Abstract
In the present study an experimental high-altitude intestinal barrier injury rat model was established by simulating an acute hypoxia environment, to provide an experimental basis to assess the pathogenesis, prevention and treatment of altitude sickness. A total of 70 healthy male Sprague-Dawley rats were divided into two groups: Control group (group C) and a high-altitude hypoxia group (group H). Following 2 days adaptation, the rats in group H were exposed to a simulated 4,000-m, high-altitude hypoxia environment for 3 days to establish the experimental model. To evaluate the model, bacterial translocation, serum lipopolysaccharide level, pathomorphology, ultrastructure and protein expression in rats were assessed. The results indicate that, compared with group C, the rate of bacterial translocation and the apoptotic index of intestinal epithelial cells were significantly higher in group H (P<0.01). Using a light microscope it was determined that the intestinal mucosa was thinner in group H, there were fewer epithelial cells present and the morphology was irregular. Observations with an electron microscope indicated that the intestinal epithelial cells in group H were injured, the spaces among intestinal villi were wider, the tight junctions among cells were open and lanthanum nitrate granules (from the fixing solution) had diffused into the intestinal mesenchyme. The expression of the tight junction protein occludin was also decreased in group H. Therefore, the methods applied in the present study enabled the establishment of a stable, high-altitude intestinal barrier injury model in rats.
Keywords: hypoxia, intestinal barrier, model
Introduction
High altitude is a low-pressure and hypoxic environment and it has been demonstrated that acute hypoxia can cause injury to a number of organs (1–3). The specialized blood supply and anatomical structure of the intestine increases its sensitivity to hypoxia, which makes the intestine vulnerable to the effects of hypoxic stress (4–6). It has been demonstrated that there are varying degrees of human gastrointestinal tract injury that occur in high-altitude hypoxic environments (7–9). As the largest bacteria and endotoxin library in the body, once the barrier function of the intestine is damaged, a large number of bacteria and endotoxins may translocate to other tissues or organs via the blood stream (10,11). This induces a cascade of inflammatory mediators, the onset of systemic inflammatory response syndrome and multiple organ dysfunction syndrome (MODS) (12,13) that may severely impact the health of individuals at high altitudes. The pathogenesis of acute hypoxia is complicated and remains unclear. There are vast plateau areas in China, where >60 million people live. When the Qinghai-Tibet railroad opened, large numbers of plainsmen began to participate in local economic construction. The safeguarding of people's safety and health is closely associated with national economic development. Research into the pathogenesis of high-altitude gastrointestinal disease may improve the quality of life of people living in the plateau areas and may provide guidance for such people with regard to prolonged, healthy living.
There are no similar conditions for assessment, and a lack of stable and repeatable experimental animal models of high-altitude intestinal injury restricts the understanding of this condition and the evaluation of potential therapeutic strategies. Thus, establishing an animal model is particularly important, as it is the basis of studying this injury. In the present study, a simulated high-altitude environment chamber combined with exercise on a treadmill was used to establish an animal model, which may provide an experimental basis for future studies.
Materials and methods
Animals and grouping
A total of 70 male Sprague-Dawley rats (7 weeks old) were purchased from the Experimental Animal Center of West China Hospital (Sichuan, China). The rats weighed 250±15 g and were randomly divided into two groups: Control group (group C) and the high-altitude acute hypoxia group (group H). The experiment was approved by the Chengdu Military General Hospital Ethics Committee (Chengdu, China).
Reagents and equipment
MacConkey agar culture medium and KTA Tachypleus amebocyte lysate were purchased from Zhanjiang Bokang Marine Biological Co., Ltd. (Guangzhou, China), terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) kit was purchased from Roche Diagnostics (cat. no. 11684817; Basel, Switzerland) and occludin rabbit monoclonal antibody (cat. no. 71-1500) was purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). A FLYDWC50-1A simulated high-altitude low-pressure chamber was purchased from Guizhou Fenglei Air Ordnance Ltd. Co. (Guizhou, China). A Tecnai 10 transmission electron microscope (Koninklijke Philips N.V., Amsterdam, Netherlands), S-3400 N scanning electron microscope (Hitachi, Ltd., Toyko, Japan) and ZH-PT experimental animal treadmill (Huaibei Zhenghua Biological Instrument Equipment Co. Ltd., Anhui, China) were also used in the present study.
Establishing the animal model
The animals were raised in the respiratory department of Chengdu Military General Hospital (Chengdu, China) at an altitude of ~500 m and a temperature of 24°C for 1 week to allow them to adapt to the environment. Food and water were provided ad libitum and a natural light cycle (12 h light/12 h dark) was adopted.
