factors in the abundance of F. prausnitzii was not discussed, and more studies are needed to determine whether there is any relationship with external factors.
E. limosum is an anaerobic Gram-positive rod present in the colon of adult humans. This species has a butyrate-producing capacity and consequently has beneficial effects in inflammatory bowel disease 35. In this study, E. limosum was detected in four infants at different time points, indicating its presence in the environment and, therefore, demonstrating that exposure to this genus occurs early in life, although members of this genus have a low capacity for colonization of the intestinal milieu until the 12th month of life.
The present study reports the characterization of the fecal microbiota in Brazilian infants, which is dominated by Bifidobacterium and Lactobacillus. The absence of Eubacterium limosum and the late colonization of F. prausnitzii are also notable. These findings suggest a lack of adult-like microbiota in infants, corroborating the results of a previous study by Ringel-Kuka et al. 36. These results contribute to observations throughout the world of the establishment of the intestinal microbiota of infants fed milk diets. The high abundance of E. coli suggests a pattern related to unhygienic conditions, as reported previously in developing countries 34. These results complement analyses of the composition of the gut microbiota in this group of Brazilian breastfed infants living in low socio-economic conditions 8,15 and highlight the influence of both diet and the environment.
AUTHOR CONTRIBUTIONS
Talarico ST performed the experiments and participated in data analysis. Santos FE performed some experiments. Brandt KG selected the children, followed the medical appointments and collected the samples. Martinez MB designed the study and participated in manuscript writing. Taddei CR designed the study, followed the experiments, conducted the data analysis and wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported by grants from the São Paulo Research Foundation (FAPESP 2011/51196-7) awarded to CRT.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and Inflamation in the intestine. Cell. 2010;140((6)):859–70. doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vrieze A, Holleman F, Zoetendal EG, De Vos WM, Hoekstra JB, Nieuwdorp M. The environment within: how gut microbiota may influence metabolism and body composition. Diabetologia. 2010;53((4)):606–13. doi: 10.1007/s00125-010-1662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemente JC, Ursell LK, Parfrey LW Knight R The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148((6)):1258–70. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91((441)):48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 5.Adlerberth I, Lindberg E, Aberg N, Hesselmar B, Saalman R, Strannegard IL, et al. Reduced enterobacterial and increased staphylococcal colonization of infantile bowel: an effect of hygienic lifestyle. Pediatr Res. 2006;59((1)):96–101. doi: 10.1203/01.pdr.0000191137.12774.b2. [DOI] [PubMed] [Google Scholar]
- 6.Rotimi VO, Duerden BI. The development of the bacterial flora in normal neonates. J Med Microbiol. 1981;14((1)):51–62. doi: 10.1099/00222615-14-1-51. [DOI] [PubMed] [Google Scholar]
- 7.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5((7)):e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taddei CR, Oliveira FF, Duarte RT, Talarico ST, Takagi EH, Ramos CarvalhoII, et al. High abundance of Escherichia during the establishment of fecal microbiota in Brazilian children. Microb Ecol. 2014;67((3)):624–34. doi: 10.1007/s00248-014-0381-x. [DOI] [PubMed] [Google Scholar]
- 9.Salminen S, Gueimonde M. Gut microbiota in infants between 6 and 24 months of age. Nestle Nutr Workshop Ser Pediatr Program. 2005;56:43–51. doi: 10.1159/000086235. [DOI] [PubMed] [Google Scholar]
- 10.Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, et al. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. 2010;51((1)):77–84. doi: 10.1097/MPG.0b013e3181d1b11e. [DOI] [PubMed] [Google Scholar]
- 11.Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23((2)):366–84. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection trough production of acetate. Nature. 2011;469((7331)):543–7. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 13.Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, et al. Low Counts of Faecalibacterium prausnitzii in Colitis Microbiota. Inflamm Bowel Dis. 2009;15((8)):1183–9. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 14.Roger LC, McCartney AL. Longitudinal investigation of the faecal microbiota of healthy full-term infants using fluorescence in situ hybridization and denaturing gradient gel electrophoresis. Microbiol. 2010;156((Pt 11)):3317–28. doi: 10.1099/mic.0.041913-0. [DOI] [PubMed] [Google Scholar]
