Abstract
Background
Approximately 75% of medical inpatients at Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi are HIV seropositive, and a third of these patients are on antiretroviral therapy (ART). Malawi guidelines recommend targeted viral load (VL) testing for patients on ART for at least one year who report excellent adherence and present with a WHO clinical stage 3 or 4 HIV disease. A switch to second-line ART is only indicated if a VL result >5000 copies/mL confirms treatment failure.
Methods
During an audit of targeted VL testing at QECH, all adult medical admissions were screened to identify those in need of VL testing. Daily review of inpatient notes ascertained whether VL testing was ordered and carried out. At 8 weeks post-discharge the laboratory database was checked for results and was triangulated with the HIV outpatient database to ascertain whether patients had attended clinic, received results, and if these results had been acted upon.
Results
Out of 81 patients recruited, 63 (77%) had a VL requested. At 8 weeks post-discharge, nine patients (14%) had VL results available. The median (IQR) waiting time for those with results was 29 days (20–47). Five patients had a VL >5000 copies/mL. Of these patients, three attended clinic and one was switched to second-line ART. Of the remaining 55 patients awaiting results, the median (IQR) waiting time at the 8-week follow-up point was 72 days (67–80). At 8 weeks post-discharge, 8 patients (33%) had died.
Conclusions
Our findings demonstrate challenges with targeted VL testing at QECH. Only two-thirds of patients with clinical ART failure were identified as eligible for targeted VL testing, and of these less than one-sixth had VL results available after 8 weeks. Interventions such as point-of-care targeted VL testing could result in faster turnaround times. In the interim, we suggest further evaluation of the possibility of switching patients with clinical ART failure and a low CD4 count to second-line ART while awaiting VL results.
Introduction
The HIV seroprevalence among adult medical inpatients at Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi is approximately 75%, and about one-third of the patients with HIV are on antiretroviral therapy (ART).1 The proportion of adult medical inpatients on ART increased from 25% in 2013 to 28% in 2014 and 31% in 2015 (Peterson I, QECH electronic patient record data, 2016, personal communication). Malawi HIV guidelines recommend targeted viral load (VL) testing for patients who have been on ART for at least one year, present with a WHO stage 3 or 4 clinical event, and report good recent adherence to their ART regimen. A switch to a second-line ART regimen is only indicated after a VL result >5000 copies/mL confirms ART failure.2 QECH is a tertiary referral hospital and processes batched dried blood spot (DBS) VL samples in its central laboratory. Inpatients requiring VL testing during admission are instructed to attend the outpatient HIV clinic 3 to 4 weeks later to obtain results. There are already significant challenges in linking HIV-infected patients to regular follow-up and ART. As the Malawian ART programme continues to develop, there is a greater risk that patients established on ART will present with ART treatment failure and that the current VL testing systems may not be robust enough to meet service demands to facilitate a timely switch of ART. We therefore prospectively studied targeted VL testing among adult inpatients at QECH and report outcomes at 8 weeks post-discharge.
Methods
Ethical approval for this study was granted by the College of Medicine Research and Ethics Committee (COMREC). Over a 4-week period, all adult medical admissions were screened for the following eligibility criteria: patients on ART for at least one year and presenting with a WHO stage 3 or 4 clinical event, with self-reported excellent ART adherence (taken ART as prescribed over the previous one month). Written consent was obtained from each patient. Demographic information, duration on ART, and self-reported ART adherence data were collected through patient interviews and review of clinical files. Inpatient notes were reviewed daily to ascertain whether VL testing was ordered and carried out. At 3, 6, and 8 weeks following patient discharge, the laboratory VL database was checked for available results, which were triangulated with the HIV outpatient clinic electronic database to determine if patients had attended the HIV clinic, received their results, and were switched to second-line treatment (if indicated). Two attempts were made to contact patients via telephone, 8 weeks post-discharge, to obtain information on health status.
Results
Eighty-eight patients were screened and 85 patients consented to be enrolled in the study. Four patients were later excluded when their ultimate discharge diagnosis was no longer a WHO stage 3 or 4 condition. Data for 81 patients were included in the final analysis. The median (IQR) age was 37 years (31–43), and the median (IQR) duration on ART was 47 months (23–84). The median (IQR) CD4 count during the previous 6 months was 94.5 cells/uL (14.5–211). The most common clinical events were tuberculosis, community-acquired pneumonia, bacterial meningitis, and non-typhoidal Salmonella sepsis. Table 1 summarises the frequency of each clinical diagnosis in the study population during the data collection period.
Table 1.
