Abstract
Anabolic androgenic steroids (AAS) are performance-enhancing drugs commonly abused by atheletes. Stanozolol is a synthetic testosterone-derived anabolic steroid. Although it is well known that AAS have several side-effects, there are only few toxicological studies available on the toxic effects and mechanisms of action of stanozolol. The aim of this study was to investigate the genotoxic effects of stanozolol and to determine its effects on telomerase activity in Sprague-Dawley male rats. For this purpose, 34 male rats were divided into 5 groups as follows: i) the control group (n=5); ii) the propylene glycol (PG)-treated group (n=5); iii) the stanozolol-treated group (n=8); iv) the PG-treated group subjected to exercise (n=8); and v) the stanozolol-treated group subjected to exercise (n=8). PG is used as a solvent control in our study. Stanozolol (5 mg/kg) and PG (1 ml/kg) were injected subcutaneously 5 days/week for 28 days. After 28 days, the animals were sacrificed, and DNA damage evaluation (comet assay) and telomerase activity assays were then performed using peripheral blood mononuclear cells (PBMCs). Telomerase activity was measured by using the TeloTAGGG Telomerase PCR ELISA PLUS kit. The results of this study revealed that stanozolol treatment induced DNA damage, while exercise exerted a protective effect. Stanozolol treatment without exercise stimulation was associated with a significant increase in telomerase activity in the PBMCs.
Keywords: anabolic androgenic steroids, stanozolol, telomerase activity, DNA damage, comet assay
Introduction
Anabolic androgenic steroids (AAS) are synthetic testosterone derivatives, which are widely abused by athletes for enhancing performance and psychological well-being (1,2). These drugs have been abused for many years by athletes and teenagers for the purpose of performance enhancement (3,4).
The chemical structure of steroids, dosage and usage frequency are the main factors responsible for the long-term side-effects of AAS. At supraphysiological doses, several side-effects have been reported due to the use of AAS. These effects include the disruption of lipid metabolism, high blood pressure, cardiac disorders, testicular atrophy, erectile dysfunction, impotence, decreased sex hormone levels, menstrual irregularities, clitoral enlargement, peliosis hepatis, neoplasia, cholestasis, jaundice, acne, alopecia, aggressiveness, depressive symptoms, manic symptoms and psychosis (5). According to worldwide testing data, it has been demonstrated that testosterone, nandrolone, methandienone and stanozolol are the most commonly abused steroids (6).
Stanozolol is a synthetic 17α-alkylated derivative of testosterone, with slow hepatic degradation (7). It is medically used for the treatment of anemia and hereditary angioedema (8). Stanozolol has the largest anabolic/androgenic ratios (9). It is known that stanozolol abuse causes a wide range of adverse effects (10). The ‘Association of Racing Commissioners International’ classified stanozolol as a class III drug (11). According to the ‘2016 Prohibition List’ prepared by the World Anti-Doping Agency (WADA), stanozolol is classified as class S1.1a and it has been prohibited both in and out of competition (12). However, due to the widespread usage of stanozolol, its metabolites have been found to be present in 50% of positive doping cases (10,13). 3′-Hydroxystanozolol, 4β-hydroxystanozolol and 16β-hydroxystanozolol are the main three metabolites of stanozolol. The acceptable urinary concentrations of stanozolol and/or its metabolites are restricted to a maximum of 2 ng/ml (13). The consumption steroid hormone drugs is associated with toxicity, mutagenicity, genotoxicity and cancerogenesis, depending on several factors, including genetic and epigenetic modifications (14). Torres-Bugarín et al reported micronucleus (MN) frequency increase in buccal mucosa cells from bodybuilders who abused AAS (15). However, Hana et al indicated that testosterone synthetic derivatives had no genotoxic effects on mouse germ cells (16). Controversial results have been obtained regarding the genotoxic effects of steroids (17,18).
