Abstract
The primary active component of black pepper is piperine, which is purified and used to treat epilepsy, achieving higher efficiency when purified. The present study was conducted to evaluate whether the anticonvulsant effect of piperine ameliorates pilocarpine-induced epilepsy, and to investigate the mechanism underlying these effects. Epilepsy was induced in Sprague Dawley rats using pilocarpine. Pilocarpine-induced epilepsy in the rats was treated with 40 mg/kg piperine for 45 consecutive days. Status epilepticus and a Morris water maze test were used to analyze the anticonvulsant effects of piperine in the epileptic rats. Inflammation and oxidative stress were then measured using commercially-available kits following piperine treatment. Lastly, the activity of caspase-3 and the protein expression levels of B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X protein (Bax) were evaluated using commercially-available kits and western blot analysis, respectively. The results demonstrated that treatment with piperine was able to reduce the status epilepticus and prevented memory impairment following pilocarpine-induced epilepsy in rats. The anticonvulsant effects of piperine decreased inflammation and oxidative stress following pilocarpine-induced epilepsy in rats. The upregulated activity of caspase-3 and expression levels of Bax/Bcl-2 were suppressed following treatment with piperine in the rats with pilocarpine-induced epilepsy. These results suggest that the anticonvulsant effects of piperine ameliorate memory impairment, inflammation and oxidative stress in a rat model of pilocarpine-induced epilepsy.
Keywords: piperine, pilocarpine-induced epilepsy, inflammation, oxidative stress
Introduction
Epilepsy is a neurological disease, and usually refers to a chronic disease with various recurrent clinical manifestations, resulting from the sudden abnormal electrical impulses of neurons in the brain (1). Refractory epilepsy, also known as intractable epilepsy, refers to epilepsy occurring multiple times each month and persists for >2 years, with no improvement observed with regular treatment (2). Approximately 30% of patients with epilepsy suffer from refractory epilepsy (3). The prevalence of epilepsy is ~5% worldwide, causing a heavy financial and emotional burden to families and communities (4).
For decades, in vitro and in vivo models of epilepsy have been used to explore the molecular and cellular mechanisms underlying recurrent spontaneous epilepsy (5). Although progress has been made in the understanding of epilepsy occurrence, investigators have yet to develop reliable biomarkers or surrogate markers for epileptogenic lesions (6). At present, the pathological mechanism underlying epilepsy occurrence is poorly understood, specifically in the case of epilepsy caused by cumulative events such as head trauma, inflammation or chronic febrile seizures (7). The primary challenge for epilepsy treatment is the inherent complexity, heterogeneity and various genetic susceptibilities that epilepsy seizures exhibit (8).
Piperine is the primary active component of black pepper and belongs to the cinnamon phthalocyanine amine alkaloid family, which is widely distributed in nature, and especially present in numerous pepper plants families (9). Piperine has been demonstrated to have numerous effects, including antioxidant (10), immunomodulatory (11), anti-tumor (12) and drug metabolism promotion effects (13).
The present study aimed to evaluate the anticonvulsant effects of piperine in rats with pilocarpine-induced epilepsy. In addition, the potential effects of piperine on memory impairment, inflammation and oxidative stress in the rat model of pilocarpine-induced epilepsy were also evaluated.
Materials and methods
Drugs and chemicals
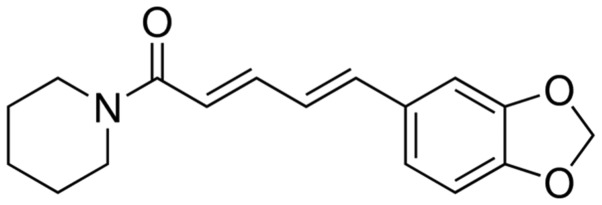
Piperine (Fig. 1) and pilocarpine was purchased from Sigma-Aldrich (St. Louis, MO, USA). Tumor necrosis factor-α (TNF-α; cat. no. H052), interleukin-1β (IL-1β; cat. no. H002), malondialdehyde (MDA; cat. no. A003-1), glutathione (GSH; cat. no. A006-2), superoxide dismutase (SOD; cat. no. A001-1), catalase (CAT; cat. no. A007-1) and caspase-3 (cat. no. H076) activity assay kits were purchased from Jiancheng Bioengineering Institute (Nanjing, China). In addition, methylscopolamine, diazepam and chloral hydrate were purchased from Nanjing Chemical Reagent Co., Ltd. (Nanjing, China).