Adaptive training on the plain
The adaptive training began at 8:00 a.m. on day 8 and was conducted as follows: A total of 10 tracks of the ZH-PT experimental animal treadmill were set to a slope degree of 0° and a rotating speed of 8 m/min. Rats exercised for 30 min, followed by a 30 min rest. During the training, rats were free to drink and had access to food ad libitum. The training was repeated 5 times in the same day, with a total training time of 2.5 h.
Intervention training stage on the plain
The present model was established based on the high-altitude pulmonary edema (HAPE) animal model described by Bai et al (14), which did not aim to exhaust the animals. In order to induce fatigue stress, the treadmill settings were adjusted. At 8:00 a.m. on day 9, the animals were put on the treadmill, the slope of the conveyor belt was set to 0° and rotation speed was set to 8 m/min. The rats ran for 15 min to adapt to the treadmill. Following this, the slope was adjusted to 10° and the rotation speed was increased to 15 m/min. The rats exercised for 4 h, followed by a 30 min rest. Rats had ad libitum access food and water during the training. The training was repeated until 8:00 a.m. on day 10, after 24 h training. If the rats stopped, they were transported to the end of treadmill where there were some electrified pillars. Thus, appropriate electrical stimulation was applied to maintain the running state. The aim was to make the exercise as consistent as possible for all animals.
Stimulated hypoxia stage
In order to stimulate acute hypoxia in group H rats, at 8:00 a.m. on day 10, rats in groups C and H were moved into separate chambers of the FLYDWC50-1A simulated high-altitude low-pressure chamber. A compression resistant door separated the chambers, with an adapter chamber in the middle and a precise control system regulated the two separate environments. Rats in group C were kept at a temperature 23–25°C, humidity of 60%, wind speed of 0.3 m/sec and a 12-h light/dark cycle. A total of 25 g/day rat food was provided for each rat (12.5 g every 12 h) and the rats were free to get water. Hypobaric hypoxia was not applied to the group C rats. For group H, the access to food and water were same to group C, but the altitude was set to 4,000 m, the chamber pressure was 40.4 kPa, the air capacity was 40 m3/h, the temperature was 25°C, the humidity was 60%, the O2 content was 18.0%, the oxygen partial pressure was 7.8 kPa, the ascending rate was 3 m/sec and the light/dark cycle was 12-h.
Sample collection
Following 72 h in the separate environments, blood and abdominal visceral tissue samples from rats in the two groups were collected. Following the induction of anesthesia (10% ethyl carbamate, 1 ml/kg body weight by intraperitoneal injection; Chengdu Kelong Chemical Co., Ltd. (Chengdu, China), samples from group H were collected within 10 min by surgical resection at a simulated altitude of 4,000 m and all operations were strictly aseptic. From each group (n=35), 20 rats were selected for sacrifice by surgical resection as described above, and the blood, liver, spleen, mesenteric lymph nodes and jejunum were collected and preserved in liquid nitrogen (−195°C). The remaining 15 rats from each group were anesthetized (10% ethyl carbamate, 1 ml/kg body weight by intraperitoneal injection) and 2 cm jejunum was removed, 10 cm from the pylorus. The jejunum was sliced into 1-mm samples, which were immersed in fixing solution for pathological observation. For each group, 5 samples were immersed in 4% paraformaldehyde fixation fluid, 5 samples were immersed in 2.5% glutaraldehyde fixation fluid, and the rest were immersed in lanthanum aldehyde fixation solution (2% triformol, 2.5% glutaraldehyde. 2% lanthanum nitrate, 0.1 M sodium cacodylate trihydrate). All of the fixing solutions were at 4°C. Samples were fixed for 24 h.
Detection indices
To detect bacterial translocation, a homogenate was produced by adding 900 µl sterile saline solution to 100 mg samples of liver, spleen and mesenteric lymph node. A total of 0.3 ml homogenate was inoculated on MacConkey culture medium (17 g peptone, 5 g pig bile salts, 5 g NaCl, 17 g agar, 1,000 ml distilled water, 10 g lactose, 10 ml 0.01% crystal violet aqueous solution, 5 ml 0.5% neutral red aqueous solution). Following incubation at 37°C for 24 h, primary biochemical identification was applied. The bacterial translocation rate (%) = positive organ number in the same group/total number of cultured organs.
Dynamic turbidimetric analysis was used to detect the level of lipopolysaccharides (LPS) in the serum. A total of 3 ml serum was collected by apex puncture and centrifuged at 4°C at 960 × g for 15 min. From this, 100 µl serum was collected and 900 µl KTA Tachypleus amebocyte lysate was added. This was mixed evenly and kept at 65°C for 20 min. Following this, 200 µl mixture was added to the primary reagent of the enzyme reaction (from the kit) and LPS content was automatically detected following 60 min using a Bacterial Endotoxin Dynamic Testing system (EDS-99; Tianjin Wireless Electronic Co., Ltd., Tianjin, China).
