Abstract
Cardiovascular diseases are common in patients with chronic kidney disease. One of the key symptoms is the calcification of the vascular smooth muscle cells (VSMCs), which is induced by dysregulated mineral metabolism with high circulating levels of inorganic phosphate (Pi) and calcium. Klotho, which was originally identified as an aging suppressor gene, has been shown to be associated with vascular calcification. Since Klotho was recently identified as a target for nuclear receptor peroxisome proliferator-activated receptor (PPAR) γ, the present study aimed to determine whether PPARγ regulates VSMC calcification through modulating the expression levels of Klotho. It was demonstrated that the expression of PPARγ was downregulated during Pi-induced VSMC calcification. In addition, treatment with PPARγ agonists inhibited the calcification and enhanced the expression of Klotho in VSMCs in a PPARγ-dependent manner. Of note, loss of Klotho expression by RNA interference abolished the ability of PPARγ activation to inhibit VSMC calcification. Furthermore, activation of Klotho as well as PPARγ inhibited the expression of Pi transporter 1/2 and reduced Pi influx into VSMCs. To the best of our knowledge, the present study was the first to demonstrate that PPARγ regulates VSMC calcification through activating Klotho.
Keywords: vascular smooth muscle cell calcification, peroxisome proliferator-activated receptor γ, Klotho, inorganic phosphate transporter 1/2
Introduction
Cardiovascular diseases are the leading cause of mortality in patients with chronic kidney disease (CKD) (1). Studies have demonstrated that declining renal function in CKD is associated with an elevated risk of cardiovascular disease (2,3). One of the key factors leading to the increased burden of cardiovascular disease is the calcification of the vascular smooth muscle cells (VSMCs) lining the vessel wall, and the severity and histoanatomic type of vascular calcification are predictors of subsequent vascular mortality (4). The prevalence and progression of vascular calcification are markedly increased in patients with advanced CKD (5). Vascular calcification in CKD patients is known as medial artery calcification, which is distinct from that found in patients with atherosclerosis, and is characterized by amorphous minerals forming along one or more elastic lamellae of the medial layer (6,7). For a long period, vascular calcification was thought to be a passive process resulting from elevated phosphate (Pi) levels and increased calcium Pi products in the plasma (8–10). However, other studies have established that vascular calcification in CKD is a highly regulated cell-mediated process that involves the entry of VSMCs into a transdifferentiation program of osteogenesis, during which numerous key regulators of bone formation and bone structural proteins are expressed (11–14).
Despite these findings, dysregulated mineral metabolism with high circulating levels of Pi and calcium have been demonstrated to be the most important factors for the initiation and progression of the calcification process in CKD patients (1,15). Extracellular Pi promotes VSMC mineralization in a concentration- and time-dependent manner by increasing Pi influx via the sodium-dependent Pi co-transporters, Pi transporter (piT)-1 and PiT-2, leading to the induction of osteoblastic differentiation factors, including core-binding factor alpha 1 (Cbfa1)/runt-related transcription factor 2 (Runx2) (16). Either the blockade of PiT-1/2 or inhibition of PiT-1/2 activity by small interfering (si)RNA prevents the induction of Cbfa1/Runx2 and osteoclast expression in VSMCs even under high extracellular Pi concentrations (17), suggesting that elevated extracellular Pi concentrations induce the mineralization of VSMCs through the activation of PiT-1/2. In addition to extracellular Pi, calcium accelerates the mineralization of VSMCs (18,19). In addition, calcium-induced mineralization has been demonstrated to be also dependent on the function of PiT-1/2 (19).
A growing body of evidence has demonstrated that the function of Klotho is associated with vascular calcification. The Klotho gene was originally identified as an aging suppressor gene in mice (20). It encodes a single span transmembrane protein and is expressed primarily in renal tubular epithelial cells (20). Studies have indicated that Klotho deficiency promotes calcification and osteoblastic differentiation of VSMCs (21,22), whereas Klotho transgenic mice have better preserved renal function and markedly less calcification compared with wild-type mice with CKD (21). It has been suggested that Klotho suppresses osteoblastic transdifferentiation and calcification of VSMCs by inhibiting PiT-1/2-dependent Pi uptake, thus repressing the expression of Cbfa1/Runx2 (21). Klotho has been identified as a target for nuclear receptor peroxisome proliferator-activated receptor (PPAR)γ (23). Thiazolidinediones, which act as PPARγ agonists, increase Klotho expression in HEK293 cells several renal epithelial cell lines at the mRNA and protein level. This induction was blocked by siRNA-mediated gene silencing of PPARγ or PPARγ antagonists, which have been shown to attenuate high glucose-induced VSMC calcification (24). However, the underlying mechanisms of the increased expression of Klotho have remained elusive.