Figure 1.
Chemical structure of piperine.
Animals
A total of 24 male Sprague-Dawley rats (230–280 g; 8-weeks-old) were housed in individual metabolic cages in a controlled environment (23±1°C; humidity, 55±5%) under a 12-h light/dark cycle. Food and water were available ad libitum. The study was carried out under the recommendations of the Sichuan University of Animal Experimentation, and the experimental procedures received prior ethical approval from the Committee for Health Guide for the Care and Use of Laboratory Animals. Prior to treatment the rats were provided with normal dry-food pellets and water-soaked food.
Induction of epilepsy in the rats using pilocarpine
Prior to treatment with 340 mg/kg of pilocarpine hydrochloride injection, the experimental rats were intraperitoneally injected with methylscopolamine (1 mg/kg) for 45 min. The rats were then evaluated for behaviors indicative of seizure activity (such as mouth and focal movements, head nodding, contra-lateral forelimb clonus, symmetrical forelimb clonus with rearing or severe seizure with rearing and falling) for 120 min after the administration of pilocarpine hydrochloride. Epileptic activity was evaluated in accordance with the Racine scale (14). When generalized seizure activity was continuously observed without normal behavior during each episode, the rats were considered suffer from seizure episodes. When the rats continuously suffered from seizure episodes for 1 h, they were intraperitoneally administered diazepam (10 mg/kg) to terminate the seizures.
Experimental procedures
The rats were randomly divided into three experimental groups: i) A control group (n=8), in which the rats were administered 0.9% normal saline; ii) an epilepsy group (n=8), in which the rats with pilocarpine-induced epilepsy were administered 0.9% saline; and iii) a piperine group (n=8), in which the rats with pilocarpine-induced epilepsy were orally administered 40 mg/kg piperine for 45 days.
Morris water maze (MWM) test
Following treatment with pilocarpine, a MWM test was used to assess the spatial memory of the rats. A circular water tank (diameter, 140 cm; depth, 60 cm) was filed with water (23±1°C), and made opaque by the addition of a white non-toxic paint (Dulux; AkzoNobel, Amsterdam, Netherlands). The rats were placed into the tank from different quadrants for 120 sec of training. Subsequently, the rats were tested for their ability to locate the hidden platform (10×10 cm) and given two trials/day. When the rat escaped onto the platform, the trial was terminated. Each trial was started and terminated manually by the experimenter, and the experiment duration was 5 days.
Sample collection and homogenization
All rats were anesthetized with 10% chloral hydrate (350 mg/kg) and sacrificed by decapitation. Hippocampus tissue samples were rapidly removed and weighed and kept at −80°C until analyzed. The brain tissue samples were homogenized using 5% w/v 20 mM RIPA lysis buffer (Beyotime Institute of Biotechnology, Haimen, China). The lysate was then subjected to centrifugation at 10,000 × g for 10 min at 4°C, and the supernatant containing the protein was collected.
Inflammation and oxidative stress
Following homogenization of the brain tissues and protein extraction, the activity levels of inflammatory cytokines (TNF-α and IL-1β) and oxidative stress-associated factors (MDA, GSH, SOD, and CAT) in the tissues were determined using ELISA kits, in accordance with the manufacturer's instructions.
Caspase-3 activity determination
Following homogenization of the brain tissues and protein extraction, the protein concentration was measured using a Bicinchoninic Acid Disodium kit (BCA; Beyotime Institute of Biotechnology). Equal amounts (20 µg) of protein were incubated with Ac-LEHD-pNA (Beyotime Institute of Biotechnology) at 37°C for 2 h in the dark. The activity of caspase-3 was determined with the appropriate ELISA kit, in accordance with the manufacturer's instructions.
Western blot analysis
Following homogenization of the brain tissues and protein extraction, the protein concentration was measured using the BCA kit and equal amounts (50 µg) of protein were separated by 10% SDS-PAGE. The protein was then electrotransferred onto nitrocellulose membranes, and blocked with 5% non-fat milk in Tris-buffered saline with Tween-20 (TBST). The membrane was incubated at 4°C overnight with anti-B-cell lymphoma 2 (Bcl-2; sc-783, dilution, 1:1,000) and anti-Bcl-2-associated X protein (Bax; ; sc-6236; dilution, 1:1,000) and anti-β-actin (sc-130656; dilution, 1:800; all from Santa Cruz Biotechnology, Inc., Dallas, TX, USA) primary antibodies. Subsequently, the membrane was incubated with goat anti-mouse immunoglobulin G secondary antibody (cat. no. 6401-05; Amyjet Scientific Inc., Wuhan, China). In order to detect the target protein, the blots were then visualized in an enhanced chemiluminescence solution (P0018A; Beyotime Institute of Biotechnology) and exposed to a ChemiDoc MP Imaging System (Bio-Rad Laboratories, Inc., Hercules, California, USA).
