Abstract
Introduction
Port site metastasis after laparoscopic surgery for cervical cancer is a rare phenomenon.
Methods
We present a case report of isolated port site recurrence 4 years following laparoscopic surgery in a patient with node-negative, clinical stage IB1 cervical adenocarcinoma.
Results
A 44 year-old woman presented with a necrotic cervical lesion. A biopsy of the mass revealed invasive endocervical adenocarcinoma. She underwent a robotic-assisted radical hysterectomy, bilateral salpingectomy, and pelvic lymph node dissection with bilateral oophoropexy. All lymph nodes were placed in an Endocatch bag prior to removal via the 12 mm assistant port. There was no clinical evidence of metastatic disease and final pathology revealed negative surgical margins and lymph nodes. Four years later, she re-presented with a soft tissue mass in her abdominal wall underlying the site of the prior laparoscopic assistant port. This was confirmed by transcutaneous biopsy to be metastatic adenocarcinoma of endocervical origin. Further work-up revealed no other evidence of metastatic disease. The recurrence was excised and all margins were negative.
Conclusion
This is the first case report describing an isolated port site recurrence in a patient who underwent robotic-assisted laparoscopic surgery for early-stage cervical adenocarcinoma with negative margins and negative lymph nodes. The mechanism underlying this isolated recurrence remains unknown.
Keywords: Cervical cancer, Laparoscopy, Minimally invasive, Recurrent, Port-site
Highlights
-
•
Port-site metastasis is a rare complication after laparoscopic surgery.
-
•
Port-site recurrence can occur in early stage, node-negative cervical cancer.
-
•
Surveillance of port sites at post-operative follow-up visits is imperative.
-
•
The mechanism underlying this isolated recurrence remains unknown.
1. Introduction
Port site metastasis (PSM) is defined by cancer growth at the site of a port incision after laparoscopic resection of malignant tumor (Abu-Rustum et al., 2004). PSM is rare, with an incidence of 1–2% following laparoscopic surgery in the setting of intraperitoneal malignancy (Ramirez et al., 2003). The risk of PSM among patients with cervical cancer has been specifically estimated to be 1.25% (Zivanovic et al., 2008), with most patients with locally advanced squamous cell cervical carcinomas in which traditional laparoscopy was performed. In the majority of cases, patients presenting with PSM had evidence of widespread metastatic disease. We present the first report of isolated PSM occurring years after robotic-assisted laparoscopic surgery in a patient with early stage, node-negative cervical adenocarcinoma.
2. Case
A 44 year-old G0 non-smoking female with cerebral palsy presented in April 2012 with a 3.5 cm necrotic endocervical lesion visualized during speculum exam. The mass was biopsied with pathology consistent with a moderately-differentiated invasive endocervical adenocarcinoma. An exam under anesthesia, cystoscopy, and proctoscopy were performed. There was a large cervical mass that encompassed the majority of the cervix but the vaginal fornices were initially noted to be free of tumor. The parametrium was also free of tumor on exam. The bladder and rectal mucosa were normal. She was clinically staged and determined to have invasive adenocarcinoma of the endocervix, FIGO Stage IB1.
In August 2012, the patient underwent a robotic-assisted type III radical hysterectomy with upper vaginectomy, bilateral salpingectomy, bilateral ureterolysis, and bilateral pelvic lymphadenectomy, with preservation and oophoropexy of both ovaries. There was no ascites present. The uterus, cervix, upper vagina, and bilateral fallopian tubes were removed intact through the vagina. All lymph nodes were placed in an Endocatch bag prior to removal. In total, 11 right pelvic lymph nodes and 9 left pelvic lymph nodes were removed. Intra-abdominal irrigation with sterile water was performed two times. The fascia of the 12 mm umbilical and assistant ports were closed and the subcutaneous tissue was irrigated. Estimated blood loss was 200 mL. Final pathology found all surgical margins and all lymph nodes to be negative for tumor. The tumor size was 3.5 cm × 2.5 cm. The closest margin distance was 1.5 cm. Approximately 1.7 cm of vagina were included with the specimen. There was no lymphovascular space invasion. Depth of cervical stromal invasion was 1.5 cm. The thickness of the cervix in the area of maximal tumor invasion was 1.8 cm. The percent of stromal invasion was 83%. There was no evidence of vascular or perineural invasion. Based on these findings, the decision was made not to administer adjuvant therapy. Over the next 3 years, she was intermittently monitored with physical exams and vaginal cytology every 3–6 months.