The present study demonstrated that the expression of PPARγ was downregulated during Pi-induced VSMC calcification. In addition, treatment with PPARγ agonists was found to inhibit the calcification and enhanced the expression of Klotho in VSMCs in a PPARγ-dependent manner. Of note, loss of Klotho expression by RNA interference abolished the ability of PPARγ activation to inhibit VSMC calcification. To the best of our knowledge, the present study was the first to demonstrate that PPARγ regulates VSMC calcification through activating Klotho.
Materials and methods
Cell culture
Vascular smooth muscle cells (VSMCs) were obtained by a previously described explant method (25,26). In brief, tissue was separated from simmental bovine aorta segments (Harbin Bin Good Animal Husbandry Co., Ltd., Harbin, China). Small pieces of tissue (1–2 mm3) were placed into a 10-cm culture dish and cultured for several weeks in Dulbecco's modified Eagle's medium (DMEM) containing 4.5 g/l glucose (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 15% fetal bovine serum and 10 mM sodium pyruvate (both Invitrogen; Thermo Fisher Scientific, Inc.) in a humidified atmosphere containing 5% CO2 at 37°C. Cells that had migrated from the explants were collected and maintained in DMEM containing 1.0 g/l glucose (Invitrogen; Thermo Fisher Scientific, Inc.), 15% FBS supplemented with 10 mM sodium pyruvate. For calcification experiments, cells of up to passage 8 were used.
Induction of calcification
VSMCs were seeded on 24-well plates at a density of 5×104/well. At confluence, cells were incubated in calcification medium (DMEM containing 15% FBS and 1 mM sodium pyruvate in the presence of 3 mM inorganic P (Pi; Invitrogen; Thermo Fisher Scientific, Inc.) for up to 9 days. Every 2 days, the medium was replaced with fresh medium. For time-course experiments, the first day of culture in calcification medium was defined as day 0. Cells incubated in calcification medium with 1 mM Pi were used as controls.
Activation of PPARγ
VSMCs were administered with various concentrations (0, 4, 8, 12, 16 or 20 µM) of the PPARγ agonists rosiglitazone (RGZ; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and thiazolidinedione (TZL; Santa Cruz Biotechnology, Inc.) in the presence of 3 mM Pi for 9 days. VSMCs were subsequently administered with RGZ or TZL in the absence or presence of PPARγ inhibitor GW9662 (10 µM; Santa Cruz Biotechnology, Inc.) to confirm the role of PPARγ in Pi-induced calcification.
Quantification of calcium deposition
Cells were decalcified with 0.6 N HCl for 24 h. Subsequently, the cells were solubilized with 0.1 N NaOH/0.1% SDS. The calcium content was determined colorimetrically by the o-cresolphthalein complexone method (Calcium kit; Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The protein content was measured with a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Inc.). The calcium content was normalized to protein content.
Von Kossa staining
Cells were fixed with 0.1% glutaraldehyde in Pi-buffered saline for 15 min at room temperature, and were then washed 3 times with double distilled H2O. Cells were incubated with 5% silver nitrate solution and exposed to sunlight for 30 min, washed with distilled water for 5 min and treated with 5% sodium thiosulfate for 2 min. Images of calcium particles were captured at 40x magnification (C-5060; Olympus Corp., Tokyo, Japan) under a microscope (TH4-200; Olympus Corp.).
Alkaline phosphatase staining and activity assay
Alkaline phosphatase (ALP) staining and activity assay were performed using a 5-bromo-4-chloro-3-indolyl Pi/nitro-blue tetrazolium Alkaline Phosphatase Staining kit and an Alkaline Phosphatase Assay kit (Beyotime Institute of Biotechnology, Shanghai, China), respectively, according to the manufacturer's protocol. Images of samples stained for ALP were captured at 40x magnification (C-5060; Olympus Corp.) under a microscope (TH4-200; Olympus Corp.). ALP activity was normalized to the total protein concentration determined using the BCA protein assay (Thermo Fisher Scientific, Inc.).
