Abstract
Multiple myeloma is a relatively uncommon plasma cell malignancy. Preclinical and clinical studies have suggested that aspirin might modify the risk of multiple myeloma. We performed a systematic review and meta-analysis of studies to examine the association between regular aspirin use and risk of multiple myeloma. Five observational studies including 332,660 adults were evaluated. The pooled estimate had a hazard ratio of 0.90 (95% confidence interval =0.58−1.39; P=0.638). Odds ratios from the two case-control studies were similar. The findings demonstrated that there was no significant association between aspirin use and the risk of multiple myeloma.
Keywords: Multiple myeloma, Aspirin, Analgesics, Neoplasms, Meta-analysis
Highlights
-
•
This is a systematic review of aspirin use on the incidence risk of multiple myeloma.
-
•
There is no evidence that aspirin modifies the risk of multiple myeloma.
-
•
More studies are needed to assess the impact of aspirin on the risk of multiple myeloma.
1. Introduction
Multiple myeloma is a malignant plasma cell disorder, comprising approximately 10% of all haematological malignancies, and its incidence is increasing [1]. The pathogenesis is complex, culminating in malignant transformation of clonal plasma cells. The aetiology is not well established, although a number of case-control and cohort studies have reported on the possible associations.
Previous studies reported no consistent association between multiple myeloma and socioeconomic status, income, and education [2], [3]. However, a familial history of myeloma in a first-degree relative has been reported to increase the risk of myeloma by 2–6 times [4], [5]. A previous study reported that the incidence of multiple myeloma was approximately 60% lower in a Chinese population compared with a non-Chinese population, and that the lower rates were maintained in migrants, showing a strong genetic component as evidenced by ethnic differences [6]. Furthermore, an association between increasing body mass index (BMI) and the risk of myeloma has been detected in several studies [7], [8], [9]. Aetiological evidence on the effects of alcohol consumption and tobacco use on the risk of multiple myeloma is limited [10], [11], [12], [13], [14]. There is currently inconsistent or limited evidence regarding the association between the risk of myeloma and various factors, including reproductive and hormonal factors [15], occupational exposure [16], chronic immune stimulation [17], and autoimmune disorders [5], [18].
Non-steroidal anti-inflammatory drugs (NSAIDs) are a class of drugs that inhibit cyclooxygenase (COX) activity and its production of inflammatory prostaglandins. COX-2 expression is associated with inflammation and numerous neoplasms, including multiple myeloma, and COX-2 positivity has been shown to be associated with a poor outcome [19], [20]. COX-2 is also expressed in pre-malignant neoplasms, and an animal study showed that the up-regulation of COX-2 was sufficient to stimulate the transformation of normal cells into invasive cancer and metastatic disease [21]. Chronic inflammation can activate stromal fibroblasts leading to enhanced COX-2 expression and the secretion of inflammatory prostaglandins. In turn, stromal cells expressing COX-2 and inflammatory prostaglandins can induce hematopoietic neoplasms to become malignant [22].
Aspirin, which is a commonly used drug, can irreversibly inactivate COX-1 and COX-2 via covalent bond formation [23]. Aspirin may also inhibit nuclear factor-kappaB [24] and interleukin-6 [25], which have been implicated in the development of multiple myeloma. Epidemiological studies have shown that regular aspirin use may be associated with a lower risk of Hodgkin lymphoma [26], [27], and non-Hodgkin lymphoma [28], [29]. Studies investigating the risk of multiple myeloma have suggested that aspirin might be chemopreventive [30], whereas others have shown no beneficial effect [31], [32], [33], [34]. In order to understand the association, and to evaluate the magnitude and quality of the supporting evidence, we performed a systematic review and meta-analyses of observational studies that evaluated the effect of regular aspirin use on the risk of developing multiple myeloma.
2. Materials and methods
2.1. Search strategy
Studies were identified from EMBASE and MEDLINE databases via the OVID platform, the Cochrane Central Register of Controlled Trials, ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization International Clinical Trials Registry Platform. Search terms for each database are shown in the Appendix. We did not apply limits to the language, date, or study size in our search, although only journal articles in English were included in the analysis. We performed the final search of all databases on 11 April 2016.