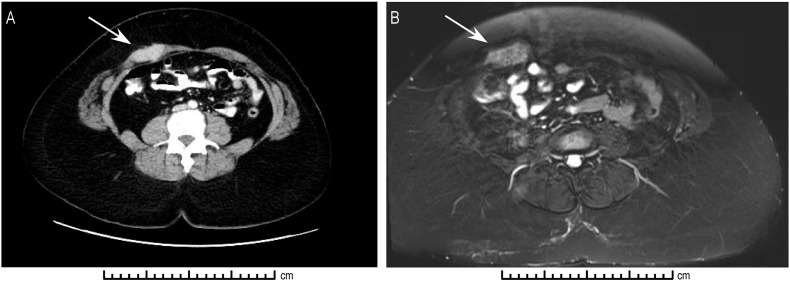
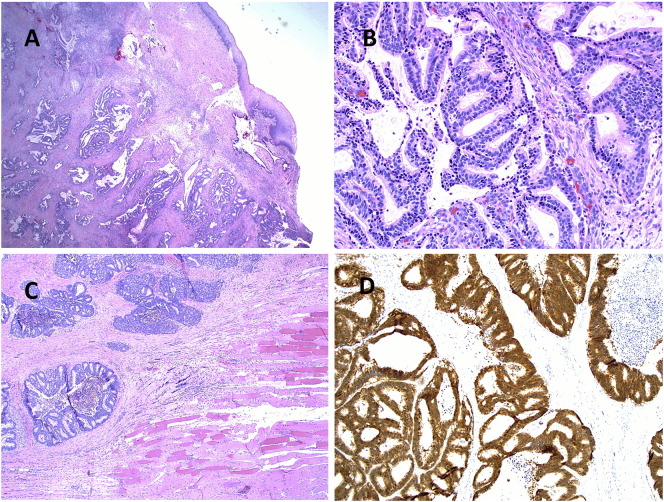
In October 2015, during a routine follow-up visit, the patient reported right periumbilical pain. On physical exam, she was noted to have a firm, tender area on her right abdominal wall, located lateral to the umbilicus. No other masses were palpable and she had no inguinal lymphadenopathy. She underwent a CT scan of her abdomen and pelvis, which revealed a new 4.4 cm hyperdense mass within the right rectus sheath, in the area of her prior 12 mm assistant port site through which a Ligasure device, Endocatch bag, and laparoscopic suction were used. This was re-demonstrated on MRI (Fig. 1). No additional masses were visualized. She then underwent a CT-guided core needle biopsy of the mass, with pathology consistent with metastatic adenocarcinoma of endocervical origin (Fig. 2). The remainder of her work-up was negative, so this was determined to be an isolated port-site recurrence. Given the isolated recurrence, the decision was made to proceed with surgical resection of the mass.
Fig. 1.
Radiologic demonstration of 2.0 cm × 4.1 cm × 3.1 cm abdominal port site recurrence. A: Computed tomographic imaging demonstrating hyperdense lesion in right rectus muscle. B: T2-weighted magnetic resonance imaging demonstrating hyperintense lesion in right rectus muscle.
Fig. 2.
Histopathology of Cervical Adenocarcinoma. A: Low power view (H.E. 4 ×) of invasive endocervical adenocarcinoma involving the ectocervix. B: Characteristic histological features of usual type endocervical adenocarcinoma (H.E. 20 ×). C: Recurrent metastatic endocervical carcinoma involving fibrous and skeletal muscle tissue at the port site (H.E. 4 ×). D: Carcinoma cells diffusely positive for P16 immunohistochemistry confirming HPV related cervical adenocarcinoma.