Reverse-transcription quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA from VSMCs was isolated using TRIzol reagent (Invitrogen). Complementary DNA was generated from 2 µg RNA with Oligo-dT primers by using the SuperScript III First Stand Synthesis System (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Real-time PCR was performed in a 20-µl reaction mixture containing 1 µl cDNA, 9 µl water and 10 µl 2X TaqMan PreAmp Master Mix (Invitrogen). Amplification was performed in an ABI 7300 system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with Taqman green fluorescence (Invitrogen). The forward and reverse PCR primers, and their respective probes were as follows: PPARγ (forward: 5′-CATAATGCCATCAGGTTTGG-3′; reverse: 5′-GTCAGCAGACTCTGGGTTCA-3′; probe: 5′-CTTCTCGGCCTGTGGCATGC-3′), Klotho (forward: 5′-GGTGGATGTCATTGGGTACA-3′; reverse: 5′-AGAGTCCACGTCTGATGCTG-3′; probe: 5′-CGGTGCCACTCGAAGCCATC-3′), Runx2 (forward: 5′-ATGGTTAATCTCCGCAGGTC-3′; reverse: 5′-TGGTGTCACTGTGCTGAAGA-3′; probe: 5′-CCAGCCACCGAGACCAACAGA-3′), Msh homeobox 2 (Msx2) (forward: CCAGCTCTCTGAACCTCACA-3′; reverse: 5′-AGTTCTGCCTCCTGCAGTCT-3′; probe: 5′-CGCCTTGGCTCTTCGGTTCTG-3′), osteocalcin (OCN) (forward: 5′-GAGCTCAACCCTGACTGTGA-3′; reverse: 5′-CTAGACTGGGCCGTAGAAGC-3′; probe: 5′-CCACATCGGCTTCCAGGAAGC-3′), matrix gla protein (MGP) (forward: 5′-GAGAACTCAACAAGCCTCA-3′; reverse: 5′-TCGTAGGCAGCATTGTATCC-3′; probe: 5′-CAAGCTTCCCGGTTGAGCTCG-3′), SM22α (forward: 5′-AGCAAGCTGGTCAATAGCCT-3′; reverse: 5′-CTCCATCTGCTTGAAGACCA-3′; probe: 5′-CGGGCACCTTCACTGGCTTG-3′) and α-actin (forward: 5′-GGTGACTCTCCAAGGTGGAT-3′; reverse: 5′-TGCAAAGGCTGAACAAACTC-3′; probe: 5′-CCTTGGCTGGGCATCACCCT-3′), PiT1 (forward: 5′-GATGTCCACAGCAACAGGAC-3′; reverse: 5′-ACTCAACGTGTGGTCAGGAA-3′; probe: 5′-AAGCTCCCTGCCATCACGCC-3′), PiT2 (forward: 5′-GGGAGGGTTGACCTGTTAGA-3′; reverse: 5′-AGTCGTACTTCCTCCCGCTA-3′; probe: 5′-AGCAACCAGGGATGCTCCGC-3′). β-actin (forward: 5′-AGCAGATGTGGATCAGCAAG-3′; reverse: 5′-TAACAGTCCGCCTAGAAGCA-3′; probe: 5′-CCTCCATCGTCCACCGCAAA-3′). All primers were obtained from Thermo Fisher Scientific, Inc., and the following thermocycling conditions were used: 95°C for 8 min, 40 cycles of 95°C for 20 sec, and 60°C for 1 min. The relative expression levels of mRNA were calculated using the 2−ΔΔCt methods as described previously (27).
Western blot analysis
Cells were lysed in radioimmunoprecipitation buffer supplemented with a protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific, Inc.). The protein concentration was determined using the BCA protein assay. Proteins were subjected to SDS-polyacrylamide gel electrophoresis and subsequently transferred onto a nitrocellulose membrane. Following blocking with 3% bovine serum albumin, the membrane was incubated with primary antibodies, including anti-PPARγ (1:500; ab66343; Abcam, Cambridge, UK), anti-Klotho (1:1,000; ab203576; Abcam) and anti-β-actin (1:500; ab1801; Abcam) overnight at 4°C. Subsequently, membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:10,000; BM2006; Wuhan Boster Biological Technology, Ltd., Wuhan, China) for 1 h at room temperature. Protein bands were detected using an enhanced chemiluminescence western blotting detection kit (Thermo Fisher Scientific, Inc.), followed by exposure of the membranes to X-ray film. Image J software (version 1.140; US National Institutes of Health, Bethesda, MD, USA) was used to quantify the blot intensity.
Small interfering RNA (siRNA) and transfection
PPARγ siRNA, Klotho siRNA and control scrambled siRNA (Invitrogen; Thermo Fisher Scientific, Inc.) were transfected with Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Cells were seeded in 24-well plates at 5×104/well and were grown overnight to reach ~80% confluence. The cells were transfected with 30 pmol siRNA and incubated for 48 h, and used for subsequent experiments after the transfection efficiency was confirmed by western blot analysis.
Statistical analysis
Values are expressed as the mean ± standard error of the mean. Differences among groups were analyzed by analysis of variance followed by Bonferroni post-hoc analyses as appropriate. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SPSS 18.0 Version software (SPSS, Inc., Chicago, IL, USA).