Pathological observation by light microscopy
Perfusion in situ combined with post-fixation was used to prepare the light microscope specimens. Animals were anesthetized (10% ethyl carbamate, 1 ml/kg body weight, by intraperitoneal injection), the right auricle was opened and the left ventricle was intubated. For perfusion, 200 ml normal saline at 4°C and 100 ml 4% triformol were used. When there was clear effluent from the right auricle and the liver was hard, the perfusion was deemed successful. The 2 cm of jejunum was cut into 1-mm sections, following fixation using 4% triformol at 4°C for 24 h, the specimens were dehydrated with 95% ethyl alcohol and embedded in paraffin. Then, the samples were cut into 4-µm sections and stained with hematoxylin and eosin (H&E). The changes in the intestinal epithelium villi and mucous layer were observed using a CX23 optical microscope (Olympus Corporation, Tokyo, Japan); 10 high-power fields were selected in every section to detect the height and surface area of villi using the following formula: S=2πrh, where r, radius of villus and h, height of villus. The mean value was noted.
Pathological observation by scanning electron microscopy
The perfusion and fixation solutions were changed to 2.5% glutaradehyde solution in order to observe the samples using a scanning electron microscope, but other protocols were the same as previously stated for light microscopy. The samples were placed in 2.5% glutaradehyde solution at 4°C, and prepared as specimens according to the routine specimen preparation method (15), prior to observation under the scanning electron microscope.
Pathological observation by transmission electron microscopy
In order to observe samples using a transmission electron microscope, perfusion and the fixation solutions were changed to lanthanum aldehyde fixation solution (2% triformol, 2.5% glutaraldehyde, 2% lanthanum nitrate, 0.1 M sodium cacodylate trihydrate), as lantham nitrate was used as the tracer. The samples were put into lanthanum aldehyde solution for fixation at 4°C for 24 h and prepared as specimens according to the routine specimen preparation (15), prior to observation under the transmission electron microscope.
Apoptosis of intestinal epithelial cells observed by histochemistry
A TUNEL assay was used to detect apoptotic epithelial cells. The samples were routinely fixed in 4% triformol at 4°C, embedded and cut into 5-µm paraffin slices, according to the manufacturer's protocol (Roche Diagnostics). A stained nucleus, in blue, was defined as positive for apoptosis. Every section was observed under a light microscope, at a magnification of ×400, to count the cells (total cells of 4 fields/4), and the apoptotic index was calculated as follows: Apoptotic cell number/total cell number.
Western blot analysis to detect occuludin expression
Total proteins from the jejunum tissue were extracted from a 50-mg sample and protein concentration was detected using a bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of Biotechnology, Haimen, China). The protein was diluted to 5 µg/µl and 5X SDS loading buffer was added prior to heating to 100°C for 3 min to degenerate the proteins. A total of 20 µg/µl protein solution was loaded in each well; this then underwent electrophoresis on a 5% SDS stacking gel and a 10% SDS separating gel at 90 V for 100 min. This was transferred to PVDF membrane by 100 V for 50 min, and put into 5% skimmed milk powder solution to block for 1 h at 25°C. Then, occludin primary antibodies (71–1500; dilution 1:1,000) and β-actin primary antibodies (sc47778; dilution 1:3,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) were added and the samples were incubated at 4°C overnight. Following washing with Tris-buffered saline and Tween-20, the corresponding secondary antibodies (ZB-2301 and ZB-2305; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) for occludin and β-actin respectively, were added and the membrane was incubated at 25°C for 1 h. The proteins were then developed using a BeyoEcl Plus kit (P0018; Beyotime Institute of Biotechnology Beijing, China). The relative grey value was used to quantify occludin expression, using ImageJ software, v2.1.4.7 (National Institutes of Health, Bethesda, MD, USA) according to the following formula: Relative grey value = grey value of occludin band/grey value of β-actin band.
Statistical analysis
SPSS, version 18.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Enumeration data were presented as percentages and the measurement data were presented as mean ± standard deviation. The comparison among groups was completed by independent-sample t-test and the comparison of ratio was completed by χ2 analysis. P<0.05 was considered to represent a statistically significant difference.
Results
Translocation of bacteria
There was no bacterial translocation detected in group C and the bacterial translocation was significantly higher in group H (P<0.01; Table I).
Table I.
Change of bacterial translocation in the two groups.
| Group | Organ number (n) | Blood | Liver | Spleen | Lymph node | Translocation rate (%) |
|---|---|---|---|---|---|---|
| C | 80 | 0 | 0 | 0 | 0 | 0 |
| H | 80 | 3 | 6 | 5 | 7 | 26.25a |
P<0.01 vs. group C. C, control group; H, high-altitude acute hypoxia group.