Patient non-compliance contributed to multiple delays in care during the workup.
In September 2016, the patient underwent radical abdominal wall resection of the mass with a 2 cm circumferential margin, including resection of the involved anterior abdominal wall, fascia, rectus muscle, posterior rectus sheath, peritoneum, and attached omentum. The abdomen and pelvis were explored and no masses were appreciated. The large, approximately 9 cm fascial defect was repaired with Composix 2.0 mesh. On final pathology, the tumor was confirmed to be metastatic cervical adenocarcinoma. Tumor margins and pelvic washings were negative. She was discharged to a short-term rehabilitation facility in stable condition. As of this writing she remains disease free.
3. Discussion
The pathophysiology of PSM is unknown, though several mechanisms have been proposed. Hematogenous spread, direct wound implantation by malignant cells, “chimney effect” from rapid desufflation of CO2 gas through the port sites, surgical technique, and laparoscopic instrumentation have all been suggested as potential etiologies (Ramirez et al., 2003).
The usual presentation of PSM in gynecologic cancers is in the setting of advanced disease and generalized or multi-focal disease recurrence, suggesting that intraperitoneal metastasis is a risk factor (Zivanovic et al., 2008, Martinez-Palones et al., 2005). Other studies have suggested that presence of ascites greatly increases the risk for PSM (Nagarsheth et al., 2004). Three similar cases of PSM following laparoscopic surgery for early stage cervical adenocarcinoma have been described, including two with isolated recurrence. Most case reports, however, suggest that PSM heralds very advanced disease with poor prognosis. None of these findings were seen in our patient, who presented with early stage disease without ascites or evidence of metastases.
In 1999, Lane and Tay (1999) reported a case of isolated PSM in a patient who underwent laparoscopy for stage IB cervical adenocarcinoma. Following tumor resection, this patient remained disease free for 19 months of follow-up. This patient, however, did not undergo robotic-assisted surgery, and her lymph nodes were notably positive. Lavie et al. (1999) described a case of isolated PSM nine months after laparoscopic-assisted vaginal hysterectomy, bilateral salpingo-oophorectomy, and bilateral pelvic lymph node dissection in a patient with node-negative Stage IA1 cervical adenocarcinoma. Our case is distinct in that our patient underwent a robotic surgery and involved bilateral ovarian preservation and suspension. Finally, Sert et al. (2010) published a case of PSM following robotic-assisted laparoscopic surgery for node-negative stage IB1 cervical adenocarcinoma. This patient, however, had evidence of widespread metastases at the time of her re-presentation, including involvement of the bladder mucosa and parametrium.
This is the first report of isolated PSM several years following robotic-assisted laparoscopic type III radical hysterectomy, upper vaginectomy, bilateral salpingectomy and pelvic lymph node dissection with bilateral ovarian preservation and oophoropexy for early stage, node-negative cervical adenocarcinoma. At initial surgery, she had no ascites, no evidence of intraperitoneal disease, and pathology specimens had negative surgical margins with negative lymph nodes and no evidence of vascular or perineural invasion. One possible explanation of PSM is the possible presence of microscopic ovarian metastases, given that this patient had both ovaries preserved and underwent ovarian suspension. Ovarian metastasis has been described in patients with cervical adenocarcinoma with various studies reporting incidences ranging from 1.3% to 28.6% depending on the stage of disease and the involvement of the uterine cervix (Tabata et al., 1987, Toki et al., 1991, Kjorstad and Bond, 1984, Sutton et al., 1992, Brown et al., 1990). There is one case report of PSM in a woman who underwent laparoscopic ovarian transposition prior to external radiation therapy of her pelvis, followed by uterovaginal brachytherapy, and ultimately open hysterectomy, bilateral adnexectomy, and pelvic lymphadenectomy for node-positive stage IIB cervical adenocarcinoma (Picone et al., 2003). Final pathology reported microscopic tumor in one ovary. She subsequently received adjuvant external radiation therapy and re-presented five months postoperatively with abdominal wall recurrence at the site of one of her prior laparoscopic port sites. Further work-up revealed generalized metastases with peritoneal carcinomatosis resulting in her death 2 months later. While the above case illustrates the potential for ovarian metastasis to lead to PSM in patients with cervical adenocarcinoma, the likelihood of this rises substantially with advanced stage disease, presence of positive lymph nodes, and presence of intraperitoneal dissemination, none of which were seen in our patient. Therefore, while possible, microscopic ovarian metastasis is possible, it is unlikely to explain the isolated recurrence observed in our patient.