Statistical analysis
The data are presented as the mean ± standard error of the mean from ≤3 independent experiments. The data were analyzed using by a Student's t-test with the statistical software SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Anticonvulsant effects of piperine relieve pilocarpine-induced epilepsy in rats
Following the induction of epilepsy using pilocarpine, treatment with piperine significantly increased latency to first seizure (P=0.0051) and time of status epilepticus (P=0.0042), as compared with the rats with pilocarpine-induced epilepsy (Table I). Status epilepticus refers to the continuous seizure episodes subsequent to intraperitoneal injection of epileptic rats with methylscopolamine. Compared with the epileptic rats, the rats treated with piperine exhibited a significant reduction in the percentage of status epilepticus (P=0.0012; Table I). In addition, piperine significantly enhanced the survival percentage of rats with status epilepticus, as compared with the rats with pilocarpine-induced epilepsy (P=0.0009; Table I).
Table I.
Anticonvulsant effects of piperine relieve pilocarpine-induced epilepsy in rats.
| Group | Latency to first seizure | Time of SE | SE percentage | Survival percentage in SE rats |
|---|---|---|---|---|
| Epilepsy | 15.9±3.11 min | 28.7±2.10 min | 97% | 55% |
| Piperine | 46.1±2.59 mina | 51.2±2.98 mina | 51%a | 91%a |
P<0.01, vs. the epilepsy group. SE, status epilepticus.
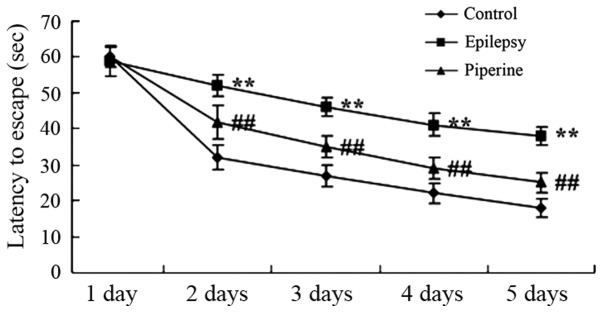
Anticonvulsant effects of piperine prevent memory impairment in rats with pilocarpine-induced epilepsy
The induction of epilepsy using pilocarpine significantly increased the escape latency in status epilepticus rats compared with the control rats (P<0.05; Fig. 2). However, piperine significantly decreased the escape latency compared with rats with pilocarpine-induced epilepsy (P<0.05; Fig. 2).
Figure 2.
Anticonvulsant effects of piperine prevent memory impairment in rats with pilocarpine-induced epilepsy. **P<0.01, vs. the control group; ##P<0.01, vs. the epilepsy group.
Anticonvulsant effects of piperine decrease the levels of inflammation in rats with pilocarpine-induced epilepsy
Following epilepsy induction using pilocarpine, the activity levels of TNF-α (P=0.0017) and IL-1β (P=0.0022) were significantly increased in the epileptic rats, as compared with the control rats (Fig. 3). However, piperine significantly reversed the increase in TNF-α (P=0.0054) and IL-1β (P=0.0043) levels, as compared with the rats with pilocarpine-induced epilepsy (Fig. 3).
Figure 3.
