Abstract
Prolyl oligopeptidase (POP) is a serine endopeptidase widely distributed in vivo with high activity in the liver. However, its biological functions in the liver have remained largely elusive. A previous study by our group has shown that POP produced N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP) and thereby exerted an anti-fibrogenic effect on hepatic stellate cells (HSCs) in vitro. It was therefore hypothesized that POP may affect the activation state of HSCs and has an important role in liver fibrosis. The HSC-T6 immortalized rat liver stellate cell line was treated with the POP inhibitor S17092 or transfected with recombinant lentivirus to overexpress POP. Cell proliferation and apoptosis were determined using a Cell Counting Kit-8 and flow cytometry, respectively. The activation status of HSCs was determined by examination of the expression of α-smooth muscle actin (α-SMA), collagen I, monocyte chemoattractant protein-1 (MCP-1), transforming growth factor (TGF)-β-Smad signaling and peroxisome proliferator activated receptor-γ (PPAR-γ). Inhibition by S17092 decreased, whereas lentiviral expression increased the activity of POP and cell proliferation, while neither of the treatments affected cell apoptosis. Of note, S17092 significantly increased, whereas POP overexpression decreased the expression of α-SMA and MCP-1 without affecting the expression of collagen I and TGF-β1. Furthermore, S17092 caused a reduction, whereas POP overexpression caused an upregulation of Smad7 protein and PPAR-γ, but not phosphorylated-Smad2/3 expression. In conclusion, POP attenuated the activation of HSCs through inhibition of TGF-β signaling and induction of PPAR-γ, which may have therapeutic potential in liver fibrosis.
Keywords: prolyl oligopeptidase, hepatic stellate cells, transforming growth factor-β-Smad signaling, peroxisome proliferator activated receptor-γ
Introduction
Prolyl oligopeptidase (POP; enzyme ID, EC 3.4.21.26; molecular weight, 80 kDa), also known as prolyl endopeptidase (Prep), is a serine endopeptidase that hydrolyzes proline-containing peptides shorter than 30 amino acids, specifically at the carboxyl terminal of internal proline residues (1). POP was first discovered in the human uterus in 1971 (2) and later it was detected in a wide range of species and in most tissues of mammals, with the highest enzyme activity generally detected in the brain (2). Several bioactive neuropeptides, such as neurotensin, bradykinin, arginine-vasopressin (AVP), thyrotropin-releasing hormone (TRH), and substance P (SP), are known to be POP substrates in vitro. However, the functions of POP in peripheral tissues have largely remained unknown (1–4). In the liver, the protein density of POP is low, while its activity is surprisingly high (5,6). It was moderately present in the cytoplasm and nuclei of hepatocytes as well as in the nuclei of Kupffer and hepatic endothelial cells (6), but its biological functions in the liver are not well studied.
Chronic liver diseases caused by various etiologies lead to liver fibrosis, which is characterized by excessive deposition of extracellular matrix (ECM) in the liver (7). The activation of HSCs has been reported to have critical roles in the development of hepatic fibrogenesis (8). It is known that the activated HSCs are the major α-smooth muscle actin (α-SMA)-producing cells and the principle source of deposited ECM, including collagen I, collagen III and proteoglycans, in liver fibrosis (9–13).
The activation of HSCs is mainly mediated by damaged hepatocyte-derived growth factors, such as transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), endothelin (ET), fibroblast growth factor (FGF) and connective tissue growth factor (CTGF) (14). Among these growth factors, TGF-β1 is recognized as a major profibrogenic cytokine by promoting and maintaining HSC activation, proliferation, as well as collagen production and deposition through the TGF-β/Smad pathway (15–18).
Peroxisome proliferator activated receptor-γ (PPAR-γ) was initially identified as a key regulator of adipogenesis (19), while increasing evidence has confirmed that PPAR-γ is a key factor in HSC activation and phenotypic alteration, maintaining HSCs in a quiescent phase, and suppressing the production of type I collagen, α-SMA and TGF-β1. Thus, PPAR-γ has an important role in reducing and preventing liver fibrosis (20,21). PPAR-γ can disrupt the TGF-β signaling pathway and Smad-dependent promoter activity, directly antagonizes the activation and/or function of Smad3 in fibroblasts without affecting the protein expression of stimulatory Smad3. However, PPAR-γ can increase the expression of inhibitory Smad7 (22–24). PPAR-γ can also restore the ability of HSCs to accumulate retinyl palmitate, a feature of quiescent HSCs (25–27). Together with a decrease in α-SMA expression, re-expression of PPAR-γ is thought to be an indicator of attenuated HSC activation and even transition to the quiescent state.
A previous study by our group showed that N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP), a tetrapeptide hydrolyzed from thymosin-β4 by POP (28), exerts an anti-fibrogenic effect on HSCs in vitro (29,30). Other studies also showed that thymosin-β4 has a protective effect against carbon tetrachloride-induced acute hepatotoxicity and inhibits HSC activation (31,32). However, whether POP can directly affect the activation of HSCs has remained elusive. POP was found to participate in liver inflammation (33) and regulate hepatocyte proliferation and differentiation (34,35). It was therefore hypothesized that POP may have an important role in regulating the functions of HSCs and inhibiting liver fibrosis.
To test this hypothesis, a pharmacological inhibitor of POP activity, S17092, was employed (36), as well as a lentiviral overexpression method to induce the protein expression of POP in the HSC-T6 immortalized rat liver stellate cell line. The results demonstrated that POP can attenuate HSC activation and may protect against liver fibrosis.