2.2. Inclusion and exclusion criteria
First, titles and abstracts were reviewed to exclude studies unrelated to the objective of this meta-analysis. Articles were considered for full reading if authors reported data from an original peer-reviewed study (i.e., not case reports, comments, letters, meeting abstracts, or review articles), and if the study design included a prospective or retrospective cohort, or if it was a case-control study. Full texts of the selected studies were then retrieved and read in full in an unblinded and independent manner by two authors (S.F.L. and T.Y.N.). Studies were considered eligible for full data extraction if they met the following criteria: i) evaluated and clearly defined exposure to aspirin; (ii) reported the risk of multiple myeloma incidence in adults (18 years or older); and (iii) reported relative risk, odds ratios (ORs), or hazard ratios (HRs), or provided data for their calculation. We excluded studies that did not provide quantification data or sufficient statistical parameters for analysis.
Two independent authors (S.F.L. and T.Y.N.) assessed the methodological quality of the studies using the Newcastle-Ottawa scale [35], [36]. In this scale, studies were scored across three categories by answering certain questions: the selection (four questions) and comparability (two questions) of study groups, and the determination of the outcomes or exposures of interest (three questions). All questions were given a score of one except for the comparability of study groups, in which separate points were awarded for controlling for age and sex (maximum score of two). Any discrepancy was resolved via a consensus.
2.3. Data Extraction
Two reviewers (S.F.L. and T.Y.N.) independently performed data extraction. We used a customized form to record the first author of the study, year of publication, study design, country of study population, duration of follow-up, outcome measures, dose and duration of aspirin use (if reported), information regarding exposure ascertainment and outcome assessment, the total number of people in each group (exposed and non-exposed), and effect estimates and 95% confidence intervals (CIs), with and without adjustment for confounding factors. When data for men and women were reported separately, the data were pooled to obtain a summary estimate. For analysis, a reference group was composed of patients with multiple myeloma who were not exposed to aspirin. We derived standard deviations and standard errors from the p-values, according to the instructions in the Cochrane Handbook for Systematic Reviews of Interventions [37]. Conflicts in data extraction were resolved by a consensus.
2.4. Outcomes assessment
The primary outcome was the risk of multiple myeloma in adults based on usage of aspirin, as compared with non-users. Aspirin use for subclinical symptoms of early myeloma was a concern, and the latency period for the development of myeloma is largely unknown; thus, when data on duration of aspirin use were available, the myeloma risks associated with the longest duration of exposure to aspirin were assessed, to minimise the risk of reverse causality.
2.5. Statistical analysis
We used the random-effects model to calculate the meta-analytic estimate of risk of multiple myeloma and 95% CIs [38]. Outcomes were relatively rare events; HRs were considered approximations of relative risks. Adjusted estimates were used in the analysis to account for confounding variables. Heterogeneity between study-specific estimates was assessed using two methods [39], [40]. First, the Cochran Q statistical test for heterogeneity, which assesses the null hypothesis that all studies in a meta-analysis have the same underlying magnitude of effect, was performed. A p-value was quoted as an indication of the extent of inter-study variability. It is widely accepted that the Cochran Q statistical test has poor power when the number of studies is small; thus, a p-value of <0.10 was considered to indicate significant heterogeneity. Second, to estimate the proportion of total variation across studies due to heterogeneity rather than chance, the I2 statistic was calculated. Higgins et al. [40] provided an informal categorisation of I2 with values of 25%, 50%, and 75% representing 'low', 'moderate', and 'high' levels of heterogeneity, respectively.
Publication bias was evaluated quantitatively using the Egger regression test (wherein publication bias is present if p≤0.10), and qualitatively using funnel plots of the logarithmic HRs versus their standard errors [41], [42]. Sensitivity analyses were performed to explain statistical heterogeneity if necessary.
All p-values were two tailed. For all tests (except for heterogeneity and publication bias), a p-value of <0.05 was considered statistically significant. Analysis and reporting were performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines [43]. All analyses and graphs were produced using Stata version 12 software (Stata, College Station, TX, USA) [44].