Results
PPARγ expression is downregulated in VSMCs with Pi-induced calcification
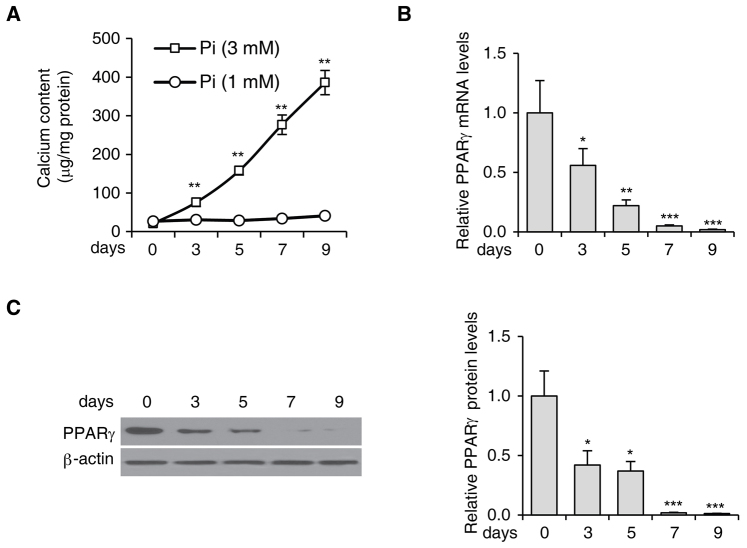
In order to investigate the role of PPARγ activation in VSMC calcification, the expression levels of PPARγ during Pi-induced VSMC calcification were examined. While low ambient Pi (1 mM) had little effect on calcium deposition, high ambient Pi (3 mM) markedly increased calcium deposition in VSMCs in a time-dependent manner. The calcium content in these cells was elevated from 21±3.4 µg/ml on day 0 to 386±31.7 µg/ml on day 9 (Fig. 1A). During Pi-induced calcification in VSMCs, the mRNA (Fig. 1B) and the protein levels (Fig. 1C) of PPARγ were significantly downregulated.
Figure 1.
PPARγ expression is downregulated in VSMCs with Pi-induced calcification. (A) Calcium content in VSMCs treated with 1 or 3 mM Pi for 0, 3, 5, 7 and 9 days. (B) PPARγ mRNA levels in VSMCs treated with 3 mM Pi at the indicated time points, analyzed by reverse-transcription quantitative polymerase chain reaction. (C) The protein levels of PPARγ in VSMCs treated with 3 mM Pi as determined by western blot analysis. Quantified PPARγ protein levels are shown in the right panel. β-actin was used as the loading control. *P<0.05, **P<0.01 and ***P<0.001 vs. cells at day 0. Values are expressed as the mean ± standard error of the mean of three independent experiments. VSMC, vascular smooth muscle cell; PPAR, peroxisome proliferator-activated receptor; Pi, inorganic phosphate.
PPARγ agonists inhibit Pi-induced VSMC calcification
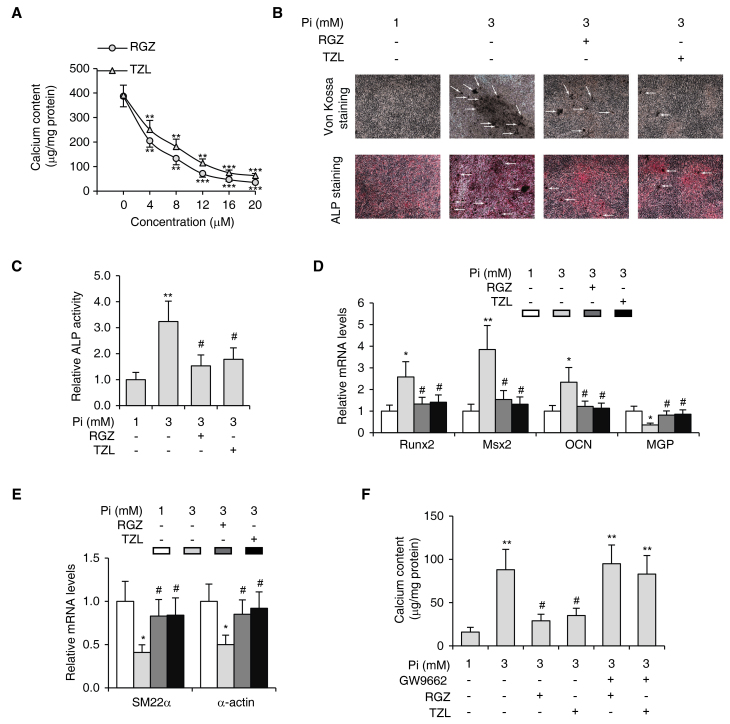
To examine whether PPARγ activation can suppress the calcification of VSMCs, the cells were treated with various concentrations of the PPARγ agonists RGZ and TZL in the presence of 3 mM Pi for 9 days. The results showed that RGZ and TZL decreased Pi-induced calcium deposition in VSMCs in a dose-dependent manner, with a mean effective dose of 7.14 µM for RGZ and 8.98 µM for TZL (Fig. 2A). Consistently, Von Kossa staining (28), ALP staining (29) (Fig. 2B) and the ALP activity assay (Fig. 2C) also revealed that Pi-induced calcification in VSMCs was inhibited by RGZ and TZL.
