Abstract
AIM
To determine how statins, testosterone (T) replacement therapy (TRT) and phosphodiesterase 5-inhibitors (PDE5I) influence age related mortality in diabetic men.
METHODS
We studied 857 diabetic men screened for the BLAST study, stratifying them (mean follow-up = 3.8 years) into: (1) Normal T levels/untreated (total T > 12 nmol/L and free T > 0.25 nmol/L), Low T/untreated and Low T/treated; (2) PDE5I/untreated and PDE5I/treated; and (3) statin/untreated and statin/treated groups. The relationship between age and mortality, alone and with T/TRT, statin and PDE5I treatment was studied using logistic regression. Mortality probability and 95%CI were calculated from the above models for each individual.
RESULTS
Age was associated with mortality (logistic regression, OR = 1.10, 95%CI: 1.08-1.13, P < 0.001). With all factors included, age (OR = 1.08, 95%CI: 1.06-1.11, P < 0.001), Low T/treated (OR = 0.38, 95%CI: 0.15-0.92, P = 0.033), PDE5I/treated (OR = 0.17, 95%CI: 0.053-0.56, P = 0.004) and statin/treated (OR = 0.59, 95%CI: 0.36-0.97, P = 0.038) were associated with lower mortality. Age related mortality was as described by Gompertz, r2 = 0.881 when Ln (mortality) was plotted against age. The probability of mortality and 95%CI (from logistic regression) of individuals, treated/untreated with the drugs, alone and in combination was plotted against age. Overlap of 95%CI lines was evident with statins and TRT. No overlap was evident with PDE5I alone and with statins and TRT, this suggesting a change in the relationship between age and mortality.
CONCLUSION
We show that statins, PDE5I and TRT reduce mortality in diabetes. PDE5I, alone and with the other treatments significantly alter age related mortality in diabetic men.
Keywords: Type 2 diabetes, Mortality, Gompertz-Makeham equation, Phosphodiesterase 5 inhibitors, Male hypogonadism, Statins, Testosterone replacement therapy
Core tip: We have described a study of men with type 2 diabetes showing that mortality rates are in accordance with the pattern described nearly 200 years ago by Benjamin Gompertz. The data show that statin, phosphodiesterase 5 inhibitors (PDE5I) and testosterone replacement in hypogonadal men reduce all-cause mortality. PDE5I, alone and in combination with the other 2 agents alters the association between age and mortality, thus improving prognosis. The graphical illustrations adopted in this paper communicate the impact of medical intervention very effectively to patients and this could potentially improve compliance leading to significant clinical benefit.
INTRODUCTION
In 1825, Benjamin Gompertz described a law defining human mortality based on the equation, μ(x) = α x eβx, with μ(x) being the mortality rate at age x years and, α and β (considered to be the ageing rate) being constants[1]. Subsequently, Makeham proposed a modified Gompertz equation, μ(x) = α x eβx + y, that included a factor (y) describing extrinsic mortality thereby allowing the effect of age -independent and -dependent factors on mortality to be studied[1,2]. Avoidance of external factors such as conflict or starvation results in the age-independent component having less impact on death rate and therefore, mortality increases exponentially with age. It has been suggested that β is similar for all populations and it is the intercept (α) that varies[3]. In developed countries, the age-independent intercept is influenced by medical care as many treatments have a significant impact on mortality though factors encountered in early life can also influence later outcomes[4].
Mortality in type 2 diabetes (T2DM) is associated with age and therapy. Patients have a 1.5-2.5 fold higher mortality than the general population[5,6] with men and women suffering a mean 7.5 years and 7 years reduction in life expectancy respectively[7]. This increase in mortality appears related to the age at diagnosis, being lower in patients diagnosed their late 70 s compared with those aged in their mid-40 s[8].