- 15.Brandt K, Taddei CR, Takagi EH, Oliveira FF, Duarte RT, Irino I, et al. Establishment of the bacterial fecal community during the first month of life in Brazilian newborns. Clinics. 2012;67((2)):113–23. doi: 10.6061/clinics/2012(02)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi H, Takahashi R, Nishi T, Sakamoto M, Benno Y. Molecular analysis of jejunal, ileal, caecal and recto-sigmoidal human colonic microbiota using 16S rRNA gene libraries and terminal restriction fragment length polymorphism. J Med Microbiol. 2005;54((Pt 11)):1093–101. doi: 10.1099/jmm.0.45935-0. [DOI] [PubMed] [Google Scholar]
- 17.Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, et al. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol. 1998;64((2)):795–9. doi: 10.1128/aem.64.2.795-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, Ahrne S, Antonsson M, Molin G. T-RFLP combined with principal component analysis and 16S rRNA gene sequencing: an effective strategy for comparison of fecal microbiota in infants of different ages. J Microbiol Methods. 2004;59((1)):53–69. doi: 10.1016/j.mimet.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol. 2004;97((6)):1166–77. doi: 10.1111/j.1365-2672.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- 20.Lemos LN, Fulthorpe RR, Roesch LF. Low sequencing efforts bias analyses of shared taxa in microbial communities. Folia Microbiol. 2012;57((5)):409–13. doi: 10.1007/s12223-012-0155-0. [DOI] [PubMed] [Google Scholar]
- 21.Maukonen J, Saarela M. Eubacterium. In: Liu D, editor. Molecular Detection of Human Bacterial Pathogens. CRC Press; 2011. pp. 391–403. [Google Scholar]
- 22.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118((2)):511–21. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 23.De Leoz ML, Kalanetra KM, Bokulich NA, Strum JS, Underwood MA, German JB, et al. Human milk glycomics and gut microbial genomics in infant feces show a correlation between human milk oligosaccharides and gut microbiota: a proof-of-concept study. J Proteome Res. 2015;14((1)):491–502. doi: 10.1021/pr500759e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185((5)):385–94. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furet JP, Firmesse O, Gourmelon M, Bridonneau C, Tap J, Mondot S, et al. Comparative assessment of human and farm animal faecal microbiota using real-time quantitative PCR. FEMS Microbiol Ecol. 2009;68((3)):351–62. doi: 10.1111/j.1574-6941.2009.00671.x. [DOI] [PubMed] [Google Scholar]
- 26.Suau A, Bonnet R, Sutren M, Godon JJ, Gibson GR, Collins MD, et al. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999;65((11)):4799–807. doi: 10.1128/aem.65.11.4799-4807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Favier CF, Vaughan EE, de Vos WM, Akkermans ADL. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002;68((1)):219–26. doi: 10.1128/AEM.68.1.219-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopkins MJ, Macfarlane GT, Furrie E, Fite A, Macfarlane S. Characterisation of intestinal bacteria in infant stools using real-time PCR and northern hybridisation analyses. FEMS Microbiol Ecol. 2005;54((1)):77–85. doi: 10.1016/j.femsec.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Martín R, Jimenez E, Heilig H, Fernandez L, Marín ML, Zoetendal EG, et al. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl Environ Microbiol. 2009;75((4)):965–9. doi: 10.1128/AEM.02063-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholtens PA, Oozeer R, Martin R, Amor KB, Knol J. The early settlers: intestinal microbiology in early life. Annu Rev Food Sci Technol. 2012;3:425–47. doi: 10.1146/annurev-food-022811-101120. [DOI] [PubMed] [Google Scholar]
- 31.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107((26)):11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funkhouser LJ, Bordenstein SR. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 2013;11((8)):e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pop M, Walker AW, Paulson J, Lindsay B, Antonio M, Hossain MA, et al. Diarrhea in young children from low-income countries leads to large-scale alterations in intestinal microbiota composition. Genome Biol. 2014;15((6)):R76. doi: 10.1186/gb-2014-15-6-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin A, Bik EM, Costello EK, Dethlefsen L, Haque R, Relman DA, et al. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PLoS One. 2013;8((1)):e53838. doi: 10.1371/journal.pone.0053838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Possemiers S, Rabot S, Espin JC, Bruneau A, Philippe C, González-Sarrías A, et al. Eubacterium limosum activates isoxanthohumol from hops (Humulus lupulus L.) into the potent phytoestrogen 8-prenylnaringenin in vitro and in rat intestine. J Nutr. 2008;138((7)):1310–6. doi: 10.1093/jn/138.7.1310. [DOI] [PubMed] [Google Scholar]
- 36.Ringel-Kulka T, Cheng J, Ringel Y, Saloj�rvi J, Carroll I, Palva A, et al. Intestinal microbiota in healthy U.S. young children and adults-a high throughput microarray analysis. PLoS One. 2013;8((5)):e64315. doi: 10.1371/journal.pone.0064315. [DOI] [PMC free article] [PubMed] [Google Scholar]