3. Results
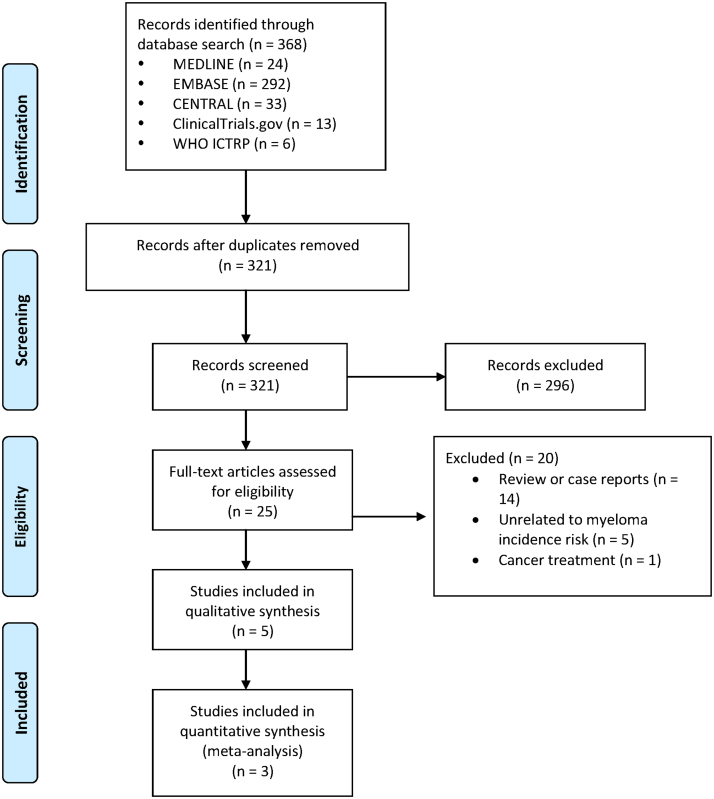
Fig. 1.
PRISMA flow diagram of included studies.
3.1. Characteristics and quality of the included studies
Table 1.
Characteristics of included studies assessing the risk of multiple myeloma associated with regular aspirin use.
| Author (year) | Location/setting and design | Journal | Total subjects | Myeloma cases | Time period | Exposure ascertainment | Outcome assessment | Exposure: aspirin use |
Adjusted for confoundersa | |
|---|---|---|---|---|---|---|---|---|---|---|
| Stratified by duration of use | Stratified by frequency of Use | |||||||||
| Moysich et al. (2007) | USA/ hospital-based case-control | Leukemia Research | 600 | 117 | 1982–1998 | Self-administered questionnaire | Hospital tumour registry | Yes | Yes | Yes |
| Teras et al. (2013) | USA/population-based prospective cohort | Cancer Epidemiology, Biomarkers & Prevention | 149,570 | 211 | 1992–2007 | Self-administered questionnaire | Self-reported with independent validation | Yes | Yes | Yes |
| Birmann et al. (2013) | USA/population-based prospective cohort | Cancer Prevention Research | 116,781 | 328 | 1984–2008 | Self-administered questionnaire | Self-reported with independent validation | Yes | Yes | Yes |
| Landgren et al. (2006) | USA/population case-control | Cancer Epidemiology, Biomarkers & Prevention | 870 | 179 | 1996–2002 | In-person interview | Cancer registry | No | No | Yes |
| Walter et al. (2011) | USA/population-based prospective cohort | Journal of Clinical Oncology | 64,839 | 66b | 2000–2008 | Self-administered questionnaire | Cancer registry | Yes | Yes | Yes |
- Moysich: age, smoking status, and year of completing the questionnaire.
- Teras: age, sex, family history of hematopoietic cancers, race/ethnicity, alcohol intake, education, smoking status, body mass index (BMI), time spent immobile, diabetes status, rheumatoid arthritis status (starting in 2001), cholesterol-lowering drug use, panadol use, and postmenopausal hormone use.
- Birmann: age, sex, BMI, use of panadol, and use of ibuprofen.
- Landgren: age, race/ethnicity, education, and BMI.
- Walter: age, sex, race/ethnicity, education, self-reported health, history of rheumatoid arthritis, history of non-rheumatoid arthritis or chronic neck or back joint pain, history of migraines or frequent headaches, history of fatigue/lack of energy, and a family history of hematopoietic cancers.
66 patients had plasma cell disorders, most of which were myelomas.
The overall methodological quality of this body of evidence was moderate-to-high. Supplementary Tables 1 and 2 show the performance of studies on the Newcastle-Ottawa scale. For the majority of the studies, exposure was ascertained via questionnaires and interviews; outcome assessments were based on electronic databases. The duration and adequacy of follow-up in cohort studies, and the nonresponse rate in case-control studies, were often reported.
The majority of the studies were adjusted for the following confounders: age [30], [31], [32], [33], [34], sex [30], [33], [34], use of panadol/NSAIDs [30], [34], race/ethnicity [31], [33], [34], BMI [30], [31], [34], education [31], [33], [34], and family history of haematopoietic cancer [33], [34].