Detection of serum endotoxin
The serum LPS concentration in group C was 0.051±0.011 EU/ml and the LPS concentration in group H was 0.803±0.115 EU/ml, a statistically significant difference (P<0.01; Fig. 1).
Figure 1.
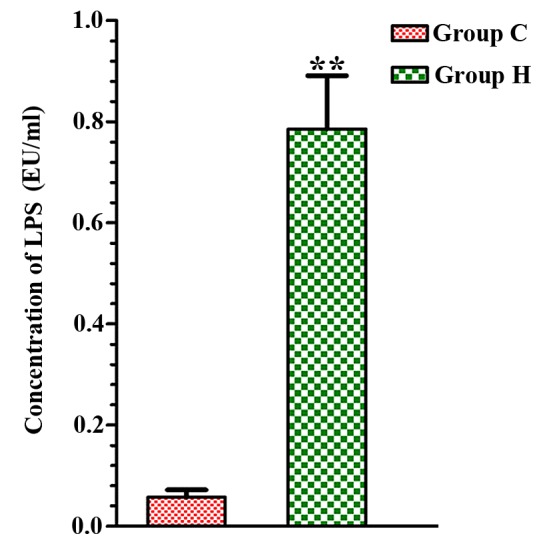
Change of LPS concentration in serum of the two groups. **P<0.01 vs. group C. LPS, lipopolysaccharides; Group C, control group; group H, high-altitude acute hypoxia group; EU, endotoxin unit.
Pathological results
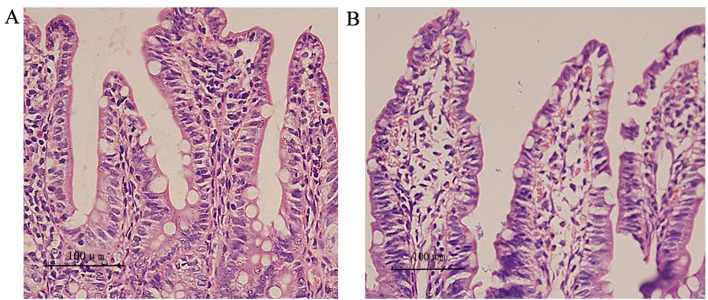
The results from light microscopy demonstrate that in group C, the epithelial mucous was smooth, the villi were arranged regularly and there was an even distribution of goblet cells among epithelial cells (Fig. 2A). In group H, the intestinal epithelial mucous was thinner, the villi reduced, the arrangement disordered and there was extracapillary inflammatory cell infiltration and red blood cell exudation (Fig. 2B). Compared with group C, the villi height, surface area and mucous thickness were significantly decreased in group H (P<0.05; Table II).
Figure 2.
Hematoxylin and eosin staining of the intestine of the two groups (magnification, ×400). (A) Group C: Epithelial mucous was smooth, the villi arranged regularly and goblet cells were evenly distributed among epithelial cells. (B) Group H: Intestinal epithelial mucous was thin, the number of villi was reduced, there was disordered arrangement and extracapillary inflammatory cell infiltration and red blood cell exudation was detected. Scale bar, 100 µm. Group C, control group; group H, high-altitude acute hypoxia group.
Table II.
Change of height, surface area of villi and thickness of mucous layer in the two groups (mean ± standard deviation, n=5).
| Group | Height of villi (µm) | Surface area of villi (µm2) | Thickness of mucous layer (µm) |
|---|---|---|---|
| C | 259±13 | 16,198±1085 | 402±9 |
| H | 217±21a | 13,423±996a | 336±7a |
P<0.05 vs. group C. C, control group; H, high-altitude acute hypoxia group.
Scanning electron microscopy
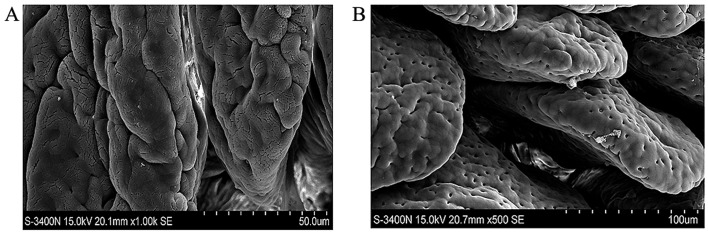
Scanning electron microscopy demonstrated that for group C, the villi were smooth, arranged regularly and morphology was plump (Fig. 3A), however, in group H, the epithelial microvilli were shorter, areas had shrunk and broken. Furthermore, there was space widening (Fig. 3B).
Figure 3.
Scanning electron microscope results of intestinal epithelium. (A) Group C: Villi were smooth, arranged regularly and had a plump morphology. (B) Group H: Epithelial microvilli were shorter, part of them had shrunk and fallen off and there was more space between them. Group C, control group; group H, high-altitude acute hypoxia group.