Telomeres play a key role in cell aging and DNA damage mechanisms. The loss of telomere function causes several adverse effects, such as genomic instability, aneuploidy and even cancer. It has been reported that tumor tissues express elevated levels of telomerase compared to normal tissues (19). Several parameters can affect telomere and telomerase functions, including oxidative stress, DNA damage, dietary supplementations and environmental/occupational exposures (20,21). The inhibition of telomerase activity is known to be associated with genomic instability, and plays an important role in DNA damage mechanisms and cancer development. It has been demonstrated that the expression of telomerase enzyme protects cells from DNA-damaging events, and it has been shown that this protective function is independent from its telomere synthesis function (22).
In this study, we investigated the DNA-damaging potential of stanozolol and its effects on telomerase activity in stanozolol-treated Sprague-Dawley rats. The role of exercise in DNA damage and telomerase activity was also assessed, as the main target population of stanozolol users are usually physically active individuals.
Materials and methods
Animal Study
A total of 34 Sprague-Dawley male rats were divided into 5 groups as follows: i) the control group (n=5); ii) the propylene glycol (PG)-treated group (n=5); iii) the stanozolol-treated group (n=8); iv) the PG-treated group subjected to exercise (n=8); and v) the stanozolol-treated group subjected to exercise (n=8) (Table I). The mice were injected with 1 ml/kg PG as a solvent control. Stanozolol (5 mg/kg) was injected subcutaneously for 28 days for 5 days per week. Swimming was selected as a mode of exercise. The mice were subjected to exercise (swimming) 1 week prior to the commencement of the treatments so that the animals may be able to adapt. During the 28 days of the experiment, the animals subjected to 20 min of swimming for 5 days per week. After 28 days, the animals were lightly anesthetized and euthanized by cervical dislocation. Peripheral blood samples were collected from the tail vein of the rats for the determination of telomerase activity and DNA damage by comet assay. Peripheral blood mononuclear cells (PBMCs) were isolated by using Histopaque 107 (MP Biomedicals LLC, Solon-Ohio, USA). Heparin-treated blood was diluted with an equal volume of PBS and layered carefully over density gradient media and centrifuged at 200 × g for 3 min at 4°C. During centrifugation, cells migrate differentially and layers are formed. PBMCs were retrieved from just above the boundary between plasma and Histopaque. Comet assay and telomerase activity assays were all performed using the PBMCs. Cell viability was examined by the trypan blue exclusion assay before performing comet assay and the viability results were >90% for each sample (data not shown).
Table I.
Experimental groups in this study.
| Animal groupsa | No. of rats | Procedures undertaken |
|---|---|---|
| Group I | 5 | No injection, no exercise |
| Group II | 5 | Subcutaneous injection of 1 ml/kg PG |
| Group III | 8 | Subcutaneous injection of 5 mg/kg stanozolol |
| Group IV | 8 | Subcutaneous injection of 1 ml/kg PG, exercise 20 min/day, 5 day/week (swimming) |
| Group V | 8 | Subcutaneous injection of 5 mg/kg stanozolol, exercise 20 min/day, 5 day/week (swimming) |
Group I, control; group II, PG; group III, stanozolol; group IV, PG treatment and exercise; group V, stanozolol treatment and exercise; PG, propylene glycol
Rat care, handling, and all of the experimental procedures employed were in accordance with the instructions of the Ethics Committee on Animal Experimentation of Istanbul University, HADYEK and approved with no. 2013/100.