Materials and methods
Cell culture
HSC-T6, which is an immortalized rat liver stellate cell line that has a stable phenotype and biochemical characteristics compared to primary stellate cells (13,37), was obtained from the Cell Bank of the Chinese Academy of Science (Shanghai, China). HSC-T6 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) in a humidified incubator at 37°C with 5% CO2.
Infection with recombinant lentivirus
A recombinant lentivirus overexpressing POP was constructed by Genechem (Shanghai, China) according to the mRNA sequence of the rat Prep gene in GenBank (NM_031324). Cytomegalovirus-driven green fluorescent protein (GFP) reporter gene, which was included in the recombinant lentivirus, was used for detecting the transduction efficiency. HSC-T6 cells were infected with empty lentivirus (mock) or POP-expressing lentivirus at a multiplicity of infection of 10 in 1 ml of an enhanced infection solution (Eni.S) containing 5 µg/ml polybrene (an infection enhancer; Genechem) for 12 h.
Cell proliferation assay
The proliferation rate of HSC-T6 cells was detected using a Cell Counting Kit-8 (CCK-8; Dojindo, Shanghai, China) according to the manufacturer's instructions. In brief, HSC-T6 cells were seeded on 96-well plates at a density of 1×103 cells per well. After culturing for 24 h, cells were treated with increasing concentrations of S17092 (Sigma; Merck Millipore, Darmstadt, Germany) (0, 5, 10, 25, 50 or 100 µg/ml) or vehicle (dimethyl sulfoxide; Sigma, Merck Millipore) for 24 or 48 h. For viral infection, HSC-T6 cells were seeded at a density of 2×103 cells per well, infected with mock-transfected or POP-expressing lentivirus for 12 h, and the transfection mixture was subsequently replaced with normal culture medium and cells were cultured for an additional 24 or 48 h. CCK-8 stain was added and the absorbance (optical density) at 450 nm was detected using a microplate reader (uQuant; Biotek, Winooski, VT, USA).
Cell apoptosis detection
Cell apoptosis was determined using an Annexin V-phycoerythrin (PE)/7-aminoactinomycin D (AAD) Apoptosis Detection kit (cat. no. 559763; Becton-Dickinson, San Jose, CA, USA) according to the manufacuturer's instructions. Cells (~2×105) seeded on 3.5-cm dishes were treated with S17092 (0, 5 or 10 µg/ml) for 24 h. Cells (~5×104) seeded on 3.5-cm dishes were infected with mock-transfected or POP-expressing lentivirus for 12 h, and the transfection mixture was replaced with normal culture medium and cells were cultured for an additional 24 h. Cells were subsequently harvested and stained with Annexin V-PE and 7-AAD at room temperature for 15 min. Apoptotic cells were detected using a FACScan flow cytometer and analyzed by CellQuest software (version 5.1; Becton-Dickinson).
Reverse-transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from treated cells using TRIzol (Takara Bio Inc., Dalian, China) and was reverse-transcribed into complementary DNA using primescript RT master mix (cat. no. RR036A; Takara Bio Inc.) under the following conditions: 37°C for 15 min, 85°C for 5 sec and 4°C 1 h. qPCR was performed in a 7500 Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) using the SYBR Premix Ex Taq (Tli RnaseH Plus; cat. no. RR420A; Takara Bio, Inc.) under the following conditions: 95°C for 30 sec, 95°C for 5 sec and 60°C for 34 sec (40 cycles), 95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec and 60°C for 15 sec. Primers of target genes were synthesized by Sangon Biotech (Shanghai, China) and their sequences are listed in Table I. Primer specificity was confirmed by a dissociation curve using 7500 system SDS software (Applied Biosystems). GAPDH was used as the internal control (38).
Table I.
Primers for real-time polymerase chain reaction.
| Gene | GenBank ID | Primers |
|---|---|---|
| TGF-β1 | NM_021578.2 | Forward: 5′-ATTCCTGGCGTTACCTTGG-3′ |
| Reverse: 5′-AGCCCTGTATTCCGTCTCCT-3′ | ||
| α-SMA | NM_031004.2 | Forward: 5′-TGTGCTATGTCGCTCTGGAC-3′ |
| Reverse: 5′-CCAATGAAAGATGGCTGGAA-3′ | ||
| MCP-1 | NM_031530.1 | Forward: 5′-CCCAGAAACCAGCCAACT-3′ |
| Reverse: 5′-CCAGTGAATGAGTAGCAGCAG-3′ | ||
| POP | NM_031324 | Forward: 5′-AGTGCCGTTTCTTGAGCAGT-3′ |
| Reverse: 5′-CGTCATCCGACAGAGTGTTG-3′ | ||
| Col1a1 | NM_053304.1 | Forward: 5′-TATCTGCCACAATGGCACGG-3′ |
| Reverse: 5′-GCACGGAAACTCCAGCTGAT-3′ | ||
| GAPDH | NM_017008.4 | Forward: 5′-GGGCAGCCCAGAACATCAT-3′ |
| Reverse: 5′-CCAGTGAGCTTCCCGTTCAG-3′ |
POP, prolyl oligopeptidase; TGF, transforming growth factor; SMA, smooth muscle actin; MCP, monocyte chemoattractant protein; Col1a1, collagen type I α 1 chain.