Several inter-related pathologies are associated with mortality in T2DM and accordingly, statins and in men, testosterone replacement therapy (TRT) and phosphodiesterase 5 inhibitors (PDE5I) are commonly prescribed. Since rates of cardiovascular disease (CVD) associated mortality are 2-4 times higher than in non-diabetics, reduction of serum LDL-cholesterol is a key aim[9,10]. Statins are the mainstay of lipid lowering therapy with trials demonstrating marked reductions in CVD in T2DM patients[9,11]. Male hypogonadism characterised by low serum testosterone and sexual dysfunction is common in diabetics and also linked with mortality[12-15]. Studies in men with diabetes show low serum free testosterone is associated with carotid atherosclerosis identified by carotid artery intimal thickness and plaque score[16]. All-cause mortality is greater in those with low compared with normal total testosterone levels[17]. Three studies, two in men with T2DM, suggest TRT reduces all-cause mortality[17-19]. A randomized controlled study of men with T2DM showed TRT improves symptoms of hypogonadism, the different symptoms significantly improving at varying testosterone thresholds[20]. Importantly, in T2DM patients, erectile dysfunction (ED) appears to be an independent predictor of vascular disease and mortality[13-15]. PDE5I have beneficial effects on endothelial function, insulin resistance and possess potentially cardio-protective properties[15,21,22]. Gazzaruso et al[15] showed that PDE5I use in T2DM men was associated with reductions in major adverse cardiac events and angiography confirmed coronary artery disease. Our longitudinal prospective study indicated that PDE5I use was possibly associated with reduced mortality independent of TRT and statin therapy[19]. Further, Anderson et al[23] reported a 31% reduction in all-cause mortality and 26% reduction in myocardial infarction in men with T2DM prescribed PDE5I.
We recently reported in a prospective longitudinal study of 857 men with T2DM that TRT and PDE5I were independently associated with increased survival in men with T2DM[19]. Importantly baseline body weight, body mass index, lipids, glycaemic control and hypertension were not associated with mortality. We now describe a retrospective analysis of this cohort determining the association between age and mortality, establishing whether the mortality rate follows the pattern described by Gompertz and estimating how testosterone status and treatments (statins, TRT and PDE5I) alter this relationship.
MATERIALS AND METHODS
This study is a retrospective analysis of follow-up data obtained in 857 men with T2DM screened following informed consent for the randomised double blind placebo controlled BLAST (Birmingham, Lichfield, Atherstone, Sutton and Tamworth) study designed to investigate the effects of long acting testosterone on clinical symptoms and metabolic parameters over a 30 wk treatment period[24]. The patients were listed in the registers of 5 English Midlands practices and initially screened for total testosterone (TT) and free testosterone (FT) during April 2007-April 2009. Based on total and free testosterone levels, the 857 men were classified as Normal T (total testosterone > 12 nmol/L and free testosterone > 0.25 nmol/L) or Low T (total testosterone ≤ 12 nmol/L or free testosterone ≤ 0.25 nmol/L) with a second measurement taken at least 2 wk later in men with TT ≤ 12 nmol/L, according to the Endocrine Society clinical practice guidelines[25]. The BLAST intervention study were approved by the West Midlands Regional Ethics Committee (reference: 08/H1208/30), the National Institute for Health Research (Birmingham and the Black Country Comprehensive Local Research Park - RM&G reference: 1268) and Warwickshire Primary Care Trust (reference: WAR230909) with the long term follow-up approved as an audit by all the appropriate Primary Care Trust Ethics Committees.
United Kingdom Primary care diabetes care treatment of glycaemic control, dyslipidaemia and hypertension is protocol and guideline driven as part of the Quality Outcomes Framework initiative. TRT was prescribed according to the BLAST study programme[24,26]. The United Kingdom NHS regulations allowed PDE5I prescribing for ED in men with diabetes with a suggested regime of 1 dose/wk[27].
The 857 men were categorised by statin, TRT and PDE5I treatment at death or final visit, firstly, by statin treatment; 195 men were Statin/untreated and 662 men Statin/treated, secondly by hypogonadism and TRT; 320 men were Normal T (TT > 12 nmoL/L and FT > 0.25 nmol/L)/untreated, 362 men were Low T (TT ≤ 12 nmol/L or FT ≤ 0.25 nmol/L)/untreated and 175 men were Low T/treated (TT ≤ 12 nmol/L or FT ≤ 0.25 nmol/L)/treated and thirdly, by PDE5I treatment; 682 men were PDE5I/untreated and 175 men were PDE5I/treated. Mortality data was collected from the general practice databases, hospital letters and death certificates.