Comet assay
The alkaline comet assay was performed as previously descrbibed by Singh et al (23) with slight modifications, as described in Ozcagli et al (24). Each microscopic slide was covered with 0.65% normal melting agarose (NMA). Histopaque density gradient centrifugation was used for lymphocyte isolation. The isolated cells were mixed with 0.65% low melting agarose (LMA) and embedded on NMA-covered slides. The slides were incubated overnight in cold lysing solution (1% Triton X–100 and 10% DMSO added just before use in 2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, pH 10) at 4°C. Subsequently, an electrophoresis step was performed with (1 mM Na2EDTA and 300 mM NaOH, pH 13) fresh solution following alkali exposure to 20 min for DNA unwinding. The electrophoresis step was conducted at 1.6 V/cm for 20 min (300 mA) at 4°C. Prior to ethidium bromide (EB) dye staining, the alkali conditions were neutralizated with tris buffer (0.4 M Tris, pH 7.5). All these steps were conducted under dimmed light and the slides were evaluated by one slide reader, blindly. One hundred cells per slide were analyzed under a fluorescence microscope equipped with an excitation filter of 546 nm and a barrier filter of 590 nm (Olympus BX53; Olympus Corp., Tokyo, Japan). The results were evaluated using the Comet Assay IV image analysis system (Comet Assay IV; Perceptive Instruments Ltd., Suffolk, UK) in order to score DNA damage. Tail intensity data was selected for the evaluation of DNA damage.
Telomerase activity assay
The determination of telomerase activity in the PBMCs was performed quantitatively using the teloTAGGG telomerase PCR ELISA PLUS kit (Roche Diagnostic GmbH, Mannheim, Germany). The kit protocol was followed for telomerase activity assessment as previously described (25). A total of 2×105 cells were transferred to a PCR tube for sample preparation for telomerase activity assay. Following centrifugation at 3,000 × g for 10 min, the supernatant was carefully removed and the pellets were collected. The assay principle was consisting of two steps. The first step included the addition of telomeric repeats (TTAGGG) to the 3′ end of biotin-labelled primers and product amplification by PCR. The second step consisted of ELISA reaction.
Statistical analysis
The mean, standard deviation (SD) and the median were used for the expression of tail intensity. Telomerase activity was expressed in proportion of the control group (%). The non-parametric Kruskal-Wallis test was applied for comparing the results of comet assay. The same test was also applied for the comparison of expressed telomerase activity. Non-parametric post-hoc comparisons were assessed using Dunn's test. Box and whisker plots were used for the graphical representation of the data. IBM SPSS Statistical software 21.0 (IBM SPSS, Armonk, NY, USA) was used for statistical analysis. A value of P<0.05 was set for accepting (or rejecting) the null hypothesis and was considered to indicate a statistically significant difference.
Results
The results of tail intensity and the telomerase activity (%) in the experiental groups (exposed and controls) are shown in Table II. The analysis revealed that tail intensity differed significantly between groups (P=0.032), while differences in telomerase activity were also significant (P<0.001). It can be seen that the highest values were observed in group III for both comet assay and telomerase activity assessment (5.8±1.6 and 1851.4±319.2, respectively) in comparison to the other groups (I, II, IV and V). All other values ranged between 3.5±0.6 (group V) to 5.1±1.7 (group IV) for tail intensity, while the lowest telomerase activity (%) was 100±19.4 (group I).
Table II.
Expression of tail intensity and telomerase activity (%) in the peripheral blood mononuclear cells from the rats in the experimental groups.
| Assay | Exposurea group | No. of rats | Mean ± standard deviation | Median | Kruskal-Wallis |
|---|---|---|---|---|---|
| Tail intensity | Group I | 5 | 4.0±0.5 | 4.3 | χ2=10.56 |
| Group II | 5 | 4.8±2.8 | 3.9 | df=4 | |
| Group III | 8 | 5.8±1.6 | 0.5 | P=0.032 | |
| Group IV | 8 | 5.1±1.7 | 4.85 | ||
| Group V | 8 | 3.5±0.6 | 3.6 | ||
| Telomerase activity (%) | Group I | 5 | 100.0±19.4 | 93.3 | χ2=24.65 |
| Group II | 5 | 474.9±358.2 | 597.0 | df=4 | |
| Group III | 8 | 1,851.4±319.2 | 1,909.2 | P<0.001 | |
| Group IV | 8 | 193.4±27.9 | 196.5 | ||
| Group V | 8 | 328.7±183.1 | 240.0 |
Group I, control; group II, PG; group III, stanozolol; group IV, PG treatment and exercise; group V, stanozolol treatment and exercise.