Western blot analysis
Treated cells were lysed in ice-cold radio-immunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitor phenylmethylsulfonyl fluoride (PMSF; Beyotime Institute of Biotechnology, Shanghai, China). Total protein was measured using the bicinchoninic acid protein assay (Beyotime Institute of Biotechnology). Mouse anti-α-SMA (cat. no. A5228; 1:1,000), rabbit anti-TGF-β1 (cat. no. SAB4502954; 1:1,000) and rabbit anti-POP (cat. no. SAB2104515; 1:500) were purchased from Sigma (Merck Millipore), while rabbit anti-Smad7 (cat. no. D160746; 1:1,000), rabbit anti-PPAR-γ (cat. no. AB61087; 1:1,000) were obtained from Sangon Biotech (Shanghai, China), rabbit anti-phosphorylated (p)-Smad 2/3 (cat. no. AP0326; 1:1,000) was purchased from Bioworld Technology (St. Louis Park, MN, USA), and mouse anti-beta tubulin (cat. no. AT819; 1:1,000) was purchased from Beyotime Institute of Biotechnology (Shanghai, China). A secondary horseradish peroxidase-conjugated goat anti-mouse antibody (cat. no. A9917; 1:60,000) and goat anti-rabbit antibody (cat. no. A9169; 1:50,000) were purchased from Sigma (Merck Millipore). Western blot analysis was performed as previously described (29). Immune complexes were detected using immobilon western chemiluminescent horseradish peroxidase substrate (Millipore Corp., Billerica, MA, USA). Bands were quantified using Image Lab version 2.0.1 (Bio-Rad Laboratories, Hercules, CA, USA). Tubulin was used as a loading control.
POP activity assay
Intracellular AcSDKP was measured using the AcSDKP enzyme immunoassay kit (Bertin Pharma, Montigny-le-Bretonneux, France) modified for the cells (29,39). In brief, cells were lysed with RIPA buffer containing 10 µmol/l captopril and 1 mmol/l PMSF. The cell number was determined using a Fuchs-Rosenthal counting chamber. Lysates were centrifuged at 14,000 × g for 10 min and supernatants were extracted with methanol. Samples and standards were then processed according to the manufacturer's instructions.
Statistical analysis
Results were expressed as the mean ± standard error of the mean. At least three independent experiments were performed for each assay. Data were analyzed by Student's t-test or one way analysis of variance followed by the Mann-Whitney U test using SAS software (Release 8.02 TS Level 02 M0; SAS Institute, Cary, NC, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Pharmacological inhibition of POP activity and lentiviral induction of POP protein expression in HSC-T6 cells
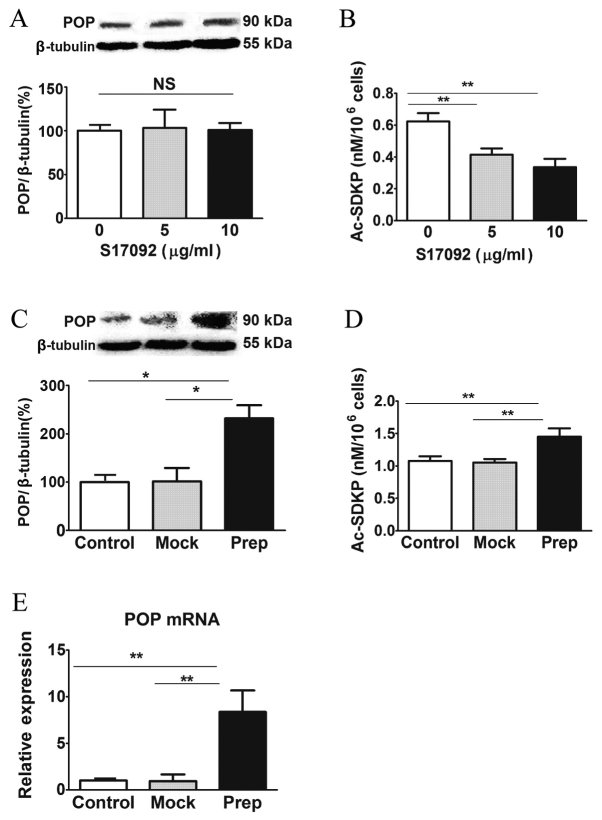
As a potent inhibitor of POP, S17092 has been considered as a promising therapeutic compound for memory impairment (40). In the present study, the effects of S17092 on HSC-T6 cells were investigated. S17092 treatment did not affect the protein levels of POP (Fig. 1A); however, it dose-dependently decreased the activity of POP as indicated by the intracellular levels of AcSDKP (Fig. 1B). To induce the activity of POP, HSC-T6 cells were transfected with a lentiviral overexpression vector. The protein levels of POP in HSC-T6 cells transduced with POP overexpression vector were ~2.5-fold increased compared with those in mock-transfected and non-transfected control HSC-T6 cells (Fig. 1C). Consistently, the intracellular levels of AcSDKP were ~1.5-fold increased, indicating that POP activity was increased (Fig. 1D). Of note, after transduction with POP-expressing lentivirus, the mRNA levels of POP were ~8-fold increased compared to those in mock- and non-transfected control HSC-T6 cells (Fig. 1E). These results showed that POP activity in HSC-T6 cells can be pharmacologically inhibited or induced using a lentivirus.
Figure 1.
POP activity inhibition by S17092 and overexpression by lentivirus in HSC-T6 cells. (A) Western blot analysis of POP protein and (B) determination of intracellular Ac-SDKP levels in HSC-T6 cells after treatment with various concentrations of POP inhibitor S17092 (0, 5 or 10 µg/ml). (C) Western blot analysis of POP protein, (D) determination of intracellular Ac-SDKP levels and (E) POP mRNA expression in HSC-T6 cells after infection with prep-or mock-lentivirus for 3 days determined by quantitative polymerase chain reaction analysis. Values are normalized to tubulin. *P<0.05, **P<0.01. NS, not significant; POP, prolyl oligopeptidase; Ac-SDKP, N-acetyl-seryl-aspartyl-lysyl-proline.