Transmission electron microscopy
Transmission electron microscope results indicated that in group C, there was a high density of lanthanum granules attached to the capillary cavity and microvilli surfaces, the tight junction between epithelial cells was closed and there was no lanthanum granule deposition in the basement membrane or extracapillary mesenchyme (Fig. 4A). The cytoplasm of epithelial cells was complete and the morphology of endoplasmic reticulum and mitochondria was normal (Fig. 4B). In group H, the tight junction among epithelial cells was open and lanthanum granules permeated into the widened tight junction (Fig. 4C). There were lanthanum granules in the extracapillary space and basement membrane, indicating continuous linear distribution. Furthermore, parts of the organelles were swollen (Fig. 4D).
Figure 4.
Transmission electron microscope results of intestinal epithelium. Group C: (A) High density of lanthanum granules attached to the capillary cavity surface and microvilli surface, the tight junction among epithelial cells was closed (indicated by the arrow) and there was no granule deposition in the basement membrane and extracapillary mesenchyme. (B) Cytoplasm of epithelial cells was complete, the morphology of endoplasmic reticulum and mitochondria was normal. (C) Group H: Tight junction among epithelial cells was open (indicated by the arrow) and lanthanum granules permeated across the widened tight junction. (D) Lanthanum granules were detected in extracapillary space and basement membrane, indicating continuous linear distribution. Parts of the organelles were swollen (indicated by the arrow). Group C, control group; group H, high-altitude acute hypoxia group.
Apoptosis of epithelial cells
In group C, there were no clearly apoptotic cells in the intestinal epithelium (Fig. 5A), and the TUNEL assay indicated that the apoptotic rate was 3.4±1.1% (data not shown). In group H, there were a large number of apoptotic cells in the intestinal epithelium (Fig. 5B) and the apoptotic rate was 18.7±1.9% (data not shown). The difference in apoptotic rates was statistically significant (P<0.01).
Figure 5.
Apoptosis of epithelial cells. (A) There was no evidence of apoptotic cells in the epithelium of group C. (B) There were a large number of apoptotic cells at the tip (indicated by the arrow) and roots of microvilli in group H. Group C, control group; group H, high-altitude hypoxia group.
Expression of the tight junction protein, occludin
Western blot analysis was used to investigate the expression of occludin (Fig. 6A), and the expression levels were quantified by densitometric analysis (Fig. 6B). Occludin expression was significantly downregulated in group H (0.42±0.011) compared with group C (1.31±0.084; P<0.05) in the tight junction of the epithelium in rats.
Figure 6.

Expression of occludin, a tight junction protein, in epithelial cells. (A) Representative western blot and (B) quantification. The western blot analysis indicates that, in group H, the expression of occludin was significantly downregulated in the tight junctions in rats (0.42±0.011 vs. 1.31±0.084; *P<0.01). Group C, control group; group H, high-altitude acute hypoxia group.
Discussion
Increasing evidence suggests that gastrointestinal tract injury causes MODS and there is an increase in the incidence of gastrointestinal diseases in patients who have travelled to a high altitude. Therefore, the initiation mechanism for altitude sickness is being investigated (16–18). In this context, a stable and effective animal model of high-altitude intestinal barrier injury is required.
There are a number of methods for establishing an animal model of gastrointestinal tract injury; however, none produce a model similar to acute hypoxia-induced intestinal barrier injury. Reasons for this include the species of animal selected; animals such as rabbits or pigs (19–21) are genetically different from humans and thus the results of these studies are not relatable to humans. Another reason is the production methods used impact the models; although the physical or chemical methods including surgery, trauma and pharmaceutical treatments (22,23) exert significant effects, models established by invasive methods do not mimic the conventional mechanism of hypoxia induced acute injury. Finally, the practical application affects the model. It has been demonstrated that when a rat is kept at a simulated 7,000 m height, the gastrointestinal tract is clearly injured (8). However, this method for establishing a model is different to the practical situation. The height limit for human survival is 5,000 m and 90% of humans live within an altitude range of 3,000–4,000 m. Thus, whether the environment at an altitude of 7,000 m induces a similar injury to an altitude of 4,000 m remains unclear. Furthermore, when producing a model at a simulated altitude of 7,000 m, the samples must be collected at an altitude <4,000 m. This may cause reperfusion injury or oxidative stress, which potentially differs from single acute hypoxia-induced intestinal injury (24). Based on previous preliminary studies (15,25), only acute hypoxia occurring at an altitude of 3,000–5,000 m does not cause injury. The present study selected rats as the experimental animal, used a high-altitude environment simulation chamber to create a specific altitude environment and increased the exercise load to establish an animal model for intestinal barrier injury following acute hypoxia.