According to the results of the pair wise comparison of tail intensity based on Dunn's test, was observed that the cells from the animals in group V exhibited significantly lower levels of tail intensity compared to those from the animals in group IV (P=0.025) and group III (P=0.002). Our results revealed that telomerase activity (%) differed significantly when group III was compared with the other groups (group I, P<0.001; group II, P=0.016; group IV, P<0.001; and group V, P=0.012). Group I exhibited a significant difference compared with the other groups apart from group IV (P=0.121). Group II did exhibit a significant difference compared to group V (P=0.292).
The expression of tail intensity is also presented in a box and whisker plot (Fig. 1A), while the telomerase expression (%) is presented in a bar chart (Fig. 1B).
Figure 1.
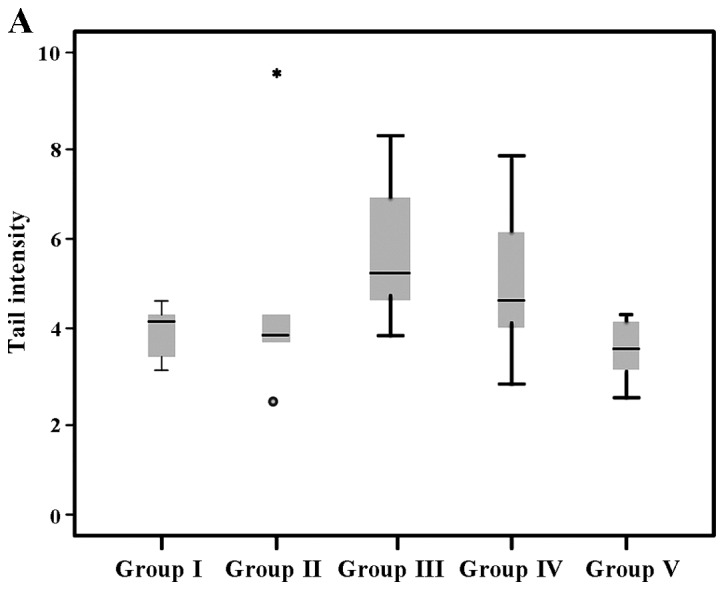
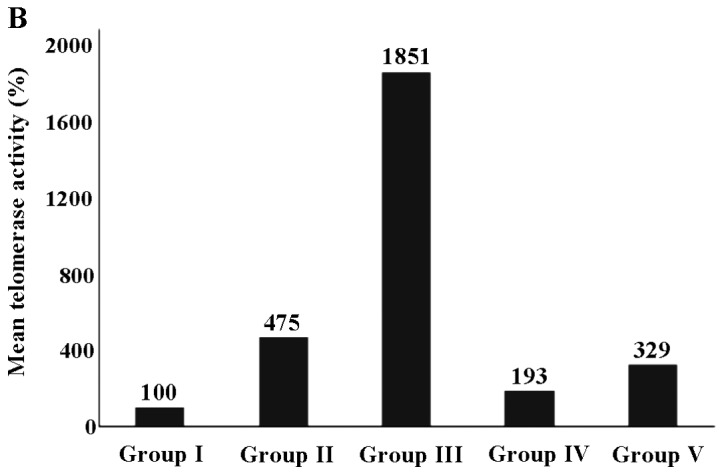
(A) Tail intensity and (B) telomerase activity (%) of stanozolol and propylene glycol (PG)-exposed groups. Group I, control; group II, PG; group III, stanozolol; group IV, PG treatment and exercise; group V, stanozolol treatment and exercise. Extreme outliers are indicated by an asterisk (*) and circle (º).