Laboratory methods
Statin and TRT prescribing was based on protocols based on laboratory measurements. Serum sex hormone binding globulin, albumin and lipids were analysed using a Roche Modular automated analyzer (Roche Diagnostics, Burgess Hill, United Kingdom). Early morning fasting TT was measured using the validated Roche common platform immunoassay. FT was calculated using the Vermeulen et al[28].
Stata version 8 (College Station, TX) was used for statistical analyses with all-cause mortality as primary end point. Differences in mortality between the alive and deceased groups were identified using χ2 (statin, PDE5I, TRT and hypogonadism) and unpaired t-test (age at death or final visit). Logistic (and logit) regression was initially carried out to study the association between death/survival (dichotomous outcome) and age at death or final follow-up visit as the independent variable. Subsequently, separate models were developed with each treatment (statins, testosterone (status and treatment) and PDE5I) being included with age at death or final visit as the independent variables. Discrete ordinal variables were factorised with one category selected as reference. In the testosterone groups, Low T/untreated was selected as reference as Normal T/untreated and Low T/treated differed by one characteristic, i.e., TT and FT concentration and TRT respectively. Patients on statins and PDE5I were compared to those not on treatment (reference groups). We estimated individual probability of mortality (and 95%CI for each man using separate logistic regression models. The statistical approaches used were reviewed by author, Professor Peter W Jones, Professor of Medical Statistics, Keele University, and considered appropriate.
RESULTS
Table 1 presents the data used to compare the age and treatment details of men who either survived or died during the study. The Table shows the mean age of the total study group and of the 754 men alive at study end and 103 deceased men. Mean age in the deceased group was significantly higher (P < 0.0001) than in survivors. Table 1 also shows the proportion of alive/deceased men treated with statin or PDE5I. In the deceased group, a significantly lower proportion of men were treated with statins (68.0%, P = 0.017) or PDE5I (2.9%, P < 0.001) compared with survivors (78.5%, 22.8% respectively). To assess the impact of hypogonadism and TRT on mortality, we stratified the 857 men into three groups; Normal T/untreated (eugonadal), Low T/untreated and Low T/treated. Table 1 shows in the deceased group that the proportions of men given TRT (5.8%, P < 0.001) or who were eugonadal (35.0%, P = 0.037) was significantly lower than that of men in the Low T/untreated group (59.2%).
Table 1.
Mortality in men with type 2 diabetes stratified by treatment with statins, testosterone status/treatment, phosphodiesterase 5-inhibitors and combinations of treatments n (%)
| Total group | Alive | Deceased | P value | |
| Patient n | 857 | 754 | 103 | |
| Mean age at death or last visit/SD (yr) | 67.4/11.6 | 66.2/11.3 | 76.2/10.2 | < 0.00011 |
| Patient numbers (%) stratified by treatment | ||||
| Statin/untreated | 195 (22.8) | 162 (21.5) | 33 (32.0) | 0.0172 |
| Statin/treated | 662 (77.3) | 592 (78.5) | 70 (68.0) | |
| Normal T/untreated | 320 (37.3) | 284 (37.7) | 36 (35.0) | < 0.0012 |
| Low T/untreated | 362 (42.2) | 301 (39.9) | 61 (59.2) | |
| Low T/treated | 175 (20.4) | 169 (22.4) | 6 (5.8) | |
| PDE5I/untreated | 682 (79.6) | 582 (77.2) | 100 (97.1) | < 0.0012 |
| PDE5I/treated | 175 (20.4) | 172 (22.8) | 3 (2.9) | |
| Not on any of the above therapeutic agents | 125 (14.6) | 92 (12.2) | 33 (32.0) | 0.0022 |
| On all 3 therapeutic agents | 45 (5.3) | 43 (5.7) | 2 (1.9) |
Unpaired t test;
χ2 analysis.