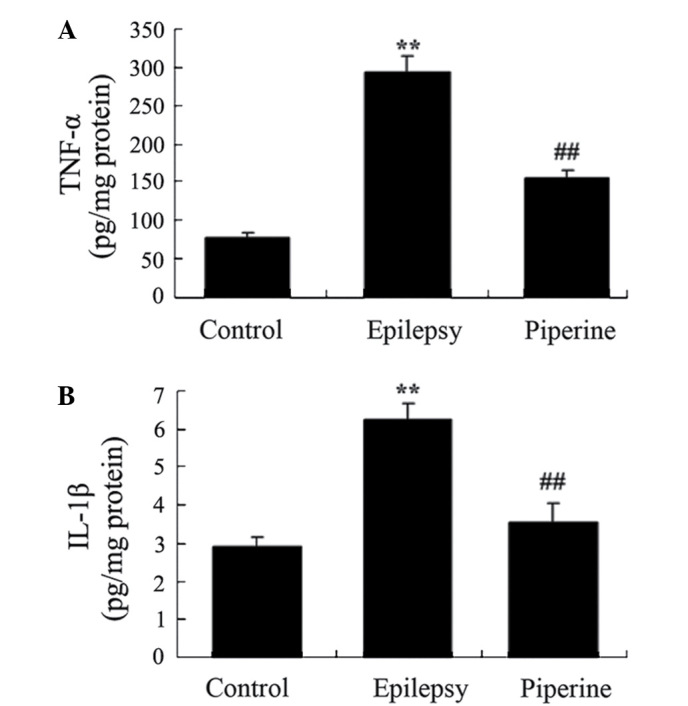
Anticonvulsant effects of piperine decrease inflammation in rats with pilocarpine-induced epilepsy. The anticonvulsant effects of piperine decreased the expression levels of (A) TNF-α and (B) IL-1β in the rats with pilocarpine-induced epilepsy. **P<0.01, vs. the control group; ##P<0.01. vs. the epilepsy group. TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β.
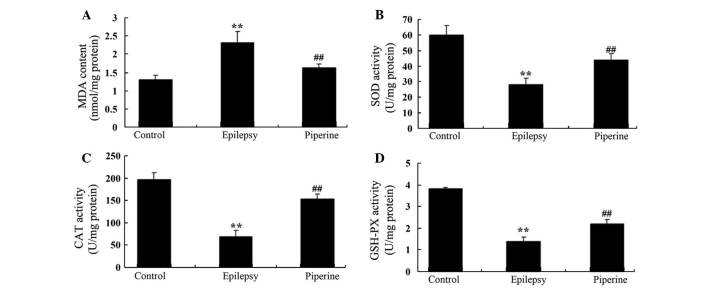
Anticonvulsant effects of piperine decrease the levels of oxidative stress in rats with pilocarpine-induced epilepsy
In the present study, pilocarpine significantly increased the activity levels of MDA, and suppressed the activity of SOD, CAT and GSH, as compared with the control rats (P=0.0067, 0.0041, 0.0023 and 0.0014, respectively; Fig. 4). Treatment with piperine significantly reversed these effects and decreased the levels of oxidative stress in rats with pilocarpine-induced epilepsy (P=0.0089, 0.0072, 0.0030 and 0.0091, respectively; Fig. 4).
Figure 4.
Anticonvulsant effects of piperine decrease the effects of oxidative stress in rats with pilocarpine-induced epilepsy. The anticonvulsant effects of piperine increased the activity levels of (A) MDA and decreased those of (B) SOD, (C) CAT and (D) GSH in the rats with pilocarpine-induced epilepsy. **P<0.01, vs. the control group; ##P<0.01, vs. the epilepsy group. MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase; GSH, glutathione.
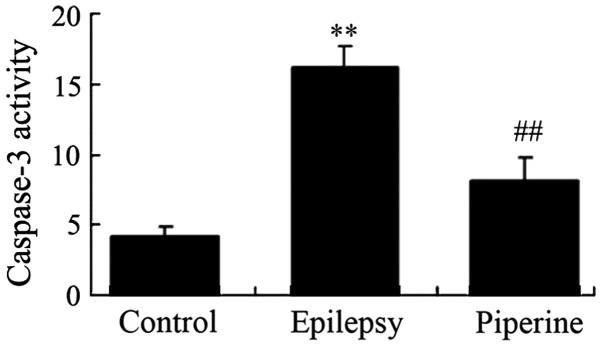
Anticonvulsant effects of piperine decrease the activity levels of caspase-3 in rats with pilocarpine-induced epilepsy
The effects of piperine on cell apoptosis are shown in Fig. 5. Piperine significantly increased caspase-3 activity levels in rats with epilepsy compared with the control rats (P=0.0010; Fig. 5). Notably, administration of piperine significantly reduced caspase-3 activity levels in rats with pilocarpine-induced epilepsy (P=0.0031; Fig. 5).
Figure 5.
Anticonvulsant effects of piperine decrease the activity levels of caspase-3 in rats with pilocarpine-induced epilepsy. **P<0.01, vs. the control group; ##P<0.01, vs. the epilepsy group.