Figure 2.
PPARγ agonists inhibit Pi-induced VSMC calcification. (A) Calcium content in VSMCs exposed to 3 mM Pi for 9 days treated with the indicated concentrations of RGZ or TZL. **P<0.01 vs. 3 mM Pi only group, ***P<0.001 vs. 3 mM Pi only group. VSMCs were subjected to Pi-induced calcification and treated with RGZ or TZL (10 µM each), followed by (B) von Kossa (upper panel) and ALP staining (lower panel; magnification, ×40; white arrows indicate calcification); (C) assessment of ALP activity; (D) determination of the mRNA expression levels of osteogenic genes Runx2, Msx2 and OCN, and calcification inhibitor MGP as well as (E) VSMC lineage markers SM22α and α-actin by reverse-transcription quantitative polymerase chain reaction analysis; and (F) assessment of the calcium content in the absence or presence of PPARγ inhibitor GW9662 (10 µM). Values are expressed as the mean ± standard error of the mean of three independent experiments. (C-F) *P<0.05, **P<0.01 vs. cells in 1 mM Pi group. #P<0.05 vs. cells in 3 mM Pi only group. VSMC, vascular smooth muscle cell; Pi, inorganic phosphate; PPAR, peroxisome proliferator-activated receptor; TZL, thiazolidinedione; RGZ, rosiglitazone; Runx2, runt-related transcription factor 2; Msx2, Msh homeobox 2; OCN, osteocalcin; MGP, matrix gla protein; ALP, alkaline phosphatase.
To further investigate whether PPARγ agonists modulate VSMC transdifferentiation, the mRNA levels of osteogenic genes and smooth muscle lineage markers were examined in VSMCs by RT-qPCR. While Pi significantly increased the expression of the osteogenic differentiation marker genes Runx2, Msx2 and OCN, the expression of these osteogenic genes was suppressed by RGZ and TZL (Fig. 2D). Conversely, the mRNA levels of calcification inhibitor gene MGP and smooth muscle differentiation marker genes SM22α and α-actin were significantly reduced during Pi-induced calcification, but were upregulated by the treatment with PPARγ agonists (Fig. 2E).
In order to confirm that the inhibition of calcification by RGZ and TZL was specifically mediated by PPARγ, VSMCs were treated with a selective PPARγ antagonist, GW9662, together with RGZ or TZL. In the presence of GW9662, neither RGZ nor TZL inhibited the calcification of VSMCs (Fig. 2F). Taken together, these results indicated that activation of PPARγ specifically inhibited Pi-induced calcification in VSMCs.
PPARγ regulates the expression of Klotho in VSMCs
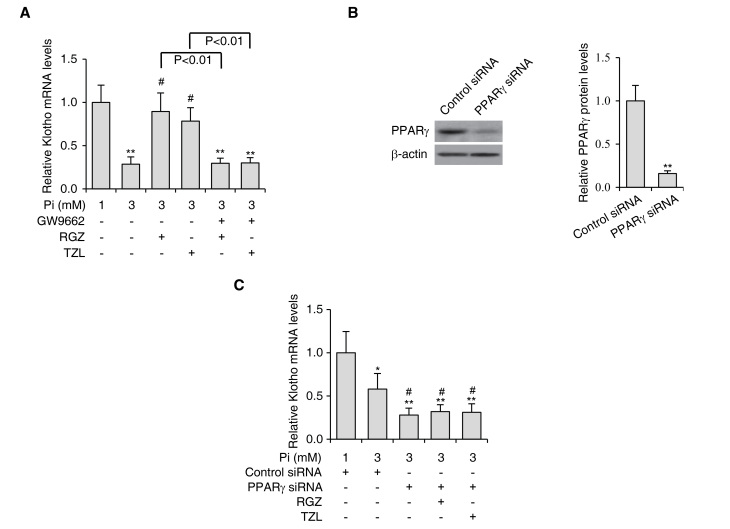
Since it has been reported that Klotho is a target gene of PPARγ (23) and that the deficiency of Klotho may cause vascular calcification (21,22), it was hypothesized that activation of PPARγ inhibits Pi-induced calcification in VSMCs via stimulation of Klotho. To test this hypothesis, Klotho expression levels were first analyzed in VSMCs treated with PPARγ agonists RGZ and TZL during Pi-induced calcification. It was found that the expression levels of Klotho were significantly reduced in the presence of Pi, but were upregulated by RGZ and TZL treatment (Fig. 3A). As expected, treatment with PPARγ inhibitor GW9662 prevented RGZ and TZL from increasing the expression levels of Klotho (Fig. 3A).