Importantly, in two of the treatment groups the age at final visit of survivors varied; PDE5I treatment (PDE5I/untreated: Mean age = 67.2 ± 10.1 years, PDE5I/treated: Mean age = 62.7 ± 10.0 years, P < 0.0001) and TRT (Low T/untreated: 67.3 ± 11.3 years, Low T/treated: 61.8 ± 10.9 years, P < 0.0001) patients. No corresponding difference in age at final visit in survivors was observed in the Statin/untreated vs Statin/treated and Normal T/untreated vs Low T/untreated groups. Age at death did not significantly differ with statin (Statin/untreated: Mean age = 77.0 ± 10.5 years, Statin/treated: Mean age = 75.8 ± 10.1 years, P = 0.56) or PDE5I treatment (PDE5I/untreated: Mean age = 76.4 ± 10.1 years, PDE5I/treated: Mean age = 67.0 ± 13.3 years, P = 0.11). Importantly, only 3 patients on PDE5I treatment died during follow-up (Table 1). Interestingly, age at death varied between the testosterone groups (Normal T/untreated: Mean age = 73.9 ± 10.6 years vs Low T/untreated: Mean age = 78.4 ± 8.9 years, P = 0.0.028, Low T/untreated: Mean age = 78.4 ± 8.9 vs Low T/treated: Mean age 66.3 ± 13.1 years, P = 0.0034).
As age at death or final visit differed between the treatment and testosterone status groups we used logistic regression analyses to see if the associations in Table 1 were independent. Table 2 shows age is associated with mortality regardless of the other factors added to regression models (Models a-e). Significant reduction in mortality was observed with TRT (Low T men - Model c) and PDE5I (Model d) treatments while the benefit due to statins approached significance (Model b). All 3 treatments were significantly associated with decreased mortality when entered together (Model e).
Table 2.
Association between age and mortality corrected for statin treatment, testosterone status/treatment and phosphodiesterase 5-inhibitors treatment
| OR (95%CI) | P value | |
| Model a | ||
| Age (yr) | 1.10 (1.08-1.13) | < 0.001 |
| Model b | ||
| Age (yr) | 1.10 (1.07-1.13) | < 0.001 |
| Statin/untreated | Reference | |
| Statin/treated | 0.63 (0.39-1.01) | 0.057 |
| Model c | ||
| Age (yr) | 1.10 (1.07-1.12) | < 0.001 |
| Normal T/untreated | 0.61 (0.42-1.07) | 0.092 |
| Low T/untreated | Reference | |
| Low T/treated | 0.31 (0.13-0.75) | 0.009 |
| Model d | ||
| Age (yr) | 1.09 (1.07-1.12) | < 0.001 |
| PDE5I/untreated | Reference | |
| PDE5I/treated | 0.16 (0.051-0.54) | 0.003 |
| Model e | ||
| Age (yr) | 1.08 (1.06-1.11) | < 0.001 |
| Statin/untreated | Reference | |
| Statin/treated | 0.59 (0.36-0.97) | 0.038 |
| Normal T/untreated | 0.69 (0.43-1.10) | 0.120 |
| Low T/untreated | Reference | |
| Low T/treated | 0.38 (0.15-0.92) | 0.033 |
| PDE5I/untreated | Reference | |
| PDE5I/treated | 0.17 (0.053-0.56) | 0.004 |
PDE5I: Phosphodiesterase 5-inhibitors.
We determined if our mortality data adhered to the Gompertz-Makeham equation. Figure 1 shows mortality rate (as % and logarithmic values) plotted against age (as 5 year categories). A linear relationship was observed between Ln (mortality) and age (R2 = 0.881) suggesting that the mortality observed in our cohort did fit the pattern initially described by Gompertz.
Figure 1.
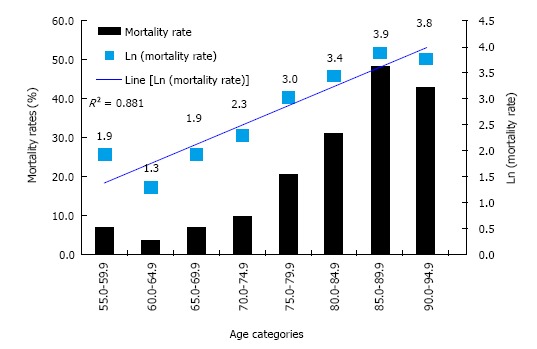
Association between Ln (mortality rate) and age.
However, our cohort is heterogeneous as it comprises men on varying treatments that influence mortality (Table 1). Figure 2 displays the relationship between mortality rates in the total group and in the different treatment and age categories (5 years selected due to lower numbers in the subgroups). As predicted in the logistic regression analyses (Table 2), statin, TRT and PDE5I treatments reduced the age related mortality, though the reduction differed in the age categories.
Figure 2.