Anticonvulsant effects of piperine decrease the expression levels of Bax/Bcl-2 in rats with pilocarpine-induced epilepsy
The effects of piperine on the Bax/Bcl-2 signaling pathway are shown in Fig. 6. Piperine significantly activated the Bax/Bcl-2 signaling pathway in the epileptic rats compared with the control rats (P<0.05; Fig. 6). However, treatment with piperine significantly downregulated Bax/Bcl-2 expression levels following the induction of epilepsy in the rats using pilocarpine (P<0.05; Fig. 6).
Figure 6.
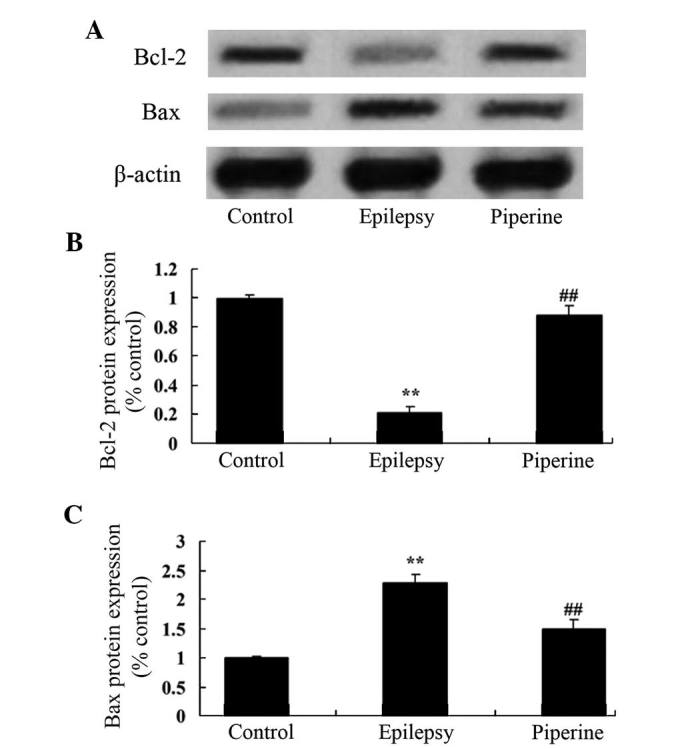
Anticonvulsant effects of piperine decrease the expression levels of Bax/Bcl-2 in rats with pilocarpine-induced epilepsy. (A) The anticonvulsant effects of piperine decrease the protein expression levels of Bax and Bcl-2, as determined by western blotting. Quantification of the protein expression levels of (B) Bcl-2 and (C) Bax in rats with pilocarpine-induced epilepsy. Bcl-2, B cell lymphoma 2; Bax, Bcl-2-associated X protein. **P<0.01 vs. the control group; ##P<0.01 vs. the epilepsy group.
Discussion
Epilepsy is a chronic neurological disorder caused by the repeated and excessive abnormal electrical impulses of brain neurons (1). The prevalence of epilepsy in the global population is ~5%, with up to 10 million patients with epilepsy in China alone (15). Numerous factors can lead to the occurrence of epilepsy, including traumatic brain injury, cerebral hemorrhage, intracranial infections, brain tumors, cerebral cortex abnormalities and brain surgery (16). A total of 70% of patients with epilepsy experience an improvement in their symptoms with the existing anti-epilepsy drugs, although there are still 30% of patients that do not have satisfactory results (17). In these cases, surgery is a good option, although many patients are not eligible for surgery if the correct brain regions cannot be located, if limited neuronal impulses are present, or if the surgical risks are too high (18). Therefore, there is an urgent requirement for novel and effective anti-epilepsy drugs or therapeutic approaches. The results of the present study suggested that the potential anticonvulsant effect of piperine relieved status epilepticus and prevented memory impairment following the induction of epilepsy in rats using pilocarpine. Pal et al (19) reported that piperine protects against epilepsy-associated depression, although the mechanism underlying these effects remains unclear. In addition, Rinwa et al (20) suggested that piperine enhanced chronic stress-induced cognitive impairment in mice. These results indicate that piperine is an effective drug for the treatment of status epilepticus and other neurologic diseases.