POP activity is essential for the proliferation of HSC-T6 cells but does not affect apoptosis
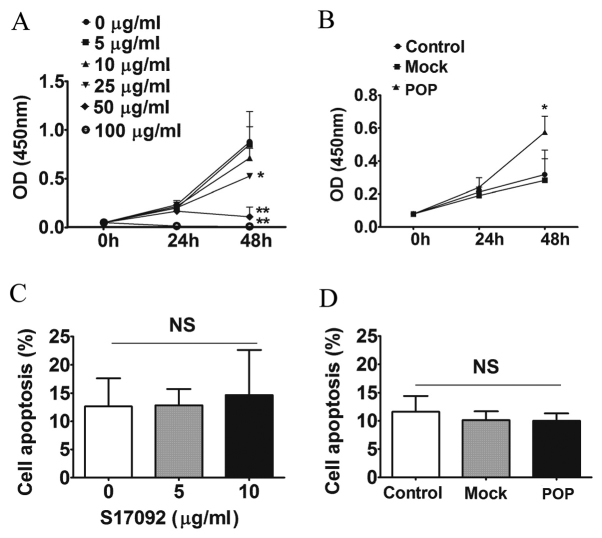
To investigate the effect of POP on the growth of HSC-T6 cells, cell proliferation and apoptosis were tested under pharmacological inhibition and lentiviral induction conditions. S17092 inhibited the proliferation of HSC-T6 cells in a dose-dependent manner (Fig. 2A), whereas POP overexpression promoted cell proliferation by 80% compared to mock- and non-transfected control HSC-T6 cells after 48 h of incubation (P<0.05) (Fig. 2B). However, S17092 or lentiviral induction of POP did not affect the apoptotic rate of HSC-T6 cells (Fig. 2C and D). These results suggested that POP increases the proliferation of HSCs in vitro.
Figure 2.
Cell prolifeation and apoptosis after POP inhibiton or overexpresion in HSC-T6 cells. Effects of (A) various concentrations of POP-inhibitor S17092 or (B) lentivirus-mediated overexpression of POP in HSC-T6 cells as determined using a Cell Counting Kit-8. The OD at 450 nm is proportional to the number of viable cells. Cell apoptosis after (C) treatment with S17092 (0, 5 or 10 µg/ml) for 24 h or (D) transduction with mock-transfected or POP-expressing lentivirus for 3 days. *P<0.05, **P<0.01 vs. control group. NS, not significant; POP, prolyl oligopeptidase; OD, optical density; mock, empty lentivirus.
POP inhibits the expression of α-SMA and MCP-1 without affecting collagen I and TGF-β1
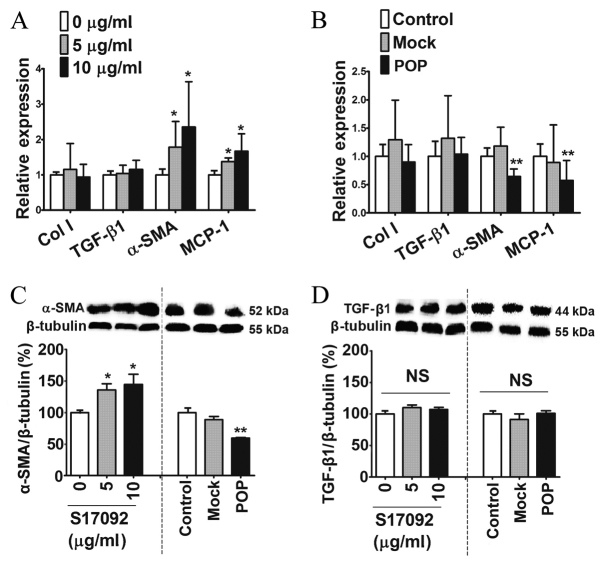
To assess the potential influence of POP on liver fibrosis, several markers for the activation of HSCs were tested after pharmacological inhibition or lentiviral induction of POP. Compared with the vehicle, S17092 dose-dependently increased the mRNA expression of α-SMA and MCP-1 in HSC-T6 cells at the concentration of 5 and 10 µg/ml without affecting collagen I and TGF-β1 mRNA expression (Fig. 3A). By contrast, POP overexpression in HSC-T6 resulted in a significant decrease in the expression of α-SMA and MCP-1 mRNA but not of collagen I and TGF-β1 mRNA when compared to mock- and non-transfected control HSC-T6 cells (Fig. 3B). Western blot analysis further confirmed that the protein levels of α-SMA were dose-dependently increased by S17092 and significantly decreased after lentiviral induction (Fig. 3C). However, the protein levels of TGF-β1 were not affected by either S17092 or lentiviral vector. These results indicated that POP reduces the expression of pro-fibrotic genes.
Figure 3.
Effects of POP on the activation status and fibrogenic properties of HSC-T6 cells. mRNA expression levels of α-SMA, collagen I, MCP-1 and TGF-β1 in HSC-T6 cells (A) treated with S17092 (0, 5 or 10 µg/ml) for 24 h or (B) infected with mock- or POP-expressing lentivirus for 3 days. Western blot analysis of (C) α-SMA and (D) TGF-β1 in HSC-T6 cells treated with S17092 (0, 5 or 10 µg/ml) for 24 h or transduced with mock-transfected or POP-expressing lentivirus for 3 days. *P<0.05, **P<0.01. NS, not significant. TGF, transforming growth factor; SMA, smooth muscle actin; MCP, monocyte chemoattractant protein; POP, prolyl oligopeptidase mock, empty lentivirus.