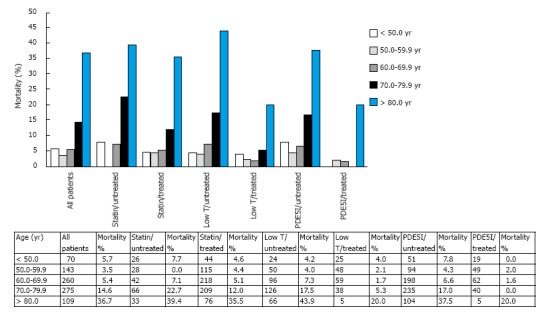
Mortality rates by age at death or final visit observed in the total group and treatment categories. PDE5I: Phosphodiesterase 5-inhibitors.
To further graphically demonstrate the impact of statin, TRT and PDE5I on mortality, the probability of mortality of each patient together with the 95%CI were estimated from the logistic regression analyses in Table 2. Figures 3 show the probability of mortality plotted against the age of the patient in the total cohort, by the treatment groups and by treatment combination (men on all 3 treatments vs not on any of the treatments). In the statin (Figure 3B) and TRT (Figure 3C) plots some overlap in the 95%CI is seen between treated compared to untreated men. For PDE5I (Figure 3D) and combination treatments (Figure 3E) no overlap of 95%CI values was observed after 50 years of age indicating the relationship between mortality and age is significantly altered.
Figure 3.
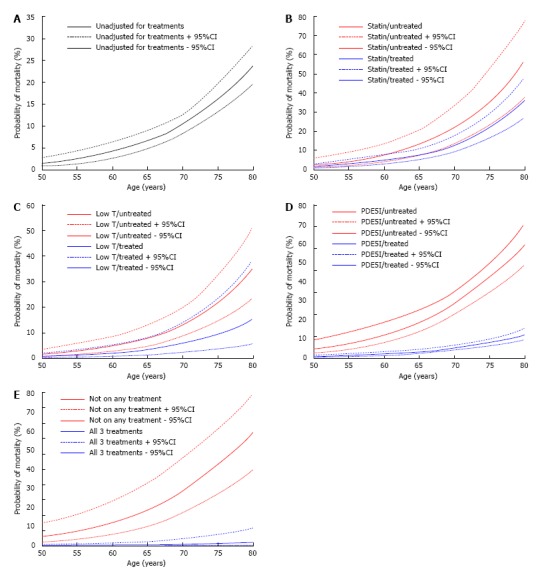
Association between probability of mortality and age. The estimated mortality probability and 95%CI from the fitted logistic regression (Table 2) were calculated from the logistic regression analyses seen in Table 2 and plotted against age at death or final visit in the following groups. Age was restricted to between 50-80 years due to reduced patient numbers in the treatment (Low T/treated and PDE5I/treated) groups (> 80 years) and the exponential pattern only being evident in the total group over the age of 50 years (Figure 1). A: Total group (from Model a in Table 2); B: Men stratified by statin treatment (from Model b in Table 2); C: Men stratified by testosterone treatment (from Model c in Table 2); D: Men stratified by PDE5I treatment (from Model d in Table 2); E: Men on all and none of the above treatments (from Model e in Table 2). PDE5I: Phosphodiesterase 5-inhibitors.
DISCUSSION
In a recent longitudinal study we showed that in men with T2DM, hypogonadism is associated with increased mortality compared to eugonadal men. Importantly TRT abolished this increase in mortality[19]. PDE5I (HR = 0.21, P = 0.009) and possibly statin (HR = 0.69, P = 0.086) use were also observed to reduce mortality[19]. Our aim in this paper was to determine how these three commonly used treatments influence the association between age and mortality in T2DM men. Our approach was to determine the probability of a patient in each treatment group living or dying at a particular age. Importantly, the Gompertz-Makeham law accurately describes the association between age and mortality in subjects aged approximately between 30-80 years, an age range that encompasses most of our study group.
Data on the United Kingdom Government Web Archive (http://webarchive.nationalarchives.gov.uk/20160105160709/ http://www.ons.gov.uk/ons/rel/vsob1/death-reg-sum-tables/2013/sty-mortality-rates-by-age.html - accessed 2016 Oct 24) shows firstly, the relevance of the Gompertz model; mortality rates between 1963 and 2013 demonstrate an exponential pattern similar to that described nearly 200 years ago by Gompertz. Secondly, the Archive data show that while the adult mortality rate had fallen during 1963-2013, possibly because of a reduction in CVD resulting from statin use and smoking cessation, it still demonstrated an exponential pattern in both genders.