The occurrence and the development of epilepsy are closely associated with immune regulation and inflammation. IL-1β is an important inflammatory mediator in acute stress reactions and damage, and increased IL-1β levels in the brain may increase the inflammation of the brain tissue, thereby causing convulsions (21). IL-1β is also able to activate endothelial cells and neutrophils, enhance the expression of adhesion molecules, promote the release of other cytokines such as IL-6, IL-8, and granulocyte-macrophage colony-stimulating factors, and cause various inflammatory responses together with TNF-α, thus inducing or aggravating epileptic convulsions (22). TNF-α is a pro-inflammatory cytokine that is rarely expressed in normal brain tissue. TNF-α levels increase rapidly in various central nervous system disorders, such as cerebral ischemia and epilepsy (23). TNF-α regulates synaptic transmission, specifically TNF-α released by glial cells. Synaptic transmission can be accelerated through AMPA receptors in neurons. Through its binding to different receptors and the adjustment of interaction with the glutamic acid system, the nervous excitability can be affected, thereby affecting the sensitivity of epilepsy occurrence (24).
Oxidative stress caused by the excessive release of free radicals participates in the pathogenesis of numerous neurodegenerative diseases (25). However, the association between oxidative stress and epilepsy has only recently been recognized. Accumulating evidence suggests that oxidative stress is not only a consequence of the onset of epilepsy, but may also be involved in the occurrence of epilepsy (24). Therefore, antioxidants that reduce oxidative stress have recently attracted attention in the treatment of epilepsy (26). However, oxidative stress damage has been shown to occur in all models of epilepsy seizures (27). In the present investigation, the results demonstrated an increase in TNF-α, IL-1β and MDA expression levels, and a reduction in SOD, CAT and GSH expression levels in the rats with pilocarpine-induced epilepsy. In addition, the results suggested that treatment with piperine reversed these effects. Concordant with these results, Umar et al (28) reported that piperine suppresses oxidative stress and inflammation in collagen-induced arthritis. Pragnya et al (29) revealed that piperine reduces oxidative stress in sodium valproate-induced autism in BALB/C mice.
Apoptosis is a cell death process triggered by internal and external factors, and is not only necessary to maintain normal physiological function, but also closely associated with the occurrence of certain diseases (30). Previous studies have demonstrated that epilepsy causes a loss of neurons via neuronal apoptosis (31). The mechanism underlying the loss of neurons may be that apoptotic stimuli stimulate various receptors including NMDA and p75NTR, causing the activation of apoptosis-associated genes, such as p53 (32). This would in turn lead to a series of biochemical changes within the cell. For instance, calcium overload is caused through NMDA receptor, which then activates the endonuclease that Ca2+ and Mg2+ are dependent on and causes DNA breakage. p75NTR-mediated signals may be associated with JNK activation and cause the phosphorylation of the c-Jun end. Caspase protease is a common transduction pathway that induces apoptosis in epilepsy (33). In the present study, cell apoptosis levels increased following the induction of epilepsy in the rats. However, the upregulation of caspase-3 activity and Bax/Bcl-2 expression levels was suppressed by treatment with piperine. Shrivastava et al (34) reported that piperine exerts a protective effect on 6-OHDA-induced Parkinson's disease via its anti-apoptotic and anti-inflammatory properties.
In conclusion, the results of the present study suggested that piperine exerts anticonvulsant effects in pilocarpine-induced epileptic rats. In addition, piperine attenuates memory impairment, neuronal inflammation and neuronal oxidative stress in the hippocampus. The beneficial effect of piperine in the treatment of epilepsy is associated with the suppression of caspase-3 activity and Bax/Bcl-2 levels in the pilocarpine-induced epileptic rats. Therefore, the results of this study further demonstrated the anticonvulsant effects of piperine. Future studies are required in order to complete our understanding of the underlying mechanism.