POP increases Smad7 and PPAR-γ without affecting p-Smad 2/3
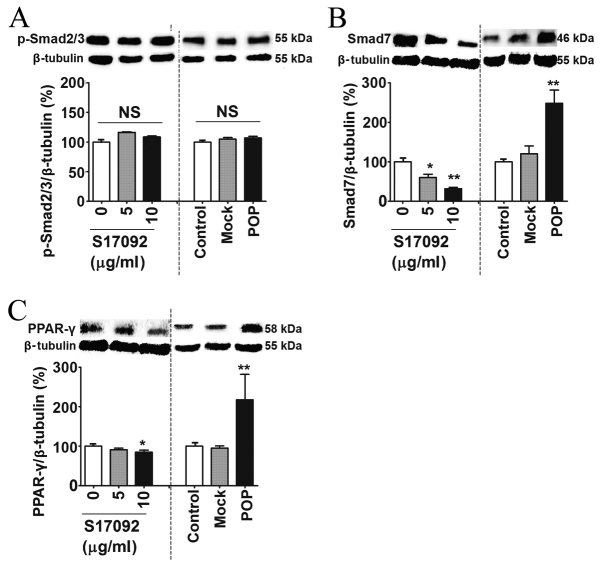
Smad proteins are intracellular mediators of the signal transduction of TGF-β (41). The protein levels of p-Smad2/3 were not affected by either S17092 treatment or POP overexpression (Fig. 4A). However, the Smad7 protein was significantly downregulated in S17092-treated cells (Fig. 4B). By contrast, Smad7 was significantly upregulated in lentivirus-induced cells compared to mock- and non-transfected control HSC-T6 cells (Fig. 4B). Of note, PPAR-γ was also markedly decreased following S17092 treatment and increased in following lentiviral induction (Fig. 4C). These results showed that POP affects the Smad7 and PPAR-γ signaling pathways.
Figure 4.
Effects of POP on Smad proteins and PPAR-γ signaling in HSC-T6 cells. Western blot analysis of (A) p-Smad 2/3, (B) Smad7 and (C) PPAR-γ in HSC-T6 cells treated with S17092 (0, 5 or 10 µg/ml) for 24 h or transduced with mock-transfected or POP-expressing lentivirus for 3 days. *P<0.05, **P<0.01. NS, not significant; POP, prolyl oligopeptidase; PPAR, peroxisome proliferator activated receptor; p-Smad, phosphorylated Smad; mock, empty lentivirus.
Discussion
The present study showed that POP attenuated the activation of HSC-T6 cells, as indicated by the inhibited expression of MCP-1 and α-SMA. This activated phenotype may be caused by increased Smad7 and PPAR-γ levels.
Liver fibrosis is associated with complex molecular and cellular mechanisms, while HSCs are considered to have a pivotal role in this process, mainly participating in intrahepatic inflammation and excessive deposition of ECM in the liver (15,42). The results of the present study showed that POP decreased α-SMA, which is a marker for HSC activation. Furthermore, in HSC-T6 cells, POP also inhibited MCP-1, which is a crucial pro-inflammatory and pro-fibrotic cytokine mainly produced by activated HSCs in the liver (43). These results suggested that POP attenuates HSC activation and thus their pro-fibrotic features.
TGF-β signaling is critical in promoting liver fibrosis (15,16). While, according to the results of the present study, inhibition by S17902 and POP overexpression did not affect TGF-β1 expression and p-Smad2/3 levels in HSC-T6 cells, POP participated in the regulation of Smad7 levels. Following POP inhibition, Smad7 was decreased, while it was increased after POP overexpression. These data suggested an inhibitory effect of POP on TGF-β1 signaling. Smad7 is an inhibitory peptide, which blocks TGF-β1 signaling by physical interaction with the activated TGF-β receptor 1 and prevention of the docking and phosphorylation of Smad2/3, thus inhibiting its pro-fibrogenic and pro-inflammatory activities and HSC activation (44,45).
Apart from de novo expression of α-SMA, reduced PPAR-γ expression is also a marker for HSC activation (20,21). The results of the present study showed that POP inhibitor significantly decreased, while lentivirus-mediated overexpression of POP increased the expression of PPAR-γ. Together with decreased α-SMA levels in HSCs, this indicated attenuated HSC activation.
The decrease of intracellular Ac-SDKP after POP inhibition by S17092 as well as the increase of intracellular Ac-SDKP after vector-mediated overexpression of POP demonstrated that POP activity was successfully manipulated in HSC-T6 cells. It may be speculated that the effects of POP on HSCs may be mediated via Ac-SDKP, since a previous study has demonstrated the anti-inflammatory and anti-fibrotic effects of Ac-SDKP on HSCs (29). However, there are differences between the effects of POP and Ac-SDKP on HSCs. In the present study, POP inhibited TGF-β1 signaling via increasing Smad7 without affecting TGF-β1 and p-Smad2/3. This effect is different from that of Ac-SDKP, which decreases the expression of TGF-β1 and p-Smad2/3 without affecting Smad7 (29). Furthermore, Ac-SDKP inhibits HSC proliferation (29), which is in contrast to the stimulatory effect of POP on the proliferation of HSCs, as shown by decreased cell proliferation after POP inhibition and increased cell proliferation after POP overexpression in the present study. Indeed, this difference has also been shown in other cell types, such as cancer cells and nervous tissue (46–48). In fact, POP was found to be important in promoting hepatocyte proliferation and liver regeneration (34,49,50). The effect may be mediated by POP present in cell nuclei, and to not be associated with its catalytic activity but with protein-protein interactions to regulate gene expression and protein secretion (35,51–53). It has been reported that POP binds to growth-associated protein 43 to control growth cone and synaptic function (46). Of note, HSC activation is generally characterized by increased expression of α-SMA and proliferation (54). However, after activation, α-SMA expression in HSCs in the S-phase of the cell cycle is low or not present (55). Recent studies showed that POP inhibitors impeded cell growth via reducing the expression of retinoblastoma protein (pRb) and Ki-67 and increasing the expression of p53, p27kip1 and pRb2/p130 in cancer cells (47,48). Collectively, the effects of POP on HSCs may not or not exclusively be due to increases in Ac-SDKP. However, it is difficult to separate the effects of POP and Ac-SDKP on HSCs since the mechanisms of the effect of Ac-SDKP on HSCs are currently elusive and no inhibitor or reliable neutralizing antibody is available to investigate these.