In this study, we used a retrospective approach based on logistic regression to study the impact of treatment on the association between age and mortality. Accordingly, we compared results from this study with those from a previous prospective longitudinal analysis[19]. As expected, similar independent associations between the treatments and mortality were observed (Table 2, model e) enabling us to examine the impact of treatment on the relationship between age and probability of mortality. The relationship between age and mortality remained significant regardless of which (single or combination) treatment factors were added to regression models.
Life tables derived from data from an adult population will reflect a combination of phenotypes related to lifestyle, pathology, therapy and genetic factors that confer varying risks of dying at a particular age. Thus, we did not expect such a close fit (R2 = 0.881) as that observed when the mortality rates of our total cohort were transformed logarithmically and plotted against age. It was tempting to carry out a similar exercise in the treatment and non-treatment subgroups but small patient numbers and low mortality rates in the testosterone and PDE5I treated men prevented this. Greater patient numbers with higher event rates would have permitted a study of these subgroups.
Using logistic regression to estimate probability of mortality for each individual patient allowed graphic demonstration of the impact of treatment on age, the most significant predictor of death. Whilst statin use and TRT did not show a statistically significant effect on the relationship between age and mortality, PDE5I treatment and combination (statin, TRT and PDE5I) treatment clearly did with no overlap of 95%CI. These results are compatible with studies showing that in T2DM patients, treatment with vardenafil results in improved endothelial parameters including flow-mediated dilation, interleukin-6 and testosterone levels[29] and indicate that a randomised controlled trial (RCT) is required for PDE5I and TRT in men with T2DM. It would be interesting if some of the large RCTs carried out showing statin-associated reductions in all-cause mortality such as 4S were analysed to establish ways in which the relationship between age and mortality may have been altered.
There are limitations to our study. The TRT arm was based on an intention to treat. The age of onset and duration of diabetes as well as exposure to statins and PDE5I were not documented. Data on the type of drug and dose was not completely recorded. We assumed that statin and PDE5I treatments were protocol driven. However, it is possible that patient selection, especially with PDE5I prescribing existed. It is believed however, that the principal reason for PDE5I prescribing is ED which has been established as a significant predictor of CVD and all-cause mortality.
Despite their limitations, our findings are important. We showed that mortality rates in men with T2DM follow the pattern described by Gompertz. We confirmed that statin, TRT and PDE5I reduce mortality in this cohort and have described how they influenced the relationship between age and mortality. We believe that our approach of communicating the effectiveness of an intervention by determining the probability of mortality at different ages is easy to understand and could be used by clinicians to improve patient compliance and lead to clinical benefit.
Our study examines the relationship between age and mortality in men with diabetes. Age was related to mortality in accordance with the Gompertz-Makeham law. We show that statin, TRT and PDE5I treatments impacted all-cause mortality and PDE5I treatment alone and in combination with statin and TRT significantly altered the relationship between age and mortality.
COMMENTS
Background
As far as the authors are aware the Gompertz-Makeham association has not been used to demonstrate the impact of medical treatments on the relationship between age and mortality.
Research frontiers
There is considerable debate regarding the effects of testosterone replacement therapy and phosphodiesterase 5-inhibitors (PDE5I) treatment in diabetic men. Although no randomised controlled studies exist longitudinal observational studies have shown potential benefits.
Innovations and breakthroughs
Having established that statin, PDE5I treatment and testosterone replacement therapy reduced mortality in the cohort, the studied the influence of these agents on this association. The data are presented in a novel graphical manner, clearly demonstrating the impact of these agents on mortality. The authors suggest the graphical illustrations in this paper will communicate the benefit of these interventions to patients and have a major positive bearing on patient compliance.
Peer-review
This paper is a very interesting retrospective study investigating whether the mortality rate follows the pattern described by Gompertz and estimating how testosterone status and treatments (statins, testosterone replacement therapy and phosphodiesterase 5 inhibitors) alter the mortality rate. A substantial and extremely meticulous work has been done and the findings are consistent.