A number of risk reducing strategies have been proposed to prevent PSM, including en bloc tumor resection, use of a specimen retrieval bag, copious irrigation of the abdominal cavity with sterile water, slow desufflation of pneumoperitoneum, fascial closure of all port sites greater than 5 mm, limited and careful introduction and removal of laparoscopic instruments, changing of possibly contaminated gloves after removal of specimens, and irrigation of port site wounds with povidone-iodine (Tjalma, 2003). Many of these strategies were employed in our case. Without knowing the underlying mechanism behind this isolated port-site recurrence, it is difficult to ascertain what additional measures could have been employed to prevent it.
In terms of treatment and management of PSM, in addition to tumor resection, adjuvant therapy, including chemotherapy and abdominal wall radiation, should be considered.
While there is little available data comparing surgical resection alone versus surgery with adjuvant therapy, chemoradiotherapy has been shown to have both curative and palliative effects, leading to reduction in tumor size and relief of abdominal pain associated with abdominal wall metastases (Kim et al., 2013). Additionally, a case series of PSM after minimally invasive hysterectomy for endometrial cancer at MD Anderson Cancer Center demonstrated excellent response with high rates of local control following surgery and adjuvant radiotherapy (Grant et al., 2015). However, this case series was small, including only 7 patients, and there was no comparison group that received surgery without radiation. Given the limited data available, the optimal combination of multimodal therapy is unknown. Our patient was offered adjuvant radiotherapy and declined, opting instead for close surveillance.
This case demonstrates the potential for PSM to occur several years following robotic surgery, even in patients with low risk, including early stage disease, negative surgical margins, negative lymph nodes, and absence of intraperitoneal disease. These patients should be closely monitored for recurrent disease with continued vigilance and long-term follow-up, including persistent attention to and thorough examination of port-sites at each outpatient visit.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
References
- Abu-Rustum N.R., Rhee E.H., Chi D.S., Sonoda Y., Gemignani M., Barakat R.R. Subcutaneous tumor implantation after laparoscopic procedures in women with malignant disease. Obstet. Gynecol. 2004;103:480–487. doi: 10.1097/01.AOG.0000114974.40512.c9. [DOI] [PubMed] [Google Scholar]
- Brown J.V., Fu Y.S., Berek J.S. Ovarian metastases are rare in stage I adenocarcinoma of the cervix. Obstet. Gynecol. 1990;76:623–626. [PubMed] [Google Scholar]
- Grant J.D., Garg A.K., Gopal R., Soliman P.T., Jhingran A., Eifel P.J., Klopp A.H. Isolated port-site metastases after minimally invasive hysterectomy for endometrial cancer: outcomes of patients treated with radiotherapy. Int. J. Gynecol. Cancer. 2015;25:869–874. doi: 10.1097/IGC.0000000000000424. [DOI] [PubMed] [Google Scholar]