In conclusion, the present study showed that POP attenuated HSC activation and decreased its pro-inflammatory and pro-fibrotic features, strongly suggesting a protective effect of POP against liver fibrosis. Further studies are required to investigate specific mechanisms of POP in regulating HSC proliferation and activation and its possible anti-fibrotic effect in vivo.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant nos. 81170,410 to Y.-W.C., 81260081 to Y.-N.D., and 81070322 to J.-G.F.), the New Talented Young Medical Specialist Cultivating Program of Shanghai (no. XYQ2011010 to Y.-W.C.), the Key Research Program for Colleges and Universities of Xinjiang Uygur Autonomous Region (no. XJEDU2012I001 to Y.-N.D.), and by the State Key Development Program for Basic Research of China (no. 2012CB517501 to J.-G.F.).
References
- 1.Gass J, Khosla C. Prolyl endopeptidases. Cell Mol Life Sci. 2007;64:345–355. doi: 10.1007/s00018-006-6317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter R, Shlank H, Glass JD, Schwartz IL, Kerenyi TD. Leucylglycinamide released from oxytocin by human uterine enzyme. Science. 1971;173:827–829. doi: 10.1126/science.173.3999.827. [DOI] [PubMed] [Google Scholar]
- 3.Irazusta J, Larrinaga G, González-Maeso J, Gil J, Meana JJ, Casis L. Distribution of prolyl endopeptidase activities in rat and human brain. Neurochem Int. 2002;40:337–345. doi: 10.1016/S0197-0186(01)00078-X. [DOI] [PubMed] [Google Scholar]
- 4.Myöhänen TT, Venäläinen JI, Garcia-Horsman JA, Piltonen M, Männistö PT. Cellular and subcellular distribution of rat brain prolyl oligopeptidase and its association with specific neuronal neurotransmitters. J Comp Neurol. 2008;507:1694–1708. doi: 10.1002/cne.21642. [DOI] [PubMed] [Google Scholar]
- 5.Myohanen TT, Pyykkö E, Männistö PT, Carpen O. Distribution of prolyl oligopeptidase in human peripheral tissues and in ovarian and colorectal tumors. J Histochem Cytochem. 2012;60:706–715. doi: 10.1369/0022155412453051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myohanen TT, Venäläinen JI, García-Horsman JA, Piltonen M, Männistö PT. Distribution of prolyl oligopeptidase in the mouse whole-body sections and peripheral tissues. Histochem Cell Biol. 2008;130:993–1003. doi: 10.1007/s00418-008-0468-x. [DOI] [PubMed] [Google Scholar]
- 7.Sakata K, Eda S, Lee ES, Hara M, Imoto M, Kojima S. Neovessel formation promotes liver fibrosis via providing latent transforming growth factor-β. Biochem Biophys Res Commun. 2014;443:950–956. doi: 10.1016/j.bbrc.2013.12.074. [DOI] [PubMed] [Google Scholar]
- 8.Yu FX, Teng YY, Zhu QD, Zhang QY, Tang Y. Inhibitory effects of capsaicin on hepatic stellate cells and liver fibrosis. Biochem Cell Biol. 2014;92:406–412. doi: 10.1139/bcb-2014-0036. [DOI] [PubMed] [Google Scholar]
- 9.Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 10.Atzori L, Poli G, Perra A. Hepatic stellate cell: A star cell in the liver. Int J Biochem Cell Biol. 2009;41:1639–1642. doi: 10.1016/j.biocel.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Wallace K, Alastair AD Burt, Wright Matthew MC. Liver fibrosis. Biochemical J. 2008;411:1–18. doi: 10.1042/BJ20071570. [DOI] [PubMed] [Google Scholar]
- 12.Yu F, Su L, Ji S, Zhang S, Yu P, Zheng Y, Zhang Q. Inhibition of hepatic stellate cell activation and liver fibrosis by fat-specific protein 27. Mol Cell Biochem. 2012;369:35–43. doi: 10.1007/s11010-012-1366-z. [DOI] [PubMed] [Google Scholar]
- 13.Friedman SL. Hepatic stellate cells: Protean, multifunctional and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borg BB, Seetharam A, Subramanian V, Basha HI, Lisker-Melman M, Korenblat K, Anderson CD, Shenoy S, Chapman WC, Crippin JS, Mohanakumar T. Immune response to extracellular matrix collagen in chronic hepatitis C-induced liver fibrosis. Liver Transpl. 2011;17:814–823. doi: 10.1002/lt.22303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, Kim KH, Park KK. Mechanisms of fibrogenesis in liver cirrhosis: The molecular aspects of epithelial-mesenchymal transition. World J Hepatol. 2014;6:207–216. doi: 10.4254/wjh.v6.i4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hills CE, Squires PE. The role of TGF-β and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev. 2011;22:131–139. doi: 10.1016/j.cytogfr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Dooley S, Delvoux B, Lahme B, Mangasser-Stephan K, Gressner AM. Modulation of transforming growth factor beta response and signaling during transdifferentiation of rat hepatic stellate cells to myofibroblasts. Hepatology. 2000;31:1094–1106. doi: 10.1053/he.2000.6126. [DOI] [PubMed] [Google Scholar]