Discussion
Apart from causing several adverse health effects, the DNA-damaging effects of different AAS have been studied using various techniques. Stanozolol is a widely abused steroid, particularly for the purposes of doping and improving physical status. Very limited information is available regarding the DNA-damaging effects of stanozolol, while a positive correlation between exposure to nandrolone and DNA damage has been determined (14,26,27). In previous studies, a significant increase in DNA damage was determined in blood, liver, bone marrow, brain and testicle cells in experimental animals exposed to high doses of nandrolone (14,28). In addition, in a review, Boettcher et al reported increased DNA damage with trenbolone treatment in zebrafish embryos via comet assay and the micronucleus test; however, this increase was not observed with high-dose trenbolone treatment (29 and refs therein). Our results are in accordance with the findings of these studies, demonstrating the DNA-damaging effects of stanozolol. Although exercise has been defined as a key element of a healthy lifestyle, many controversial results have also been published (30–32). Exercise-induced DNA damage has been documented in several studies in physically active/overtrained individuals or even in experimental animals by several techniques (33–35). According to our search, there was only one study available in the literature investigating stanozolol and exercise-induced genotoxicity. Martins et al demonstrated that the administration of anabolic steroids (decadurabulin and winstrol) induced chromosomal damage, which was not associated with physical activity (36). The results of our study indicated increased DNA damage in the exercise-exposed control group (PG and exercise group); however, stanozolol played a protective role in exercise-induced DNA damage in the stonozolol-exposed groups.
It is well-documented that telomerase activity plays a role in DNA damage and repair mechanisms. In addition, several pathways have been defined regarding the mechanisms of the effects of AAS on telomerase activity (20,37,38) Nourbakhsh et al demonstrated that testosterone and androstenedione both increased cell viability by upregulating telomerase activity in the OVCAR-3 cell line. This upregulation was triggered by the PI3K/Akt pathway. Quantitative PCR analyses demonstrated that the hTERT mRNA levels were significantly increased with exposure to testosterone and androstenedione (37). Zhou et al demonstrated that the testosterone derivative, estrogen, increased telomerase activity via the mitogen-activated protein kinase (MAPK) pathway in endometrial cancer cells (39). Geier et al reported significantly increased telomerase activity and gene expression with testosterone and dihydrotestosterone (DHT) treatment (40). In addition, androgen exposure has previously been reported to increase telomerase activity by higher TERT mRNA levels in PBMCs (38). According to our search, there was no study available in the literature on the effects of stanozolol on telomerase activity. There is limited evidence regarding the genotoxicity of stanozolol, while there are several studies available on the effects of other AAS. We demonstrated that stanozolol induced telomerase activity and this increase may be associated with DNA damage.
The effects of exercise on telomerase activity primarily depend on the amount of exercise and cell types. Increased telomerase enzyme activity in skeletal muscles with exercise has been shown by Ludlow et al (20). Exercise may reduce telomere shortening-associated aging; however, extreme amounts of exercise have been reported to shorten telomeres. Furthermore, the effects of exercise on telomeric genes and miRNA regulation provides insight into the mechanistic association between exercise, telomeres and health (41). Mosallanezhad et al demonstrated significantly increased levels of telomerase activity and improved telomere length in sedentary women who performed high-intensity interval training (42). In this study, we observed a decrease in telomerase activity in stanozolol-treated rats subjected to exercise. Our results are in line with those of previous findings regarding the effects of exercise on telomerase activity.
In conclusion, in this study, an increase in DNA damage was detected with exercise; however, it was observed that exercise did not have a significant DNA-damaging effect in the steroid treated group. Therefore, the preventive role of stanozolol should be taken into consideration for the interpretation of these results for physically active or overtrained individuals. There is a need for further studies in order to clarify the preventive role of stanozolol in physical activity in terms of DNA damage. Thus, the effects of exercise warrant further clarifications for the determination of the genotoxic effects of stanozolol and the mechanisms responsible for stanozolol-induced DNA damage. The DNA-damaging effects of stanozolol may be mediated through alterations in telomerase activity. However, further studies are required in order to fully elucidate the association between stanozolol use, exercise and DNA damage.
Acknowledgements
The present study was supported by Istanbul University Scientific Research Projects Department (project no. 39101-21899).