The animals were subjected to adaptive training at a normal altitude on an animal treadmill to avoid any coordination difficulty due to unfamiliar equipment. The rats received a sport load, to cause only slight fatigue stimulation and not cause pathophysiogical changes associated with the gastrointestinal tract. Notably, the sport load is similar to the fatigue status of individuals who go to high-altitude regions by railway or road, making the model more applicable to humans.
Following training, the rats were kept in a simulated high-altitude environment chamber at an altitude of 7,000 m. Environmental factors were strictly controlled and food was supplied on ration to control the non-experimental factors that may alter the results. The altitude of 4,000 m is closer to the living altitude of humans. Therefore, after 3 days, the samples were collected at an altitude of 4,000 m, which ensured the safety of researchers and decreased the potential for secondary injury to rats from decreasing the altitude. This made the model more similar to the actual condition of hypoxia-induced intestinal injury.
MacConkey culture medium was used to identify the bacteria in each organ to judge whether it was from the intestine, following sample collection. As a selective culture medium, MacConkey culture medium contains the nutrient substances suitable for intestinal bacteria, which can partially inhibit gram-positive bacteria and completely inhibit the migrating growth of Proteusbacillus vulgaris (26). The addition of a pH indicator, neutral red and lactose, allows the pathogenic enterobacteria to be identified (27). The results indicated that there was bacterial translocation in group H but not in group C (P<0.05). This demonstrates that the modeling method may damage the intestinal mucous and cause intestinal bacterial translocation. The microscopy results indicated abnormalities in the intestinal epithelial ultrastructure, manifesting as epithelial villi falling off, shrinking and shortening, thinning of the epithelial mucosa, widening of inter-villi space and swelling and the disappearance of organelles in cytoplasm. Lanthanum nitrate-traced transmission microscopy was used to assess the degree of injury, as a high density of lanthanum granules was readily observed using electron microscopy. The diameter of the lanthanum granules was 3–4 nm and under normal conditions these would not enter the mesenchyme from the complete epithelium or microvascular tight junction. The results demonstrated that in group H, lanthanum granules were evenly distributed at epithelial cell tight junctions, basement membrane of vessels and intercellular space. This indicates that there was injury to the general structure and ultra micropathology of the intestinal barrier in the animal model. The lanthanum nitrate-traced transmission electron microscopy technique (28) observed that the lanthanum granules permeated into the deep layer from the opened epithelial cell tight junction. Thus, it is suggested that there is a correlation between the injury of epithelial barrier and damage to tight junction complexes. The tight junction of epithelial cells is a multi-functional complex consisting of many proteins (29,30), including occludin, claudins and actin. As a large protein family, there are a number of claudin subtypes with diverse functions (31,32). Therefore, monomeric occludin has become the preferred protein to detect (33–35). In the current study, western blot analysis performed to detect occludin in the rat intestinal epithelium following animal model establishment demonstrated that expression of occludin in tight junctions was downregulated. This indicates that the intestinal barrier function was weakened and permeability was increased, and verifies that the animal model was established successfully at a molecular level. However, it remains unclear how acute hypoxia induced damage to barrier function and this requires further analysis. In the future, occludin may be used to predict the permeability change of epithelial cells; however, further studies are required to confirm this.
In conclusion, the current study established an animal model for hypoxia-induced gastrointestinal injury in rats. The mechanism of inducement is similar to the pathophysiological process of the disease occurring under natural conditions. The establishment of this animal model may provide the basis for studies into possible treatments of intestinal barrier damage induced by hypoxia.