- 18.Nakao A, Afrakhte M, Morén A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 19.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: Opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 20.Zardi EM, Navarini L, Sambataro G, Piccinni P, Sambataro FM, Spina C, Dobrina A. Hepatic PPARs: Their role in liver physiology, fibrosis and treatment. Curr Med Chem. 2013;20:3370–3396. doi: 10.2174/09298673113209990136. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Zhang S, Chu ES, Go MY, Lau RH, Zhao J, Wu CW, Tong L, Zhao J, Poon TC, Sung JJ. Peroxisome proliferator-activated receptors gamma reverses hepatic nutritional fibrosis in mice and suppresses activation of hepatic stellate cells in vitro. Int J Biochem Cell Biol. 2010;42:948–957. doi: 10.1016/j.biocel.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Bian D, Zhang J, Wu X, Dou Y, Yang Y, Tan Q, Xia Y, Gong Z, Dai Y. Asiatic acid isolated from Centella asiatica inhibits TGF-β1-induced collagen expression in human keloid fibroblasts via PPAR-γ activation. Int J Biol Sci. 2013;9:1032–1042. doi: 10.7150/ijbs.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon KI, Kulkarni A, Woeller CF, Phipps RP, Sime PJ, Hindman HB, Huxlin KR. Inhibitory effects of PPARγ ligands on TGF-β1-induced corneal myofibroblast transformation. Am J Pathol. 2014;184:1429–1445. doi: 10.1016/j.ajpath.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh AK, Bhattacharyya S, Lakos G, Chen SJ, Mori Y, Varga J. Disruption of transforming growth factor beta signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor gamma. Arthritis Rheum. 2004;50:1305–1318. doi: 10.1002/art.20104. [DOI] [PubMed] [Google Scholar]
- 25.Blaner WS, O'Byrne SM, Wongsiriroj N, Kluwe J, D'Ambrosio DM, Jiang H, Schwabe RF, Hillman EM, Piantedosi R, Libien J. Hepatic stellate cell lipid droplets: A specialized lipid droplet for retinoid storage. Biochim Biophys Acta. 2009;1791:467–473. doi: 10.1016/j.bbalip.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S, Choi S, Lee MG, Lim C, Oh J. Retinol binding protein-albumin domain III fusion protein deactivates hepatic stellate cells. Mol Cells. 2012;34:517–522. doi: 10.1007/s10059-012-0183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kluwe J, Wongsiriroj N, Troeger JS, Gwak GY, Dapito DH, Pradere JP, Jiang H, Siddiqi M, Piantedosi R, O'Byrne SM, et al. Absence of hepatic stellate cell retinoid lipid droplets does not enhance hepatic fibrosis but decreases hepatic carcinogenesis. Gut. 2011;60:1260–1268. doi: 10.1136/gut.2010.209551. [DOI] [PubMed] [Google Scholar]
- 28.Cavasin MA, Rhaleb NE, Yang XP, Carretero OA. Prolyl Oligopeptidase is involved in release of the Antifibrotic peptide Ac-SDKP. Hypertension. 2004;43:1140–1145. doi: 10.1161/01.HYP.0000126172.01673.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YW, Liu BW, Zhang YJ, Chen YW, Dong GF, Ding XD, Xu LM, Pat B, Fan JG, Li DG. Preservation of basal AcSDKP attenuates carbon tetrachloride-induced fibrosis in the rat liver. J Hepatol. 2010;53:528–536. doi: 10.1016/j.jhep.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Xu LM, Chen YW, Ni QW, Zhou M, Qu CY, Zhang Y. Antifibrotic effect of N-acetyl-seryl-aspartyl-lysyl-proline on bile duct ligation induced liver fibrosis in rats. World J Gastroenterol. 2012;18:5283–5288. doi: 10.3748/wjg.v18.i37.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao Y, Qu C, Ge W, Wang B, Wu J, Xu L, Chen Y. Depletion of thymosin β4 promotes the proliferation, migration and activation of human hepatic stellate cells. Cell Physiol Biochem. 2014;34:356–367. doi: 10.1159/000363005. [DOI] [PubMed] [Google Scholar]
- 32.Reyes-Gordillo K, Shah R, Arellanes-Robledo J, Rojkind M, Lakshman MR. Protective effects of thymosin β4 on carbon tetrachloride-induced acute hepatotoxicity in rats. Ann N Y Acad Sci. 2012;1269:61–68. doi: 10.1111/j.1749-6632.2012.06728.x. [DOI] [PubMed] [Google Scholar]
- 33.Nozaki Y, Sato N, Iida T, Hara K, Fukuyama K, Epstein WL. Prolyl endopeptidase purified from granulomatous inflammation in mice. J Cell Biochem. 1992;49:296–303. doi: 10.1002/jcb.240490313. [DOI] [PubMed] [Google Scholar]
- 34.Yamakawa N, Shimeno H, Soeda S, Nagamatsu A. Regulation of prolyl oligopeptidase activity in regenerating rat liver. Biochim Biophys Acta. 1994;1199:279–284. doi: 10.1016/0304-4165(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 35.Tenorio-Laranga J, Männistö PT, Storvik M, Van der Veken P, García-Horsman JA. Four day inhibition of prolyl oligopeptidase causes significant changes in the peptidome of rat brain, liver and kidney. Biochimie. 2012;94:1849–1859. doi: 10.1016/j.biochi.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Bellemère G, Morain P, Vaudry H, Jégou S. Effect of S 17092, a novel prolyl endopeptidase inhibitor, on substance P and alpha-melanocyte-stimulating hormone breakdown in the rat brain. J Neurochem. 2003;84:919–929. doi: 10.1046/j.1471-4159.2003.01536.x. [DOI] [PubMed] [Google Scholar]