Glossary
Abbreviations
- AAS
anabolic androgenic steroids
- EB
ethidium bromide
- LMA
low melting agarose
- NMA
normal melting agarose
- PBMCs
peripheral blood mononuclear cells
- SD
standard deviation
- WADA
World Anti-Doping Agency
References
- 1.Sagoe D, McVeigh J, Bjørnebekk A, Essilfie MS, Andreassen CS, Pallesen S. Polypharmacy among anabolic-androgenic steroid users: A descriptive metasynthesis. Subst Abuse Treat Prev Policy. 2015;10:12. doi: 10.1186/s13011-015-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsitsimpikou C, Tsarouhas K, Spandidos DA, Tsatsakis AM. Detection of stanozolol in the urine of athletes at a pg level: The possibility of passive exposure. Biomed Rep. 2016;5:665–666. doi: 10.3892/br.2016.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darke S, Torok M, Duflou J. Sudden or unnatural deaths involving anabolic-androgenic steroids. J Forensic Sci. 2014;59:1025–1028. doi: 10.1111/1556-4029.12424. [DOI] [PubMed] [Google Scholar]
- 4.Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34:513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- 5.Büttner A, Thieme D. Side effects of anabolic androgenic steroids: Pathological findings and structure-activity relationships. Handb Exp Pharmacol. 2010;195:459–484. doi: 10.1007/978-3-540-79088-4_19. [DOI] [PubMed] [Google Scholar]
- 6.Kicman AT. Pharmacology of anabolic steroids. Br J Pharmacol. 2008;154:502–521. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dos Santos G Barbosa, Rodrigues MJ Machado, Gonçalves EM, Marcondes MC Cintra Gomes, Areas MA. Melatonin reduces oxidative stress and cardiovascular changes induced by stanozolol in rats exposed to swimming exercise. Eurasian J Med. 2013;45:155–162. doi: 10.5152/eajm.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beg T, Siddque YH, Afzal M. Chromosomal damage induced by androgenic anabolic steroids, stanozolol and trenbolone in human lymphocytes. Adv Environ Biol. 2007;1:39–43. [Google Scholar]
- 9.Helfman T, Falanga V. Stanozolol as a novel therapeutic agent in dermatology. J Am Acad Dermatol. 1995;33:254–258. doi: 10.1016/0190-9622(95)90244-9. [DOI] [PubMed] [Google Scholar]
- 10.Jalali A Shalizar, Najafi G, Hosseinchi M, Sedighnia A. Royal Jelly alleviates sperm toxicity and improves in vitro fertilization outcome in Stanozolol-treated mice. Iran J Reprod Med. 2015;13:15–22. [PMC free article] [PubMed] [Google Scholar]
- 11.Moeller BC, Sams RA, GuingabCagmat JD, Szabo NJ, Colahan P, Stanley SD. Pharmacokinetics of stanozolol in Thoroughbred horses following intramuscular administration. J Vet Pharmacol Ther. 2013;36:201–204. doi: 10.1111/j.1365-2885.2012.01393.x. [DOI] [PubMed] [Google Scholar]
- 12.World Anti-doping Agency, corp-author. https://www.wada-ama.org/ 2016 List of Prohibited Substances and Methods. doi: 10.1093/anatox/35.9.608. Accessed September 16, 2016. [DOI] [PubMed] [Google Scholar]
- 13.Deshmukh NI, Zachar G, Petróczi A, Székely AD, Barker J, Naughton DP. Determination of stanozolol and 3′-hydroxystanozolol in rat hair, urine and serum using liquid chromatography tandem mass spectrometry. Chem Cent J. 2012;6:162. doi: 10.1186/1752-153X-6-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.do Carmo CA, Gonçalves ÁLM, Salvadori DMF, Maistro EL. Nandrolone androgenic hormone presents genotoxic effects in different cells of mice. J Appl Toxicol. 2012;32:810–814. doi: 10.1002/jat.1701. [DOI] [PubMed] [Google Scholar]