References
- 1.Yang K, Su J, Lang Y, Liu SP, Yin J. Contradictory imaging and EEG results in resection surgery of bitemporal lobe epilepsy: A case report. Exp Ther Med. 2014;7:731–733. doi: 10.3892/etm.2013.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang X, Shen X, Xia Y. Electroacupuncture-induced attenuation of experimental epilepsy: A comparative evaluation of acupoints and stimulation parameters. Evid Based Complement Alternat Med. 2013;2013:149612. doi: 10.1155/2013/149612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerr EN, Blackwell MC. Near-transfer effects following working memory intervention (Cogmed) in children with symptomatic epilepsy: An open randomized clinical trial. Epilepsia. 2015;56:1784–1792. doi: 10.1111/epi.13195. [DOI] [PubMed] [Google Scholar]
- 4.Jobe PC, Dailey JW, Wernicke JF. A noradrenergic and serotonergic hypothesis of the linkage between epilepsy and affective disorders. Crit Rev Neurobiol. 1999;13:317–356. doi: 10.1615/critrevneurobiol.v13.i4.10. [DOI] [PubMed] [Google Scholar]
- 5.Cramer JA. Reflections on a career in epilepsy: An unplanned journey. Epilepsy Behav. 2016;57:217–219. doi: 10.1016/j.yebeh.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Sahoo SS, Zhang GQ, Lhatoo SD. Epilepsy informatics and an ontology-driven infrastructure for large database research and patient care in epilepsy. Epilepsia. 2013;54:1335–1341. doi: 10.1111/epi.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reilly C, Agnew R, Neville BG. Depression and anxiety in childhood epilepsy: A review. Seizure. 2011;20:589–597. doi: 10.1016/j.seizure.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Hughes JR. A review of sudden unexpected death in epilepsy: Prediction of patients at risk. Epilepsy Behav. 2009;14:280–287. doi: 10.1016/j.yebeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Song XY, Xu S, Hu JF, Tang J, Chu SF, Liu H, Han N, Li JW, Zhang DM, Li YT, Chen NH. Piperine prevents cholesterol gallstones formation in mice. Eur J Pharmacol. 2015;751:112–117. doi: 10.1016/j.ejphar.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe PB, Doucette CD, Walsh M, Hoskin DW. Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp Mol Pathol. 2013;94:109–114. doi: 10.1016/j.yexmp.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Kalia NP, Suden P, Chauhan PS, Kumar M, Ram AB, Khajuria A, Bani S, Khan IA. Protective efficacy of piperine against Mycobacterium tuberculosis. Tuberculosis (Edinb) 2014;94:389–396. doi: 10.1016/j.tube.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Xia Y, Khoi PN, Yoon HJ, Lian S, Joo YE, Chay KO, Kim KK, Jung YD. Piperine inhibits IL-1β-induced IL-6 expression by suppressing p38 MAPK and STAT3 activation in gastric cancer cells. Mol Cell Biochem. 2015;398:147–156. doi: 10.1007/s11010-014-2214-0. [DOI] [PubMed] [Google Scholar]
- 13.Chu CY, Chang JP, Wang CJ. Modulatory effect of piperine on benzo [a] pyrene cytotoxicity and DNA adduct formation in V-79 lung fibroblast cells. Food Chem Toxicol. 1994;32:373–377. doi: 10.1016/0278-6915(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 14.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Zhang C, Wang Y, Hu W, Shao X, Zhang JG, Zhang K. Prognostic factors for seizure outcome in patients with MRI-negative temporal lobe epilepsy: A meta-analysis and systematic review. Seizure. 2016;38:54–62. doi: 10.1016/j.seizure.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Xiang J, Jiang Y. Regulation of Cu-Zn superoxide dismutase on SCN2A in SH-SY5Y cells as a potential therapy for temporal lobe epilepsy. Mol Med Rep. 2014;9:16–22. doi: 10.3892/mmr.2013.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CS, Liao CH, Lin CC, Lane HY, Sung FC, Kao CH. Patients with epilepsy are at an increased risk of subsequent stroke: A population-based cohort study. Seizure. 2014;23:377–381. doi: 10.1016/j.seizure.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Lv WP, Han RF, Shu ZR. Associations between the C3435T polymorphism of the ABCB1 gene and drug resistance in epilepsy: A meta-analysis. Int J Clin Exp Med. 2014;7:3924–3932. [PMC free article] [PubMed] [Google Scholar]
- 19.Pal A, Nayak S, Sahu PK, Swain T. Piperine protects epilepsy associated depression: A study on role of monoamines. Eur Rev Med Pharmacol Sci. 2011;15:1288–1295. [PubMed] [Google Scholar]