The doses of aspirin were 81 mg or 325 mg (regular dose), as reported in three of the studies [30], [33], [34]; only the outcomes of patients who received a regular dose were included in the analysis. For the two case-control studies [31], [32], outcomes were evaluated using ORs, which are the odds of myeloma in the aspirin group compared with the non-aspirin group. An OR of <1 indicates a lower risk of myeloma in the aspirin group than in the non-aspirin group. In the three cohort studies, HRs were estimated using Cox proportional hazards models for the associations between aspirin use and risk of myeloma.
3.2. Outcome measures
Because the study design, subjects, and reported outcome measures (HRs and ORs in cohort and case-control studies, respectively) varied among studies, we could not formally pool the results from all studies. Therefore, we performed a meta-analysis of the cohort studies, which constituted the majority of participants. We also provided a narrative summary of the results.
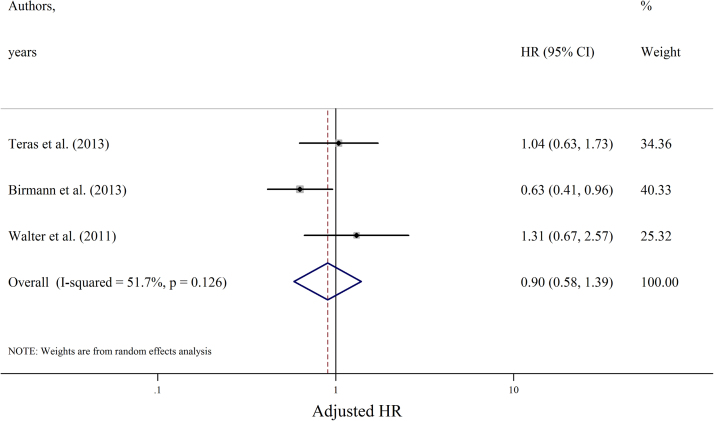
Fig. 2.
Forest plot showing studies' adjusted HRs of the association between regular aspirin use and the pooled summary HR (n=3). HR, hazard ratio.
ORs were available from the two case-control studies, which included 1470 patients and 296 cases of myeloma. Aspirin use was associated with ORs of 0.90 (95% CI =0.65–1.49) and 0.90 (95% CI 0.40–2.00) in the two studies, which were not statistically significant, similar to the pooled estimate in the meta-analysis of the cohort studies.
4. Discussion
In this systematic review of five studies analysing the effect of regular aspirin usage on the risk of multiple myeloma in >330,000 participants, we found that although there was a slight trend towards a lower risk, aspirin usage was not significantly associated with a decreased risk of multiple myeloma.
The strengths of our study included the comprehensive and simultaneous assessment of the effects of aspirin on the risk modification of multiple myeloma.
Preclinical studies have suggested that aspirin might modify the risk of cancer. The anti-neoplastic effect of aspirin is thought to be mediated via inactivation of COX-2 [23], nuclear factor-kappaB [24], and inflammatory cytokines [25], which are frequently expressed in neoplastic lesions and the tumour microenvironment. While these cancer-modifying effects are biologically plausible, clinical studies have not consistently shown these results, and this was reflected in our study. Understanding the complex relationship between aspirin and the risk of multiple myeloma is challenging. The latency period for development of myeloma can be 10–20 years, and there is a possibility of reverse causality. In addition, despite the majority of studies adjusting for numerous covariates, it was impossible to eliminate the potential of residual confounding variables, especially confounding by indication bias.
Furthermore, there were other limitations inherent to a meta-analysis of observational studies. Observational studies lack the experimental randomization of the intervention allocation, which is necessary for the optimal assessment of exposure outcomes. Secondly, the same confounders were not adjusted for in all studies.
Moderate heterogeneity was observed in a relatively small number of studies with diverse characteristics. Studies used different data collection methods (interview questionnaires and databases with variable qualities), and exposures to aspirin were defined using different frequencies and durations. Although the random effects model was applied to estimate pooled HRs and the sensitivity analysis did not support an influential effect, these approaches might not have fully accounted for the heterogeneity introduced by the differences in defining disease, data collection, and drug use.
Based on the results of this study, the chemopreventive effects of aspirin use in multiple myeloma are questionable. This question is difficult to address fully based on retrospective studies due to confounding by indication and reverse causality. The performance of a randomised trial assessing the effects of aspirin on the incidence of multiple myeloma would be challenging due to the large sample size and duration of follow-up required. Further prospective observational studies, with sound methodology and adequate sample sizes, are required to assess the impact of aspirin usage on the risk of multiple myeloma.