- 15.Torres-Bugarín O, Covarrubias-Bugarín R, Zamora-Perez AL, Torres-Mendoza BMG, García-Ulloa M, Martínez-Sandoval FG. Anabolic androgenic steroids induce micronuclei in buccal mucosa cells of bodybuilders. Br J Sports Med. 2007;41:592–596. doi: 10.1136/bjsm.2006.032474. discussion 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hana HY, Khalil WK, Elmakawy AI, Elmegeed GA. Androgenic profile and genotoxicity evaluation of testosterone propionate and novel synthesized heterocyclic steroids. J Steroid Biochem Mol Biol. 2008;110:284–294. doi: 10.1016/j.jsbmb.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Ho SM, Roy D. Sex hormone-induced nuclear DNA damage and lipid peroxidation in the dorsolateral prostates of Noble rats. Cancer Lett. 1994;84:155–162. doi: 10.1016/0304-3835(94)90370-0. [DOI] [PubMed] [Google Scholar]
- 18.Ragnotti G, Presta M, Maier JAM, Rusnati M, Mazzoleni G, Legati F, Chiesa R, Braga M, Calovini D. Critical role of gonadal hormones on the genotoxic activity of the hepatocarcinogen DL-ZAMI 1305. Cancer Lett. 1987;36:253–261. doi: 10.1016/0304-3835(87)90018-8. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Qi Y, Ge Y, Duplessis T, Rowan BG, Ip C, Cheng H, Rennie PS, Horikawa I, Lustig AJ, et al. Telomerase as an important target of androgen signaling blockade for prostate cancer treatment. Mol Cancer Ther. 2010;9:2016–2025. doi: 10.1158/1535-7163.MCT-09-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludlow AT, Ludlow LW, Roth SM. Do telomeres adapt to physiological stress? Exploring the effect of exercise on telomere length and telomere-related proteins. Biomed Res Int. 2013;2013:601368. doi: 10.1155/2013/601368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu H, Guo D, Li K, PedersenWhite J, StallmannJorgensen IS, Huang Y, Parikh S, Liu K, Dong Y. Increased telomerase activity and vitamin D supplementation in overweight African Americans. Int J Obes. 2012;36:805–809. doi: 10.1038/ijo.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleisig HB, Hukezalie KR, Thompson CAH, AuYeung TTT, Ludlow AT, Zhao CR, Wong JMY. Telomerase reverse transcriptase expression protects transformed human cells against DNA-damaging agents, and increases tolerance to chromosomal instability. Oncogene. 2016;35:218–227. doi: 10.1038/onc.2015.75. [DOI] [PubMed] [Google Scholar]
- 23.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 24.Ozcagli E, Sardas S, Biri A. Assessment of DNA damage in postmenopausal women under hormone replacement therapy. Maturitas. 2005;51:280–285. doi: 10.1016/j.maturitas.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Tsitsimpikou C, Tzatzarakis M, Fragkiadaki P, Kovatsi L, Stivaktakis P, Kalogeraki A, Kouretas D, Tsatsakis AM. Histopathological lesions, oxidative stress and genotoxic effects in liver and kidneys following long term exposure of rabbits to diazinon and propoxur. Toxicology. 2013;307:109–114. doi: 10.1016/j.tox.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed MA. Amelioration of nandrolone decanoate-induced testicular and sperm toxicity in rats by taurine: Effects on steroidogenesis, redox and inflammatory cascades, and intrinsic apoptotic pathway. Toxicol Appl Pharmacol. 2015;282:285–296. doi: 10.1016/j.taap.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Pozzi R, Fernandes KR, de Moura CFG, Ferrari RAM, Fernandes KPS, Renno ACM, Ribeiro DA. Nandrolone decanoate induces genetic damage in multiple organs of rats. Arch Environ Contam Toxicol. 2013;64:514–518. doi: 10.1007/s00244-012-9848-2. [DOI] [PubMed] [Google Scholar]
- 28.Hemmersbach P, Grosse J. Nandrolone: A multi-faceted doping agent. Handb Exp Pharmacol. 2010;195:127–154. doi: 10.1007/978-3-540-79088-4_6. [DOI] [PubMed] [Google Scholar]