References
- 1.Lazaridis C. Plateau waves of intracranial pressure and mechanisms of brain hypoxia. J Crit Care. 2014;29:303–304. doi: 10.1016/j.jcrc.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Salinas CE, Blanco CE, Villena M, Giussani DA. High-altitude hypoxia and echocardiographic indices of pulmonary hypertension in male and female chickens at adulthood. Circ J. 2014;78:1459–1464. doi: 10.1253/circj.CJ-13-1329. [DOI] [PubMed] [Google Scholar]
- 3.Oddo M, Nduom E, Frangos S, MacKenzie L, Chen I, Maloney-Wilensky E, Kofke WA, Levine JM, LeRoux PD. Acute lung injury is an independent risk factor for brain hypoxia after severe traumatic brain injury. Neurosurgery. 2010;67:338–344. doi: 10.1227/01.NEU.0000371979.48809.D9. [DOI] [PubMed] [Google Scholar]
- 4.Adak A, Maity C, Ghosh K, Mondal KC. Alteration of predominant gastrointestinal flora and oxidative damage of large intestine under simulated hypobaric hypoxia. Z Gastroenterol. 2014;52:180–186. doi: 10.1055/s-0033-1336007. [DOI] [PubMed] [Google Scholar]
- 5.Moonen RM, Kessels CG, Zimmermann LJ, Villamor E. Mesenteric artery reactivity and small intestine morphology in a chicken model of hypoxia-induced fetal growth restriction. J Physiol Pharmacol. 2012;63:601–612. [PubMed] [Google Scholar]
- 6.Yeh KY, Yeh M, Polk P, Glass J. Hypoxia-inducible factor-2α and iron absorptive gene expression in Belgrade rat intestine. Am J Physiol Gastrointest Liver Physiol. 2011;301:G82–G90. doi: 10.1152/ajpgi.00538.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu C, Sun R, Qiao X, Xu C, Shang X, Niu W, Chao Y. Effect of vitamin e supplementation on intestinal barrier function in rats exposed to high altitude hypoxia environment. Korean J Physiol Pharmacol. 2014;18:313–320. doi: 10.4196/kjpp.2014.18.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou QQ, Yang DZ, Luo YJ, Li SZ, Liu FY, Wang GS. Over-starvation aggravates intestinal injury and promotes bacterial and endotoxin translocation under high-altitude hypoxic environment. World J Gastroenterol. 2011;17:1584–1593. doi: 10.3748/wjg.v17.i12.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinmore AJ, Edwards JS, Menzies IS, Travis SP. Intestinal carbohydrate absorption and permeability at high altitude (5,730 m) J Appl Physiol (1985) 1994;76:1903–1907. doi: 10.1152/jappl.1994.76.5.1903. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez de Medina F, Romero-Calvo I, Mascaraque C, Martínez-Augustin O. Intestinal inflammation and mucosal barrier function. Inflamm Bowel Dis. 2014;20:2394–2404. doi: 10.1097/MIB.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Zhang W, Zuo L, Dong J, Zhu W, Li Y, Gu L, Gong J, Li Q, Li N, Li J. Intestinal dysbacteriosis contributes to decreased intestinal mucosal barrier function and increased bacterial translocation. Lett Appl Microbiol. 2014;58:384–392. doi: 10.1111/lam.12201. [DOI] [PubMed] [Google Scholar]
- 12.Balzan S, de Almeida Quadros C, de Cleva R, Zilberstein B, Cecconello I. Bacterial translocation: Overview of mechanisms and clinical impact. J Gastroenterol Hepatol. 2007;22:464–471. doi: 10.1111/j.1440-1746.2007.04933.x. [DOI] [PubMed] [Google Scholar]
- 13.Pathan N, Burmester M, Adamovic T, Berk M, Ng KW, Betts H, Macrae D, Waddell S, Paul-Clark M, Nuamah R, et al. Intestinal injury and endotoxemia in children undergoing surgery for congenital heart disease. Am J Respir Crit Care Med. 2011;184:1261–1269. doi: 10.1164/rccm.201104-0715OC. [DOI] [PubMed] [Google Scholar]
- 14.Bai C, She J, Goolaerts A, Song Y, Shen C, Shen J, Hong Q. Stress failure plays a major role in the development of high-altitude pulmonary oedema in rats. Eur Respir J. 2010;35:584–591. doi: 10.1183/09031936.00001709. [DOI] [PubMed] [Google Scholar]
- 15.Guo P, Luo H, Fan Y, Luo Y, Zhou Q. Establishment and evaluation of an experimental animal model of high altitude cerebral edema. Neurosci Lett. 2013;547:82–86. doi: 10.1016/j.neulet.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Adak A, Maity C, Ghosh K, Pati BR, Mondal KC. Dynamics of predominant microbiota in the human gastrointestinal tract and change in luminal enzymes and immunoglobulin profile during high-altitude adaptation. Folia Microbiol (Praha) 2013;58:523–528. doi: 10.1007/s12223-013-0241-y. [DOI] [PubMed] [Google Scholar]
- 17.Anand AC, Sashindran VK, Mohan L. Gastrointestinal problems at high altitude. Trop Gastroenterol. 2006;27:147–153. [PubMed] [Google Scholar]
- 18.Luks AM, Swenson ER. Evaluating the risks of high altitude travel in chronic liver disease patients. High Alt Med Biol. 2015;16:80–88. doi: 10.1089/ham.2014.1122. [DOI] [PubMed] [Google Scholar]