Footnotes
Supported by Bayer plc to University of Bedfordshire (ref: SOP ID: BSP-SOP-040); Bayer plc played no part in the design, conduct of the study, data collection, statistical analyses or preparation of the manuscript.
Institutional review board statement: The study was reviewed and approved by the following bodies: National Research Ethics Service (West Midlands Ethics Service) 08/H1208/30; National Institute for Health Research (Birmingham and the Black Country Comprehensive Local Research Network), RM and G reference Number 1268; Warwickshire Primary Care Trust (West Midlands (South) Comprehensive Local Research Network), reference WAR230909; Institute of Diabetes for Older People, Beds and Herts Postgraduate Medical School, University of Bedfordshire (ref: SOP ID: BSP-SOP-040); Bayer plc (ref: SOP ID: BSP-SOP-040): funding organisation; Institute for Science and Technology in Medicine, Keele University Medical School: approval for publication (Professor N Forsyth, Director of the Institute).
Informed consent statement: All participants in the intervention study (BLAST) provided informed written consent prior to study enrolment. The long term follow-up of the screened individuals receiving standard care was approved as an audit by Primary Care Trusts.
Conflict-of-interest statement: No potential conflicts of interest relevant to this article were reported.
Data sharing statement: Technical appendix, statistical code, and dataset are available from the corresponding author at (sud.ramachandran@heartofengland.nhs.uk). Participants gave informed consent for anonymous data to be shared with health authorities and published in scientific journals.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: October 31, 2016
First decision: December 1, 2016
Article in press: January 3, 2017
P- Reviewer: Dimitriadis F, Georgescu A, Li HJ S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
References
- 1.Parsonnet J, Greene KD, Gerber AR, Tauxe RV, Vallejo Aguilar OJ, Blake PA. Shigella dysenteriae type 1 infections in US travellers to Mexico, 1988. Lancet. 1989;2:543–545. doi: 10.1016/s0140-6736(89)90662-4. [DOI] [PubMed] [Google Scholar]
- 2.Hallén A. Makeham’s addition to the Gompertz law re-evaluated. Biogerontology. 2009;10:517–522. doi: 10.1007/s10522-008-9184-0. [DOI] [PubMed] [Google Scholar]
- 3.Vaupel JW. Biodemography of human ageing. Nature. 2010;464:536–542. doi: 10.1038/nature08984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltrán-Sáncheza H, Crimmins E, Finch C. Early cohort mortality predicts the rate of aging in the cohort: a historical analysis. J Dev Orig Health Dis. 2012;3:380–386. doi: 10.1017/S2040174412000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med. 2006;23:516–521. doi: 10.1111/j.1464-5491.2006.01838.x. [DOI] [PubMed] [Google Scholar]
- 6.Hansen LJ, Olivarius Nde F, Siersma V. 16-year excess all-cause mortality of newly diagnosed type 2 diabetic patients: a cohort study. BMC Public Health. 2009;9:400. doi: 10.1186/1471-2458-9-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan CL, Currie CJ, Peters JR. Relationship between diabetes and mortality: a population study using record linkage. Diabetes Care. 2000;23:1103–1107. doi: 10.2337/diacare.23.8.1103. [DOI] [PubMed] [Google Scholar]
- 8.Nwaneri C, Bowen-Jones D, Cooper H. Screening for type 2 diabetes and population mortality over 10 years. Lancet. 2013;381:901–902. doi: 10.1016/S0140-6736(13)60665-0. [DOI] [PubMed] [Google Scholar]
- 9.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 10.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 11.Pyŏrälä K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S) Diabetes Care. 1997;20:614–620. doi: 10.2337/diacare.20.4.614. [DOI] [PubMed] [Google Scholar]
- 12.Hackett GI, Cole NS, Deshpande AA, Popple MD, Kennedy D, Wilkinson P. Biochemical hypogonadism in men with type 2 diabetes in Primary Care Practice. Br J Diabetes Vasc Dis. 2009;5:226–231. [Google Scholar]