- 37.Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, Blaner WS. An immortalized rat liver stellate cell line (HSC-T6): A new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41:882–893. [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Liu JM, Lawrence F, Kovacevic M, Bignon J, Papadimitriou E, Lallemand JY, Katsoris P, Potier P, Fromes Y, Wdzieczak-Bakala J. The tetrapeptide AcSDKP, an inhibitor of primitive hematopoietic cell proliferation, induces angiogenesis in vitro and in vivo. Blood. 2003;101:3014–3020. doi: 10.1182/blood-2002-07-2315. [DOI] [PubMed] [Google Scholar]
- 40.Morain P, Lestage P, De Nanteuil G, Jochemsen R, Robin JL, Guez D, Boyer PA. S 17092: A prolyl endopeptidase inhibitor as a potential therapeutic drug for memory impairment. Preclinical and clinical studies. CNS Drug Rev. 2002;8:31–52. doi: 10.1111/j.1527-3458.2002.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284–292. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi SS, Diehl AM. Epithelial-to-mesenchymal transitions in the liver. Hepatology. 2009;50:2007–2013. doi: 10.1002/hep.23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577–594. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 44.Pohlers D, Brenmoehl J, Löffler I, Müller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G. TGF-beta and fibrosis in different organs-molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746–756. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Miyazono K, ten Dijke P, Heldin CH. TGF-beta signaling by Smad proteins. Adv Immunol. 2000;75:115–157. doi: 10.1016/S0065-2776(00)75003-6. [DOI] [PubMed] [Google Scholar]
- 46.Di Daniel E, Glover CP, Grot E, Chan MK, Sanderson TH, White JH, Ellis CL, Gallagher KT, Uney J, Thomas J, et al. Prolyl oligopeptidase binds to GAP-43 and functions without its peptidase activity. Mol Cell Neurosci. 2009;41:373–382. doi: 10.1016/j.mcn.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki K, Sakaguchi M, Tanaka S, Yoshimoto T, Takaoka M. Prolyl oligopeptidase inhibition-induced growth arrest of human gastric cancer cells. Biochem Biophys Res Commun. 2014;443:91–96. doi: 10.1016/j.bbrc.2013.11.051. [DOI] [PubMed] [Google Scholar]
- 48.Sakaguchi M, Matsuda T, Matsumura E, Yoshimoto T, Takaoka M. Prolyl oligopeptidase participates in cell cycle progression in a human neuroblastoma cell line. Biochem Biophys Res Commun. 2011;409:693–698. doi: 10.1016/j.bbrc.2011.05.066. [DOI] [PubMed] [Google Scholar]
- 49.Agirregoitia N, Casis L, Gil J, Ruiz F, Irazusta J. Ontogeny of prolyl endopeptidase and pyroglutamyl peptidase I in rat tissues. Regul Pept. 2007;139:52–58. doi: 10.1016/j.regpep.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Matsubara Y, Ono T, Tsubuki S, Irie S, Kawashima S. Transient up-regulation of a prolyl endopeptidase activity in the microsomal fraction of rat liver during postnatal development. Eur J Biochem. 1998;252:178–183. doi: 10.1046/j.1432-1327.1998.2520178.x. [DOI] [PubMed] [Google Scholar]
- 51.Myöhänen TT, García-Horsman JA, Tenorio-Laranga J, Männistö PT. Issues about the physiological functions of prolyl oligopeptidase based on its discordant spatial association with substrates and inconsistencies among mrna, protein levels and enzymatic activity. J Histochem Cytochem. 2009;57:831–848. doi: 10.1369/jhc.2009.953711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tenorio-Laranga J, Männistö PT, García-Horsman JA. Hunting for peptide substrates of prolyl oligopeptidase: Classical versus non-classical bioactive peptides. CNS Neurol Disord Drug Targets. 2011;10:319–326. doi: 10.2174/187152711794653841. [DOI] [PubMed] [Google Scholar]
- 53.Matsuda T, Sakaguchi M, Tanaka S, Yoshimoto T, Takaoka M. Prolyl oligopeptidase is a glyceraldehyde-3-phosphate dehydrogenase-binding protein that regulates genotoxic stress-induced cell death. Int J Biochem Cell Biol. 2013;45:850–857. doi: 10.1016/j.biocel.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007;131:1728–1734. doi: 10.5858/2007-131-1728-HSCALF. [DOI] [PubMed] [Google Scholar]
- 55.Mashimo Y, Mochida S, Inao M, Yamaoka M, Nagoshi S, Matsui A, Fujiwara K. Decreased expression of smooth muscle α actin in activated rat hepatic stellate cells at the S-phase of the cell cycle in vitro. Hepatol Res. 1999;15:22–31. doi: 10.1016/S1386-6346(99)00012-1. [DOI] [Google Scholar]