5. Conclusion
This systematic review of existing studies did not support the existence of a protective or harmful association between regular aspirin use and the risk of multiple myeloma.
Source of funding
None.
Acknowledgement
None.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.lrr.2017.02.002.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Pasqualetti P. Socioeconomic status and survival in multiple myeloma. Minerva Med. 1990;81(10):713–716. [PubMed] [Google Scholar]
- 3.Baris D. Socioeconomic status and multiple myeloma among US blacks and whites. Am. J. Public Health. 2000;90(8):1277–1281. doi: 10.2105/ajph.90.8.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriksson M., Hallberg B. Familial occurrence of hematologic malignancies and other diseases in multiple myeloma: a case-control study. Cancer Causes Control. 1992;3(1):63–67. doi: 10.1007/BF00051914. [DOI] [PubMed] [Google Scholar]
- 5.Landgren O. Familial characteristics of autoimmune and hematologic disorders in 8406 multiple myeloma patients: a population-based case-control study. Int. J. Cancer. 2006;118(12):3095–3098. doi: 10.1002/ijc.21745. [DOI] [PubMed] [Google Scholar]
- 6.Chan V. Lower incidence of plasma cell neoplasm is maintained in migrant Chinese to British Columbia: findings from a 30-year survey. Leuk. Lymphoma. 2011;52(12):2316–2320. doi: 10.3109/10428194.2011.601475. [DOI] [PubMed] [Google Scholar]
- 7.Friedman G.D., Herrinton L.J. Obesity and multiple myeloma. Cancer Causes Control. 1994;5(5):479–483. doi: 10.1007/BF01694762. [DOI] [PubMed] [Google Scholar]
- 8.Brown L.M. Diet and nutrition as risk factors for multiple myeloma among blacks and whites in the United States. Cancer Causes Control. 2001;12(2):117–125. doi: 10.1023/a:1008937901586. [DOI] [PubMed] [Google Scholar]
- 9.Calle E.E. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 10.Linet M.S. Is cigarette smoking a risk factor for non-Hodgkin's lymphoma or multiple myeloma? Results from the Lutheran Brotherhood cohort study. Leuk. Res. 1992;16(6–7):621–624. doi: 10.1016/0145-2126(92)90011-u. [DOI] [PubMed] [Google Scholar]
- 11.Friedman G.D. Cigarette smoking, leukemia, and multiple myeloma. Ann. Epidemiol. 1993;3(4):425–428. doi: 10.1016/1047-2797(93)90071-b. [DOI] [PubMed] [Google Scholar]
- 12.Brown L.M. Multiple myeloma among Blacks and Whites in the United States: role of cigarettes and alcoholic beverages. Cancer Causes Control. 1997;8(4):610–614. doi: 10.1023/a:1018498414298. [DOI] [PubMed] [Google Scholar]
- 13.Adami J. Smoking and the risk of leukemia, lymphoma, and multiple myeloma (Sweden) Cancer Causes Control. 1998;9(1):49–56. doi: 10.1023/a:1008897203337. [DOI] [PubMed] [Google Scholar]
- 14.Nieters A., Deeg E., Becker N. Tobacco and alcohol consumption and risk of lymphoma: results of a population-based case-control study in Germany. Int. J. Cancer. 2006;118(2):422–430. doi: 10.1002/ijc.21306. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez E. Hormone replacement therapy and cancer risk: a systematic analysis from a network of case-control studies. Int. J. Cancer. 2003;105(3):408–412. doi: 10.1002/ijc.11083. [DOI] [PubMed] [Google Scholar]
- 16.Fritschi L., Siemiatycki J. Lymphoma, myeloma and occupation: results of a case-control study. Int. J. Cancer. 1996;67(4):498–503. doi: 10.1002/(SICI)1097-0215(19960807)67:4<498::AID-IJC6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 17.Bourguet C.C., Logue E.E. Antigenic stimulation and multiple myeloma. A prospective study. Cancer. 1993;72(7):2148–2154. doi: 10.1002/1097-0142(19931001)72:7<2148::aid-cncr2820720714>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Cuzick J., De Stavola B.L. Autoimmune disorders and multiple myeloma. Int. J. Epidemiol. 1989;18(1):283. doi: 10.1093/ije/18.1.283. [DOI] [PubMed] [Google Scholar]
- 19.Ladetto M. Cyclooxygenase-2 (COX-2) is frequently expressed in multiple myeloma and is an independent predictor of poor outcome. Blood. 2005;105(12):4784–4791. doi: 10.1182/blood-2004-11-4201. [DOI] [PubMed] [Google Scholar]