- 20.Rinwa P, Kumar A. Piperine potentiates the protective effects of curcumin against chronic unpredictable stress-induced cognitive impairment and oxidative damage in mice. Brain Res. 2012;1488:38–50. doi: 10.1016/j.brainres.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Dundar NO, Aktekin B, Ekinci NC, Sahinturk D, Yavuzer U, Yegin O, Haspolat S. Interleukin-1beta secretion in hippocampal sclerosis patients with mesial temporal lobe epilepsy. Neurol Int. 2013;5:e17. doi: 10.4081/ni.2013.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta RA, Motiwala MN, Dumore NG, Danao KR, Ganjare AB. Effect of piperine on inhibition of FFA induced TLR4 mediated inflammation and amelioration of acetic acid induced ulcerative colitis in mice. J Ethnopharmacol. 2015;164:239–246. doi: 10.1016/j.jep.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 23.Tiwari P, Dwivedi R, Mansoori N, Alam R, Chauhan UK, Tripathi M, Mukhopadhyay AK. Do gene polymorphism in IL-1beta, TNF-alpha and IL-6 influence therapeutic response in patients with drug refractory epilepsy? Epilepsy Res. 2012;101:261–267. doi: 10.1016/j.eplepsyres.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Singhal V, Roshan R, Sharma A, Rembhotkar GW, Ghosh B. Piperine inhibits TNF-alpha induced adhesion of neutrophils to endothelial monolayer through suppression of NF-kappaB and IkappaB kinase activation. Eur J Pharmacol. 2007;575:177–186. doi: 10.1016/j.ejphar.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 25.Waldbaum S, Patel M. Mitochondrial dysfunction and oxidative stress: a contributing link to acquired epilepsy? J Bioenerg Biomembr. 2010;42:449–455. doi: 10.1007/s10863-010-9320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khajuria A, Thusu N, Zutshi U, Bedi KL. Piperine modulation of carcinogen induced oxidative stress in intestinal mucosa. Mol Cell Biochem. 1998;189:113–118. doi: 10.1023/A:1006877614411. [DOI] [PubMed] [Google Scholar]
- 27.Luna B, Bhatia S, Yoo C, Felty Q, Sandberg DI, Duchowny M, Khatib Z, Miller I, Ragheb J, Prasanna J, Roy D. Bayesian network and mechanistic hierarchical structure modeling of increased likelihood of developing intractable childhood epilepsy from the combined effect of mtDNA variants, oxidative damage, and copy number. J Mol Neurosci. 2014;54:752–766. doi: 10.1007/s12031-014-0364-x. [DOI] [PubMed] [Google Scholar]
- 28.Umar S, Sarwar AH Golam, Umar K, Ahmad N, Sajad M, Ahmad S, Katiyar CK, Khan HA. Piperine ameliorates oxidative stress, inflammation and histological outcome in collagen induced arthritis. Cell Immunol. 2013;284:51–59. doi: 10.1016/j.cellimm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Pragnya B, Kameshwari JS, Veeresh B. Ameliorating effect of piperine on behavioral abnormalities and oxidative markers in sodium valproate induced autism in BALB/C mice. Behav Brain Res. 2014;270:86–94. doi: 10.1016/j.bbr.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 30.Kumar A, Sasmal D, Sharma N. Immunomodulatory role of piperine in deltamethrin induced thymic apoptosis and altered immune functions. Environ Toxicol Pharmacol. 2015;39:504–514. doi: 10.1016/j.etap.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Wu X, Guo J, Yuan J. Myocyte-specific enhancer binding factor 2A expression is downregulated during temporal lobe epilepsy. Int J Neurosci. 2015:1–11. doi: 10.3109/00207454.2015.1038711. [DOI] [PubMed] [Google Scholar]
- 32.Engel T, Caballero-Caballero A, Schindler CK, Plesnila N, Strasser A, Prehn JH, Henshall DC. BH3-only protein Bid is dispensable for seizure-induced neuronal death and the associated nuclear accumulation of apoptosis-inducing factor. J Neurochem. 2010;115:92–101. doi: 10.1111/j.1471-4159.2010.06909.x. [DOI] [PubMed] [Google Scholar]
- 33.Rinwa P, Kumar A, Garg S. Suppression of neuroinflammatory and apoptotic signaling cascade by curcumin alone and in combination with piperine in rat model of olfactory bulbectomy induced depression. PLoS One. 2013;8:e61052. doi: 10.1371/journal.pone.0061052. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Shrivastava P, Vaibhav K, Tabassum R, Khan A, Ishrat T, Khan MM, Ahmad A, Islam F, Safhi MM, Islam F. Anti-apoptotic and anti-inflammatory effect of Piperine on 6-OHDA induced Parkinson's rat model. J Nutr Biochem. 2013;24:680–687. doi: 10.1016/j.jnutbio.2012.03.018. [DOI] [PubMed] [Google Scholar]