Summary of WHO stage 3 and 4 HIV diseases in the study population
| WHO stage 3 or 4 condition | Frequency |
| Tuberculosis | 32 |
| Community-acquired pneumonia | 17 |
| Non-typhoidal Salmonella sepsis | 5 |
| Bacterial meningitis | 5 |
| Disseminated Kaposi sarcoma | 4 |
| Cryptococcal meningitis | 4 |
| Oesophageal candidiasis | 4 |
| Sepsis (source unknown) | 3 |
| Other* | 7 |
Other: HIV encephalitis, HIV wasting syndrome, intracranial space occupying lesion (?lymphoma), lung abscess, E. coli sepsis, HIV-associated nephropathy, Pneumocystis jirovecii pneumonia
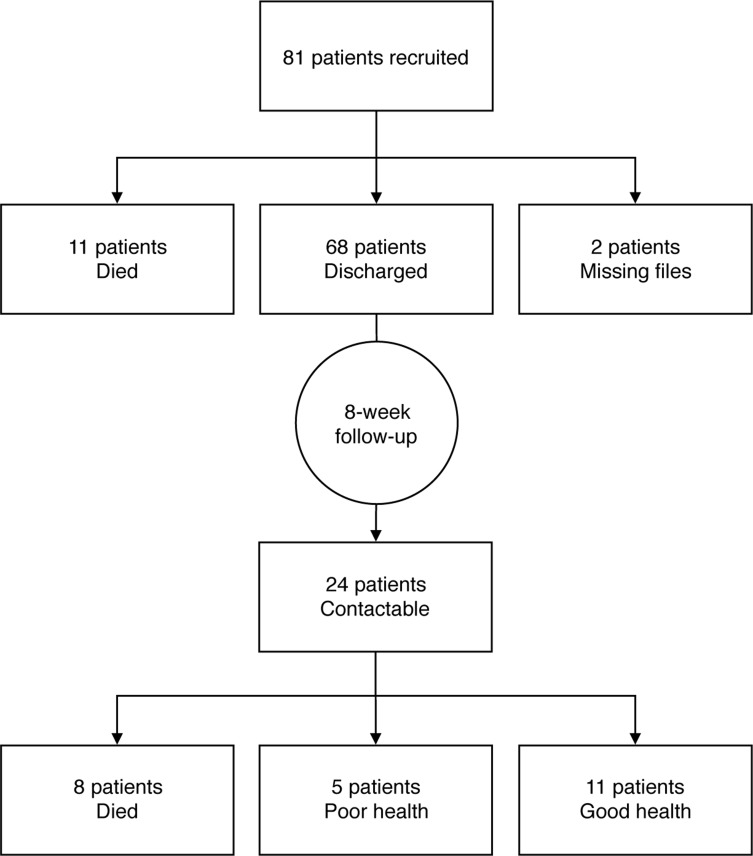
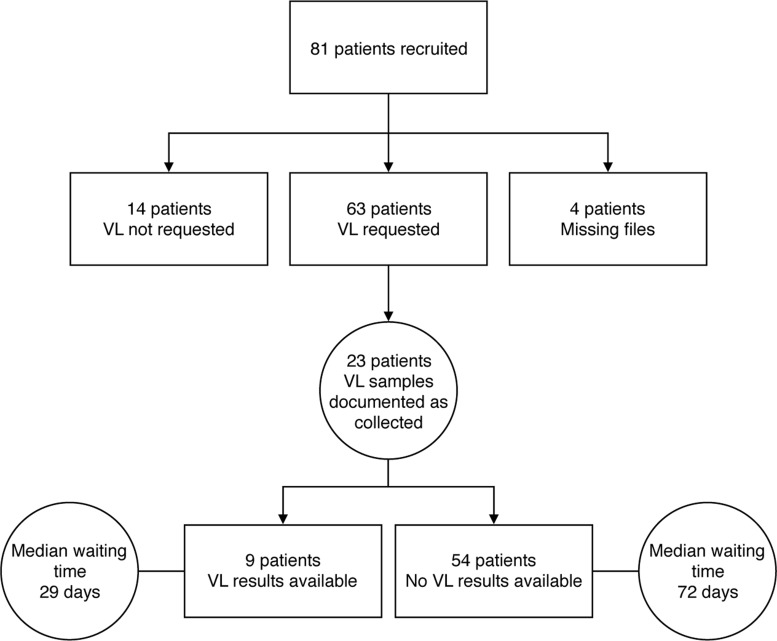
Twenty-nine patients had at least two WHO stage 3 and 4 conditions. Figure 1 demonstrates the pathway a patient needs to undergo in order to receive VL results. Figure 2 summarises the VL testing pathway experienced by the study population. During admission, 63 patients (78%) had a VL requested by their clinician and, at 8 weeks after discharge, 9 patients (14%) ultimately had a VL result available in the laboratory. The median (IQR) waiting time from blood sampling to available results was 29 days (20–47). Four of the nine patients with results had an undetectable VL. The median (IQR) VL result of the remaining 5 patients was 5.33log10 (4.57log10 to 5.46log10). Three of the five patients with a high VL attended clinic and one was switched to a second-line ART regimen. Of the remaining 55 patients awaiting results, the median (IQR) waiting time from admission to 8-week follow-up was 72 days (67–80). The laboratory reported that during the study period there was a lack of reagents and therefore a large backlog in VL test processing. Outcome data at the 8-week follow-up point were available for 24 of the 68 patients discharged alive. Of these, 8 (33%) had died and 5 (21%) reported to be in poor health. Figure 3 summarises outcome data.