- 20.Cetin M. Overexpression of cyclooxygenase-2 in multiple myeloma: association with reduced survival. Am. J. Hematol. 2005;80(3):169–173. doi: 10.1002/ajh.20460. [DOI] [PubMed] [Google Scholar]
- 21.Harris R.E. Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem. 2007;42:93–126. doi: 10.1007/1-4020-5688-5_4. [DOI] [PubMed] [Google Scholar]
- 22.Baglole C.J. More than structural cells, fibroblasts create and orchestrate the tumor microenvironment. Immunol. Investig. 2006;35(3–4):297–325. doi: 10.1080/08820130600754960. [DOI] [PubMed] [Google Scholar]
- 23.Blobaum A.L., Marnett L.J. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 2007;50(7):1425–1441. doi: 10.1021/jm0613166. [DOI] [PubMed] [Google Scholar]
- 24.Klein B. Positioning NK-kappaB in multiple myeloma. Blood. 2010;115(17):3422–3424. doi: 10.1182/blood-2010-01-264796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawano M. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- 26.Chang E.T. Aspirin and the risk of Hodgkin's lymphoma in a population-based case-control study. J. Natl. Cancer Inst. 2004;96(4):305–315. doi: 10.1093/jnci/djh038. [DOI] [PubMed] [Google Scholar]
- 27.Chang E.T. Aspirin and other nonsteroidal anti-inflammatory drugs in relation to Hodgkin lymphoma risk in northern Denmark. Cancer Epidemiol. Biomark. Prev. 2010;19(1):59–64. doi: 10.1158/1055-9965.EPI-09-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y. Prior medical conditions and medication use and risk of non-Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control. 2004;15(4):419–428. doi: 10.1023/B:CACO.0000027506.55846.5d. [DOI] [PubMed] [Google Scholar]
- 29.Flick E.D. Use of nonsteroidal antiinflammatory drugs and non-Hodgkin lymphoma: a population-based case-control study. Am. J. Epidemiol. 2006;164(5):497–504. doi: 10.1093/aje/kwj223. [DOI] [PubMed] [Google Scholar]
- 30.Birmann B.M. Regular aspirin use and risk of multiple myeloma: a prospective analysis in the health professionals follow-up study and nurses' health study. Cancer Prev. Res. (Philos.) 2014;7(1):33–41. doi: 10.1158/1940-6207.CAPR-13-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landgren O. Risk of multiple myeloma following medication use and medical conditions: a case-control study in Connecticut women. Cancer Epidemiol. Biomark. Prev. 2006;15(12):2342–2347. doi: 10.1158/1055-9965.EPI-06-0097. [DOI] [PubMed] [Google Scholar]
- 32.Moysich K.B. Regular analgesic use and risk of multiple myeloma. Leuk. Res. 2007;31(4):547–551. doi: 10.1016/j.leukres.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 33.Walter R.B. Long-term use of acetaminophen, aspirin, and other nonsteroidal anti-inflammatory drugs and risk of hematologic malignancies: results from the prospective Vitamins and Lifestyle (VITAL) study. J. Clin. Oncol. 2011;29(17):2424–2431. doi: 10.1200/JCO.2011.34.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teras L.R. Aspirin and other nonsteroidal anti-inflammatory drugs and risk of non-hodgkin lymphoma. Cancer Epidemiol. Biomark. Prev. 2013;22(3):422–428. doi: 10.1158/1055-9965.EPI-12-1158. [DOI] [PubMed] [Google Scholar]
- 35.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 36.G.A. Wells, D. O′ Connell, J. Peterson, V. Welch, M. Losos, P. Tugwell, The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [cited 11 April 2016]; Available from: 〈http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm〉.
- 37.Higgins, J.P.T., Green, S. (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: 〈www.cochrane-handbook.org〉.
- 38.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 39.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 40.Higgins J.P. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Easterbrook P.J. Publication bias in clinical research. Lancet. 1991;337(8746):867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 42.Egger M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liberati A. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.StataCorp . StataCorp LP; College Station, TX: 2011. Stata Statistical Software: Release 12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material