Figure 3.
PPARγ agonists enhance Klotho expression in VSMCs. (A) VSMCs were subjected to Pi-induced calcification and treated with RGZ or TZL (10 µM each) in the absence or presence of PPARγ inhibitor GW9662 (10 µM). The expression levels of Klotho were determined by reverse-transcription quantitative polymerase chain reaction analysis. (B) The protein levels of PPARγ in VSMCs treated with siRNA targeting PPARγ were determined by western blot analysis. Quantified PPARγ protein levels are shown in the right panel. β-actin was used as the loading control. **P<0.01 vs. control siRNA-transfected cells. (C) Expression levels of Klotho in VSMCs subjected to Pi-induced calcification treated with RGZ or TZL (10 µM) in the absence or presence of PPARγ siRNA. Values are expressed as the mean ± standard error of the mean of three independent experiments. *P<0.05, **P<0.01 vs. cells in 1 mM Pi group. #P<0.05 vs. cells in 3 mM Pi only group. VSMC, vascular smooth muscle cell; Pi, inorganic phosphate; PPAR, peroxisome proliferator-activated receptor; TZL, thiazolidinedione; RGZ, rosiglitazone; siRNA, small interfering RNA.
Furthermore, PPARγ siRNA was introduced into VSMCs to knockdown PPARγ, and the protein levels of endogenous PPARγ were reduced by ~80% (Fig. 3B). Knockdown of PPARγ further decreased Klotho expression during VSMC calcification (Fig. 3C). Importantly, when PPARγ expression was knocked down, RGZ and TZL could no longer upregulate the expression of Klotho, suggesting that the effects of RGZ and TZL in regulating Klotho expression were specifically mediated by PPARγ.
PPARγ regulates VSMC calcification through activating Klotho
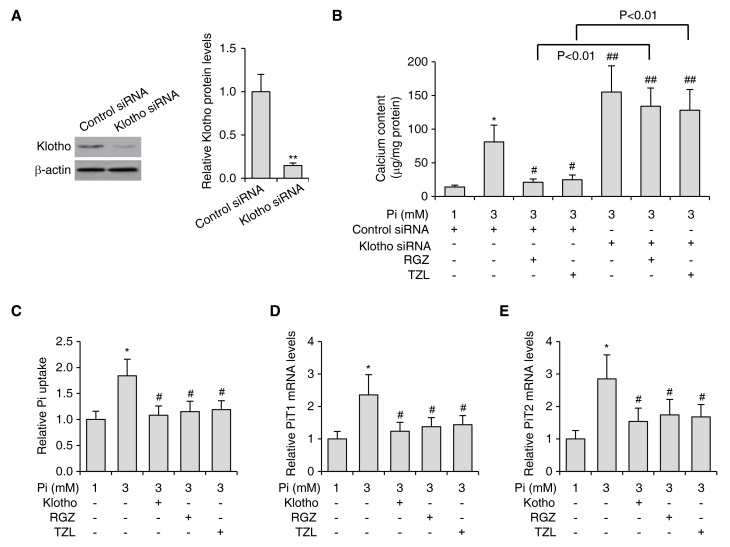
In order to determine whether the inhibitory effects on VSMC calcification by PPARγ activation were dependent on Klotho upregulation, siRNA targeting Klotho was introduced into VSMCs. With this treatment, the protein levels of endogenous Klotho were decreased by ~80% (Fig. 4A). Consistently with previously reported results, Klotho knockdown led to elevated levels of Pi-induced calcification (Fig. 4B). Of note, in VSMCs with Klotho knockdown, PPARγ agonists were no longer able to inhibit calcification (Fig. 4B), indicating that PPARγ activation inhibited VSMC calcification through activating Klotho.
Figure 4.
PPARγ regulates VSMC calcification through activating Klotho. (A) The protein levels of Klotho in VSMCs treated with siRNA targeting Klotho as determined by western blot. Quantified protein levels of Klotho are shown in the right panel. β-actin was used as the loading control. **P<0.01 vs. control siRNA-transfected cells. (B) Calcium content in VSMCs subjected to Pi-induced calcification treated with RGZ or TZL (10 µM each), in the absence or presence of Klotho siRNA. (C) Pi uptake and (D) PiT-1 mRNA levels and (E) PiT-2 mRNA levels as determined by reverse-transcription quantitative polymerase chain reaction analysis in VSMCs subjected to Pi-induced calcification and treated with RGZ (10 µM), TZL (10 µM) or soluble Klotho (0.4 nM). Values are expressed as the mean ± standard error of the mean of three independent experiments. **P<0.05 vs. control siRNA-transfected cells treated with 1 mM Pi. #P<0.05, ##P<0.01 vs. control siRNA-transfected cells treated with 3 mM Pi. VSMC, vascular smooth muscle cell; Pi, inorganic phosphate; TZL, thiazolidinedione; RGZ, rosiglitazone; siRNA, small interfering RNA; PiT, Pi transporter.