- 29.Boettcher M, Kosmehl T, Braunbeck T. Low-dose effects and biphasic effect profiles: Is trenbolone a genotoxicant? Mutat Res. 2011;723:152–157. doi: 10.1016/j.mrgentox.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Spanidis Y, Goutzourelas N, Stagos D, Mpesios A, Priftis A, BarOr D, Spandidos DA, Tsatsakis AM, Leon G, Kouretas D. Variations in oxidative stress markers in elite basketball players at the beginning and end of a season. Exp Ther Med. 2016;11:147–153. doi: 10.3892/etm.2015.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baltaci SB, Mogulkoc R, Baltaci AK. Resveratrol and exercise (Review) Biomed Rep. 2016;5:525–530. doi: 10.3892/br.2016.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powers SK, Hogan MC. Exercise and oxidative stress. J Physiol. 2016;594:5079–5080. doi: 10.1113/JP272255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gandhi G, Chopra G. DNA damage in peripheral blood leukocytes of physically active individuals as measured by the alkaline single cell gel electrophoresis assay. Environ Mol Mutagen. 2009;50:291–303. doi: 10.1002/em.20457. [DOI] [PubMed] [Google Scholar]
- 34.Pereira BC, Pauli JR, Antunes LM, de Freitas EC, de Almeida MR, de Paula Venâncio V, Ropelle ER, de Souza CT, Cintra DE, Papoti M, et al. Overtraining is associated with DNA damage in blood and skeletal muscle cells of Swiss mice. BMC Physiol. 2013;13:11. doi: 10.1186/1472-6793-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siu PM, Pei XM, Teng BT, Benzie IF, Ying M, Wong SH. Habitual exercise increases resistance of lymphocytes to oxidant-induced DNA damage by upregulating expression of antioxidant and DNA repairing enzymes. Exp Physiol. 2011;96:889–906. doi: 10.1113/expphysiol.2011.058396. [DOI] [PubMed] [Google Scholar]
- 36.Martins RA, Gomes GA, Aguiar O, Jr, Medalha CC, Ribeiro DA. Chromosome damage and cytotoxicity in oral mucosa cells after 2 months of exposure to anabolic steroids (decadurabolin and winstrol) in weight lifting. Steroids. 2010;75:952–955. doi: 10.1016/j.steroids.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Nourbakhsh M, Golestani A, Zahrai M, Modarressi MH, Malekpour Z, Karami-Tehrani F. Androgens stimulate telomerase expression, activity and phosphorylation in ovarian adenocarcinoma cells. Mol Cell Endocrinol. 2010;330:10–16. doi: 10.1016/j.mce.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, Young NS. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114:2236–2243. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou C, Steplowski TA, Dickens HK, Malloy KM, Gehrig PA, Boggess JF, Bae-Jump VL. Estrogen induction of telomerase activity through regulation of the mitogen-activated protein kinase (MAPK) dependent pathway in human endometrial cancer cells. PLoS One. 2013;8:e55730. doi: 10.1371/journal.pone.0055730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geier R, Adler S, Rashid G, Klein A. The synthetic estrogen diethylstilbestrol (DES) inhibits the telomerase activity and gene expression of prostate cancer cells. Prostate. 2010;70:1307–1312. doi: 10.1002/pros.21166. [DOI] [PubMed] [Google Scholar]
- 41.Chilton WL, Marques FZ, West J, Kannourakis G, Berzins SP, O'Brien BJ, Charchar FJ. Acute exercise leads to regulation of telomere-associated genes and microRNA expression in immune cells. PLoS One. 2014;9:e92088. doi: 10.1371/journal.pone.0092088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosallanezhad Z, Nikbakht H, Gaeini AA, Gholami M. The effect of high-intensity interval training on telomere length of leukocytes in sedentary young women. Advances in Environmental Biology. 2014:841–846. [Google Scholar]