- 13.Ma RC, So WY, Yang X, Yu LW, Kong AP, Ko GT, Chow CC, Cockram CS, Chan JC, Tong PC. Erectile dysfunction predicts coronary heart disease in type 2 diabetes. J Am Coll Cardiol. 2008;51:2045–2050. doi: 10.1016/j.jacc.2008.02.051. [DOI] [PubMed] [Google Scholar]
- 14.Dong JY, Zhang YH, Qin LQ. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2011;58:1378–1385. doi: 10.1016/j.jacc.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Gazzaruso C, Solerte SB, Pujia A, Coppola A, Vezzoli M, Salvucci F, Valenti C, Giustina A, Garzaniti A. Erectile dysfunction as a predictor of cardiovascular events and death in diabetic patients with angiographically proven asymptomatic coronary artery disease: a potential protective role for statins and 5-phosphodiesterase inhibitors. J Am Coll Cardiol. 2008;51:2040–2044. doi: 10.1016/j.jacc.2007.10.069. [DOI] [PubMed] [Google Scholar]
- 16.Fukui M, Kitagawa Y, Nakamura N, Kadono M, Mogami S, Hirata C, Ichio N, Wada K, Hasegawa G, Yoshikawa T. Association between serum testosterone concentration and carotid atherosclerosis in men with type 2 diabetes. Diabetes Care. 2003;26:1869–1873. doi: 10.2337/diacare.26.6.1869. [DOI] [PubMed] [Google Scholar]
- 17.Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169:725–733. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 18.Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050–2058. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- 19.Hackett G, Heald AH, Sinclair A, Jones PW, Strange RC, Ramachandran S. Serum testosterone, testosterone replacement therapy and all-cause mortality in men with type 2 diabetes: retrospective consideration of the impact of PDE5 inhibitors and statins. Int J Clin Pract. 2016;70:244–253. doi: 10.1111/ijcp.12779. [DOI] [PubMed] [Google Scholar]
- 20.Hackett G, Cole N, Saghir A, Jones P, Strange RC, Ramachandran S. Testosterone undecanoate improves sexual function in men with type 2 diabetes and severe hypogonadism: results from a 30-week randomized placebo-controlled study. BJU Int. 2016;118:804–813. doi: 10.1111/bju.13516. [DOI] [PubMed] [Google Scholar]
- 21.Francis SH, Corbin JD. Molecular mechanisms and pharmacokinetics of phosphodiesterase-5 antagonists. Curr Urol Rep. 2003;4:457–465. doi: 10.1007/s11934-003-0027-x. [DOI] [PubMed] [Google Scholar]
- 22.Vlachopoulos C, Ioakeimidis N, Rokkas K, Stefanadis C. Cardiovascular effects of phosphodiesterase type 5 inhibitors. J Sex Med. 2009;6:658–674. doi: 10.1111/j.1743-6109.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- 23.Anderson SG, Hutchings DC, Woodward M, Rahimi K, Rutter MK, Kirby M, Hackett G, Trafford AW, Heald AH. Phosphodiesterase type-5 inhibitor use in type 2 diabetes is associated with a reduction in all-cause mortality. Heart. 2016;102:1750–1756. doi: 10.1136/heartjnl-2015-309223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P. Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med. 2014;11:840–856. doi: 10.1111/jsm.12404. [DOI] [PubMed] [Google Scholar]
- 25.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 26.Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P, Saghir A. The response to testosterone undecanoate in men with type 2 diabetes is dependent on achieving threshold serum levels (the BLAST study) Int J Clin Pract. 2014;68:203–215. doi: 10.1111/ijcp.12235. [DOI] [PubMed] [Google Scholar]
- 27.Hackett G, Kell P, Ralph D, Dean J, Price D, Speakman M, Wylie K. British Society for Sexual Medicine guidelines on the management of erectile dysfunction. J Sex Med. 2008;5:1841–1865. doi: 10.1111/j.1743-6109.2008.00773.x. [DOI] [PubMed] [Google Scholar]
- 28.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 29.Santi D, Granata AR, Guidi A, Pignatti E, Trenti T, Roli L, Bozic R, Zaza S, Pacchioni C, Romano S, et al. Six months of daily treatment with vardenafil improves parameters of endothelial inflammation and of hypogonadism in male patients with type 2 diabetes and erectile dysfunction: a randomized, double-blind, prospective trial. Eur J Endocrinol. 2016;174:513–522. doi: 10.1530/EJE-15-1100. [DOI] [PubMed] [Google Scholar]