Figure 1.
Intended viral load testing pathway at Queen Elizabeth Central Hospital
Figure 2.
Viral load (VL) testing pathway for study patients including median waiting time for viral load results
Figure 3.
Patient outcomes at discharge and 8 weeks post-discharge
Discussion
Our findings demonstrate challenges with targeted VL testing at QECH. Even in this tertiary facility, only two-thirds of patients with clinical ART failure were identified by clinicians as eligible for targeted VL testing, and of these less than one-sixth had VL results available 8 weeks after discharge. Those with available results had waited four weeks on average. Ultimately, only one patient was switched to a second-line regimen. The mortality rate in those who could be contacted was high.
The VL testing pathways at QECH are complex, with many steps required before a patient can receive results, as demonstrated in Figure 1. Delays are experienced throughout this process. Many patients were identified as needing a VL test, but because they were too sick and did not have relatives or guardians to escort them from the inpatient wards to the laboratory, these patients did not attend the laboratory for blood sampling,. Blood sampling is not carried out on the wards because of the need for dried blood spot testing and poor linkage of patients to their results when samples are obtained during their ward stays.
This was a single-centre experience, but we believe that our findings are likely to be generalisable to inpatients at other hospitals in Malawi, particularly those where there is no onsite testing. Apart from strengthening local systems, there are wider-scale interventions that could be considered. Using a point-of-care VL testing approach for patients with clinical failure would result in faster turnaround times by circumventing the need for batch testing.3 This could be of particular benefit for admitted patients who normally receive ART from community clinics a long distance from the referral hospital. In the interim, we suggest further evaluation of the possibility of switching a patient to second-line ART, while awaiting VL results, based on the presence of a clinical stage 4 opportunistic infection and a low CD4 count.4,5 This would prevent long delays in effective ART for this high-risk patient group, who are often suffering from advanced ART failure, which exposes them to risk of further complications and death.6
References
- 1.SanJoaquin MA, Allain TJ, Molyneux ME, et al. Surveillance Programme of IN-patients and Epidemiology (SPINE): implementation of an electronic data collection tool within a large hospital in Malawi. PLoS Med. 2013;10(3):e1001400. doi: 10.1371/journal.pmed.1001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ministry of Health, Malawi, author. Ministry of Health, Malawi. Clinical management of HIV in children and adults [Internet] Lilongwe: Ministry of Health, Malawi; 2014. Feb, [cited 2016 Mar 30]. 95 p. Available from: http://www.emtct-iatt.org/wp-content/uploads/2015/09/Malawi-HIV-Guidelines-2014.pdf. [Google Scholar]
- 3.Stevens W, Gous N, Ford N, Scott LE. Feasibility of HIV point-of-care tests for resource-limited settings: challenges and solutions. BMC Med. 2014 Aug;12:173. doi: 10.1186/s12916-014-0173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mugyenyi P, Walker AS, Hakim J, et al. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010 Jan 9;375(9709):123–131. doi: 10.1016/S0140-6736(09)62067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waruru A, Muttai H, Ng'ang'a L, et al. Positive Predictive Value of the WHO Clinical and Immunologic Criteria to Predict Viral Load Failure among Adults on First, or Second-Line Antiretroviral Therapy in Kenya. PLoS One. 2016 Jul 6;11(7):e0158881. doi: 10.1371/journal.pone.0158881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paton NI, Kityo C, Hoppe A, et al. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med. 2014 Jul 17;371(3):234–247. doi: 10.1056/NEJMoa1311274. [DOI] [PubMed] [Google Scholar]