- Kim B., Huh S.J., Kim B.G. Port site metastasis after robotic-assisted laparoscopic hysterectomy for uterine cervical cancer: a case report and literature review. Taiwan J. Obstet. Gynecol. 2013;52:558–563. doi: 10.1016/j.tjog.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Kjorstad K.E., Bond B. Stage IB adenocarcinoma of the cervix: metastatic potential and patterns of dissemination. Am. J. Obstet. Gynecol. 1984;150:297–299. doi: 10.1016/s0002-9378(84)90368-5. [DOI] [PubMed] [Google Scholar]
- Lane G., Tay J. Port-site metastasis following laparoscopic lymphadenectomy for adenosquamous carcinoma of the cervix. Gynecol. Oncol. 1999;74:130–133. doi: 10.1006/gyno.1999.5379. [DOI] [PubMed] [Google Scholar]
- Lavie O., Cross P.A., Beller U., Dawlatly B., Lopes A., Monaghan J.M. Laparoscopic port-site metastasis of an early stage adenocarcinoma of the cervix with negative lymph nodes. Gynecol. Oncol. 1999;75:155–157. doi: 10.1006/gyno.1999.5502. [DOI] [PubMed] [Google Scholar]
- Martinez-Palones J.M., Gil-Moreno A., Perez-Benavente M.A., Garcia-Gimenez A., Xercavins J. Umbilical metastasis after laparoscopic retroperitoneal paraaortic lymphadenectomy for cervical cancer: a true port-site metastasis? Gynecol. Oncol. 2005;97:292–295. doi: 10.1016/j.ygyno.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Nagarsheth N.P., Rahaman J., Cohen C.J., Gretz H., Nezhat F. The incidence of port-site metastases in gynecologic cancers. JSLS. 2004;8:133–139. [PMC free article] [PubMed] [Google Scholar]
- Picone O., Aucouturier J.S., Louboutin A., Coscas Y., Camus E. Abdominal wall metastasis of a cervical adenocarcinoma at the laparoscopic trocar insertion site after ovarian transposition: case report and review of the literature. Gynecol. Oncol. 2003;90:446–449. doi: 10.1016/s0090-8258(03)00271-3. [DOI] [PubMed] [Google Scholar]
- Ramirez P.T., Wolf J.K., Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol. Oncol. 2003;91:179–189. doi: 10.1016/s0090-8258(03)00507-9. [DOI] [PubMed] [Google Scholar]
- Sert B. Robotic port-site and pelvic recurrences after robot-assisted laparoscopic radical hysterectomy for a stage IB1 adenocarcinoma of the cervix with negative lymph nodes. Int. J. Med. Robot. 2010;6:132–135. doi: 10.1002/rcs.295. [DOI] [PubMed] [Google Scholar]
- Sutton G.P., Bundy B.N., Delgado G., Sevin B.U., Creasman W.T., Major F.J., Zaino R. Ovarian metastases in stage IB carcinoma of the cervix: a Gynecologic Oncology Group study. Am. J. Obstet. Gynecol. 1992;166:50–53. doi: 10.1016/0002-9378(92)91828-x. [DOI] [PubMed] [Google Scholar]
- Tabata M., Ichinoe K., Sakuragi N., Shiina Y., Yamaguchi T., Mabuchi Y. Incidence of ovarian metastasis in patients with cancer of the uterine cervix. Gynecol. Oncol. 1987;28:255–261. doi: 10.1016/0090-8258(87)90170-3. [DOI] [PubMed] [Google Scholar]
- Tjalma W.A. Laparoscopic surgery and port-site metastases: routine measurements to reduce the risk. Eur. J. Gynaecol. Oncol. 2003;24:236. [PubMed] [Google Scholar]
- Toki N., Tsukamoto N., Kaku T., Toh N., Saito T., Kamura T., Matsukuma K., Nakano H. Microscopic ovarian metastasis of the uterine cervical cancer. Gynecol. Oncol. 1991;41:46–51. doi: 10.1016/0090-8258(91)90253-2. [DOI] [PubMed] [Google Scholar]
- Zivanovic O., Sonoda Y., Diaz J.P., Levine D.A., Brown C.L., Chi D.S., Barakat R.R., Abu-Rustum N.R. The rate of port-site metastases after 2251 laparoscopic procedures in women with underlying malignant disease. Gynecol. Oncol. 2008;111:431–437. doi: 10.1016/j.ygyno.2008.08.024. [DOI] [PubMed] [Google Scholar]