It has been proposed that Klotho inhibits Pi-induced VSMC calcification by inhibiting PiT-1/2 expression, thus preventing PiT-1/2-dependent Pi influx (21). In line with this theory, PPARγ activation induced by RGZ and TZL, as well as treatment with soluble Klotho, reduced Pi influx (Fig. 4C) and inhibited PiT-1/2 expression (Fig. 4D and E). The fact that PPARγ activation and Klotho reduced Pi influx and inhibited PiT-1/2 expression further suggested that the PPARγ activation inhibits calcification via increasing Klotho expression. Taken together, these results indicated that PPARγ activation ameliorated VSMC calcification through upregulation of Klotho.
Discussion
Patients with CKD have a disproportionately high occurrence of vascular calcification compared to the general population. One theory to explain this observation is the dysregulated calcium and Pi metabolism that is common in these patients. Klotho was recently found to be associated with vascular calcification (21,22), possibly via its function to maintain mineral homeostasis and to regulate Pi influx into cells. PPARγ has emerged as a regulator of Klotho (23). In this context, it is important to investigate whether PPARγ regulates VSMC calcification through modulating the expression levels of Klotho.
The present study revealed that the expression of PPARγ was significantly reduced during Pi-induced VSMC calcification. This finding suggested a physiological role for PPARγ in the process of VSMC calcification. In fact, PPARγ is a transcription factor known to act as a sensor that translates environmental stimuli into adaptive cellular responses, and has been shown to be a key repressor of osteoblastogenesis (30,31). In addition, a previous study demonstrated that PPARγ counteracted vascular calcification by inhibiting Wnt5a signaling in atherosclerotic lesions (32). However, the mechanism of action for PPARγ in regulating Pi-induced vascular calcification have largely remained elusive.
The present study found that treatment with PPARγ agonists inhibited calcification in VSMCs in a PPARγ-dependent manner. The calcification of VSMC was characterized not only by increased cellular calcium content, but also by phenotypic transition involving the expression of osteogenic genes. It is known that VSMCs can undergo phenotypic changes (33), and that they have important roles in the initiation and progression of vascular calcification (34), which is associated with a decrease in smooth muscle lineage markers and an increase in osteogenic markers (35). The present study also found that PPARγ regulated the gene expression of Klotho in VSMCs. Activation of PPARγ led to increased expression of Klotho, while knockdown of PPARγ resulted in decreased levels of Klotho. These results were consistent with a previous study using kidney cell lines (23).
The most important findings of the present study were that Klotho was required for the regulation of Pi-induced vascular calcification by PPARγ. It was demonstrated that knockdown of Klotho abolished the ability of activated PPARγ to inhibit calcification in VSMCs. These findings shed light on the underlying mechanisms via which PPARγ regulates Pi-induced vascular calcification. Of note, there are two major types of vascular calcification, which are distinguished by their location and association with atherosclerotic plaque formation. Atherosclerotic calcification is located in the intimal layer and is associated with cellular necrosis, lipid deposition and inflammation. As lesions progress, osteogenesis becomes increasingly evident. The other type of vascular calcification is called Monckeberg sclerosis and is characterized by amorphous mineral forming along elastic lamellae of the medial layer. This type of calcification is therefore also known as medial artery calcification, and is more prevalent in patients with CKD (6,7). The fact that Klotho is required for the regulation of Pi-induced vascular calcification by PPARγ in medial artery calcification, which is commonly seen in CKD patients, does not exclude the possibility of further molecules mediating the regulation of vascular calcification by PPARγ in atherosclerotic lesions.
In conclusion, the present study showed that PPARγ regulates Pi-induced calcification and Klotho expression in VSMCs. Moreover, loss of Klotho expression abolished the ability of activated PPARγ to inhibit VSMC calcification. To the best of our knowledge, the present study was the first to demonstrate that PPARγ regulates VSMC calcification through activating Klotho.
Acknowledgements
This work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education of China (grant no. 20122307110011), Science Projects of Heilongjiang Health Department (grant no. 2013024); and Key Projects of Natural Science Foundation of Heilongjiang Province (grant no. ZD201014).