- 19.Reino DC, Pisarenko V, Palange D, Doucet D, Bonitz RP, Lu Q, Colorado I, Sheth SU, Chandler B, Kannan KB, et al. Trauma hemorrhagic shock-induced lung injury involves a gut-lymph-induced TLR4 pathway in mice. PLoS One. 2011;6:e14829. doi: 10.1371/journal.pone.0014829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinsasser A, Levin DL, Loeckinger A, Hopkins SR. A pig model of high altitude pulmonary edema. High Alt Med Biol. 2003;4:465–474. doi: 10.1089/152702903322616218. [DOI] [PubMed] [Google Scholar]
- 21.Heath D, Williams D, Rios-Dalenz J, Gosney J. Pulmonary vascular disease in a rabbit at high altitude. Int J Biometeorol. 1990;34:20–23. doi: 10.1007/BF01045815. [DOI] [PubMed] [Google Scholar]
- 22.Liang HY, Chen T, Wang T, Huang Z, Yan HT, Tang LJ. Time course of intestinal barrier function injury in a sodium taurocholate-induced severe acute pancreatitis in rat model. J Dig Dis. 2014;15:386–393. doi: 10.1111/1751-2980.12148. [DOI] [PubMed] [Google Scholar]
- 23.He C, Yang S, Yu W, Chen Q, Shen J, Hu Y, Shi J, Wu X, Li J, Li N. Effects of continuous renal replacement therapy on intestinal mucosal barrier function during extracorporeal membrane oxygenation in a porcine model. J Cardiothorac Surg. 2014;9:72. doi: 10.1186/1749-8090-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin HJ, Wang CT, Niu KC, Gao C, Li Z, Lin MT, Chang CP. Hypobaric hypoxia preconditioning attenuates acute lung injury during high-altitude exposure in rats via up-regulating heat-shock protein 70. Clin Sci (Lond) 2011;121:223–231. doi: 10.1042/CS20100596. [DOI] [PubMed] [Google Scholar]
- 25.Luo H, Guo P, Zhou Q. Role of TLR4/NF-kB in damage to intestinal mucosa barrier function and bacterial translocation in rats exposed to hypoxia. PLoS One. 2012;7:e46291. doi: 10.1371/journal.pone.0046291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez LR, Rodrigues D, Dias CG. Evaluation of phenotypic tests to detect carbapenem-resistant Enterobacteriaceae in colonized patients hospitalized in intensive care units. Braz J Infect Dis. 2015;19:436–438. doi: 10.1016/j.bjid.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tchesnokova VL, Ottley LL, Sakamoto K, Fierer J, Sokurenko E, Liss MA. Rapid identification of rectal multidrug-resistant Escherichia coli before transrectal prostate biopsy. Urology. 2015;86:1200–1205. doi: 10.1016/j.urology.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Igawa S, Kishibe M, Murakami M, Honma M, Takahashi H, Iizuka H, Ishida-Yamamoto A. Tight junctions in the stratum corneum explain spatial differences in corneodesmosome degradation. Exp Dermatol. 2011;20:53–57. doi: 10.1111/j.1600-0625.2010.01170.x. [DOI] [PubMed] [Google Scholar]
- 29.Schneeberger EE, Lynch RD. The tight junction: A multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 30.Torres-Flores JM, Silva-Ayala D, Espinoza MA, López S, Arias CF. The tight junction protein JAM-A functions as coreceptor for rotavirus entry into MA104 cells. Virology. 2014;475:172–178. doi: 10.1016/j.virol.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Rossa J, Protze J, Kern C, Piontek A, Günzel D, Krause G, Piontek J. Molecular and structural transmembrane determinants critical for embedding claudin-5 into tight junctions reveal a distinct four-helix bundle arrangement. Biochem J. 2014;464:49–60. doi: 10.1042/BJ20140431. [DOI] [PubMed] [Google Scholar]
- 32.Izraely S, Sagi-Assif O, Klein A, Meshel T, Ben-Menachem S, Zaritsky A, Ehrlich M, Prieto VG, Bar-Eli M, Pirker C, et al. The metastatic microenvironment: Claudin-1 suppresses the malignant phenotype of melanoma brain metastasis. Int J Cancer. 2015;136:1296–1307. doi: 10.1002/ijc.29090. [DOI] [PubMed] [Google Scholar]
- 33.Cheng X, He P, Yao H, Dong Q, Li R, Shen Y. Occludin deficiency with BACE1 elevation in cerebral amyloid angiopathy. Neurology. 2014;82:1707–1715. doi: 10.1212/WNL.0000000000000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthusamy A, Lin CM, Shanmugam S, Lindner HM, Abcouwer SF, Antonetti DA. Ischemia-reperfusion injury induces occludin phosphorylation/ubiquitination and retinal vascular permeability in a VEGFR-2-dependent manner. J Cereb Blood Flow Metab. 2014;34:522–531. doi: 10.1038/jcbfm.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karagiannis GS, Schaeffer DF, Cho CK, Musrap N, Saraon P, Batruch I, Grin A, Mitrovic B, Kirsch R, Riddell RH, Diamandis EP. Collective migration of cancer-associated fibroblasts is enhanced by overexpression of tight junction-associated proteins claudin-11 and occludin. Mol Oncol. 2014;8:178–195. doi: 10.1016/j.molonc.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]