References
- 1.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. (5 Suppl 3) [DOI] [PubMed] [Google Scholar]
- 2.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 3.McCullough PA, Sandberg KR, Dumler F, Yanez JE. Determinants of coronary vascular calcification in patients with chronic kidney disease and end-stage renal disease: A systematic review. J Nephrol. 2004;17:205–215. [PubMed] [Google Scholar]
- 4.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 5.Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 6.Goodman WG, London G, Amann K, Block GA, Giachelli C, Hruska KA, Ketteler M, Levin A, Massy Z, McCarron DA, et al. Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43:572–579. doi: 10.1053/j.ajkd.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Shroff RC, Shanahan CM. The vascular biology of calcification. Semin Dial. 2007;20:103–109. doi: 10.1111/j.1525-139X.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- 8.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 9.Cozzolino M, Dusso AS, Slatopolsky E. Role of calcium-phosphate product and bone-associated proteins on vascular calcification in renal failure. J Am Soc Nephrol. 2001;12:2511–2516. doi: 10.1681/ASN.V12112511. [DOI] [PubMed] [Google Scholar]
- 10.Ganesh SK, Stack AG, Levin NW, HulbertShearon T, Port FK. Association of elevated serum PO(4), Ca × PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 11.Boström K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iimura T, Oida S, Takeda K, Maruoka Y, Sasaki S. Changes in homeobox-containing gene expression during ectopic bone formation induced by bone morphogenetic protein. Biochem Biophys Res Commun. 1994;201:980–987. doi: 10.1006/bbrc.1994.1798. [DOI] [PubMed] [Google Scholar]
- 13.Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Mönckeberg's sclerosis: Evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.CIR.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 14.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–494. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 15.Block GA, HulbertShearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 16.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.RES.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98:905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 18.Lomashvili K, Garg P, O'Neill WC. Chemical and hormonal determinants of vascular calcification in vitro. Kidney Int. 2006;69:1464–1470. doi: 10.1038/sj.ki.5000297. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Curinga G, Giachelli CM. Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int. 2004;66:2293–2299. doi: 10.1111/j.1523-1755.2004.66015.x. [DOI] [PubMed] [Google Scholar]
- 20.Kuro-o M. Klotho. Pflugers Arch. 2010;459:333–343. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- 21.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Li Y, Fan Y, Wu J, Zhao B, Guan Y, Chien S, Wang N. Klotho is a target gene of PPAR-gamma. Kidney Int. 2008;74:732–739. doi: 10.1038/ki.2008.244. [DOI] [PubMed] [Google Scholar]
- 24.Zhou YB, Zhang J, Peng DQ, Chang JR, Cai Y, Yu YR, Jia MZ, Wu W, Guan YF, Tang CS, Qi YF. Peroxisome proliferator-activated receptor g ligands retard cultured vascular smooth muscle cells calcification induced by high glucose. Cell Biochem Biophys. 2013;66:421–429. doi: 10.1007/s12013-012-9490-7. [DOI] [PubMed] [Google Scholar]
- 25.Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971;50:172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shioi A, Nishizawa Y, Jono S, Koyama H, Hosoi M, Morii H. Beta-glycerophosphate accelerates calcification in cultured bovine vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1995;15:2003–2009. doi: 10.1161/01.ATV.15.11.2003. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Rungby J, Kassem M, Eriksen EF, Danscher G. The von Kossa reaction for calcium deposits: Silver lactate staining increases sensitivity and reduces background. Histochem J. 1993;25:446–451. doi: 10.1007/BF00157809. [DOI] [PubMed] [Google Scholar]
- 29.Hui M, Li SQ, Holmyard D, Cheng P. Stable transfection of nonosteogenic cell lines with tissue nonspecific alkaline phosphatase enhances mineral deposition both in the presence and absence of beta-glycerophosphate: Possible role for alkaline phosphatase in pathological mineralization. Calcif Tissue Int. 1997;60:467–472. doi: 10.1007/s002239900264. [DOI] [PubMed] [Google Scholar]
- 30.Wan Y. PPARgamma in bone homeostasis. Trends Endocrinol Metab. 2010;21:722–728. doi: 10.1016/j.tem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007;13:1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- 32.Woldt E, Terrand J, Mlih M, Matz RL, Bruban V, Coudane F, Foppolo S, El Asmar Z, Chollet ME, Ninio E, et al. The nuclear hormone receptor PPARg counteracts vascular calcification by inhibiting Wnt5a signalling in vascular smooth muscle cells. Nat Commun. 2012;3:1077. doi: 10.1038/ncomms2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iyemere VP, Proudfoot D, Weissberg PL, Shanahan CM. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J Intern Med. 2006;260:192–210. doi: 10.1111/j.1365-2796.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- 34.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]