Abstract
Cancer-induced bone pain can severely compromise the life quality of patients, while tolerance limits the use of opioids in the treatment of cancer pain. Monocyte chemoattractant protein-1 (MCP-1) is known to contribute to neuropathic pain. However, the role of spinal MCP-1 in the development of morphine tolerance in patients with cancer-induced bone pain remains unclear. The aim of the present study was to investigate the role of spinal MCP-1 in morphine tolerance in bone cancer pain rats (MTBP rats). Bone cancer pain was induced by intramedullary injection of Walker 256 cells into the tibia of the rats, while morphine tolerance was induced by continuous intrathecal injection of morphine over a period of 9 days. In addition, anti-MCP-1 antibodies were intrathecally injected to rats in various groups in order to investigate the association of MCP-1 with mechanical and heat hyperalgesia using the paw withdrawal threshold (PWT) and thermal withdrawal latency (TWL) tests, respectively. Furthermore, MCP-1 and CCR2 expression levels were measured using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot analysis, and CCR2 expression levels were measured using RT-qPCR. The results indicated that MCP-1 and CCR2 expression levels were significantly increased in the spinal cord of MTBP rats. Intrathecal administration of anti-MCP-1 neutralizing antibodies was observed to attenuate the mechanical and thermal allodynia in MTBP rats. Therefore, the upregulation of spinal MCP-1 and CCR2 expression levels may contribute to the development of mechanical allodynia in MTBP rats. In conclusion, MCP-1/CCR2 signaling may serve a crucial role in morphine tolerance development in rats suffering from cancer-induced bone pain.
Keywords: morphine tolerance, bone cancer pain, spinal cord, monocyte chemoattractant protein-1, C-C chemokine receptor type 2
Introduction
Pain is a common and debilitating complication associated with cancer, and severely compromises the life quality of cancer patients (1). The most common source of pain is caused by the invasion of cancer cells into the bone (2). Cancer-induced bone pain involves a complex interaction of various molecular events, and combines inflammatory and neuropathic pain (3). Although opioids are presently commonly used for the treatment of cancer-induced bone pain, prolonged or high-dose opioid treatment often results in the development of tolerance and even hyperalgesia (4,5). Therefore, it is important to understand the underlying mechanisms of morphine tolerance in patients with cancer-induced pain. It has been reported that morphine tolerance and neuropathic pain share a similar cellular mechanism that is responsible for increased sensitivity to pain (6), and the activation of microglia-mediated formation contributes to morphine tolerance (7). Developing a novel strategy for reducing morphine tolerance may be valuable for the treatment of cancer-induced bone pain.
Accumulating evidence has indicated that the monocyte chemoattractant protein-1 (MCP-1) chemokine contributes to the activation of spinal microglia during pathological pain, and that along with its receptor, C-C chemokine receptor type 2 (CCR2), it serves an important role in bone cancer-induced hyperalgesia (8,9). In addition, it has been reported that MCP-1 expression increased in the spinal cord in animal models of neuropathic pain induced by peripheral nerve injury (chronic constriction injury) (10), spinal nerve ligation (11) and spinal cord contusion injuries (12–14). The role of MCP-1 in neuropathic pain has been further supported by several studies, showing that intrathecal injection of anti-MCP-1 neutralizing antibodies alleviated neuropathic pain induced by nerve injury (11,15) and surgical incision (16). Furthermore, CCR2 knockout mice failed to develop mechanical allodynia (17), suggesting that CCR2 receptors are important for the development of neuropathic pain. However, it remains to be determined whether spinal MCP-1 contributes to the development of morphine tolerance in patients suffering with cancer-induced bone pain.
In the present study, the expression levels of MCP-1 and CCR2 in the spinal cord were investigated in a model of morphine tolerance in bone cancer pain rats (MTBP rats). The current study aimed to examine whether spinal MCP-1 is involved in morphine tolerance development in rats suffering from cancer-induced bone pain.
Materials and methods
Animals
The present study was approved by the Animal Care and Use Committee of Shandong University (Jinan, China). Experiments were performed according to the Guidelines of the International Association for the Study of Pain (18). Adult female Sprague-Dawley rats (n=72; weight, 150–180 g) were purchased from the Experimental Animal Center of the Chinese Academy of Medical Sciences (Beijing, China). The animals were housed at room temperature (22–24°C) with 40–60% relative humidity and a 12/12-h light-dark cycle. Food and water were provided ad libitum to the animals.
A total of 24 rats were randomly divided into four groups (n=6 per group), as follows: Sham control (S group), morphine tolerance (M group), bone cancer pain (B group), and morphine tolerance and bone cancer pain (BM group). The intrathecal injection experiments involved the use of 48 rats were randomly divided into 6 groups (n=8 per group), as follows: Bone cancer pain (B group), morphine tolerance and bone cancer pain (BM group), intrathecal administration of anti-MCP-1 antibody in morphine tolerance and bone cancer pain (BM+Ab group), intrathecal administration of control IgG in morphine tolerance and bone cancer pain (BM+ IgG group), intrathecal administration of anti-MCP-1 antibody in bone cancer pain (B+Ab group), intrathecal administration of control IgG in bone cancer pain (B+IgG group).
MTBP rat model
Walker 256 mammary gland carcinoma cells (The Chinese Academy of Medical Sciences, Beijing, China). were prepared as previously described (19–21). Briefly, cells were collected from ascites of rats when ascites became obvious and diluted to a density of 2×107 cells/ml. Rats in the four groups were anesthetized by intraperitoneal injection of pentobarbital sodium (40 mg/kg; Sigma-Aldrich, St. Louis, MO, USA). Next, an animal model of bone cancer pain were established by injecting 10 µl Hank's balanced salt solution (Sigma-Aldrich) containing 1×105 Walker 256 cells into the tibial bone marrow cavity of the rats. Sham rats were treated by injection of 10 µl Hank's balanced salt solution into the bone cavity of the left tibia. Morphine tolerance was also induced by continuous intrathecal injection of morphine (20 µg/kg, twice a day; Sigma-Aldrich) between days 9 and 18 after cell injection, thus establishing the MTBP rat model. In the sham control group, the same volume of normal saline was intrathecally injected. All rats were sacrificed on day 18 after the initial injection.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted after sacrifice from the spinal cord (L4-L6) using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) as previously described (19,22). RNA was reverse transcribed into cDNA using a reverse transcription kit (cat no. 4368814; Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. qPCR was performed as previously described (23), using an Applied Biosystems 7500 sequence detection system (Thermo Fisher Scientific, Inc.). The reaction was performed in a final mixture volume of 50 µl [annealing buffer: KCl (250 mM), Tris (10 mM; pH 8.3), EDTA (1 mM); cDNA buffer: Tris (48 mM; pH 8.3), MgCl2 (32 mM); cDNA synthesis mix: 1.25 µl DTT (100 mM), 1 µl RNAse Block (Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA) or other RNAsin (1.25 mM), 5µl dNTPs, 0.25µl M-MuLV reverse transcriptase], containing 2 µl of cDNA. The following primers were used: MCP-1 (NM_031530) (19), 5′-AGCACCTTTGAATGTGAACT-3′ (forward) and 5′-AGAAGTGCTTGAGGTG-GTT-3′ (reverse); CCR2 (NM_021866) (3), 5′-CGCAGAGTT-GACAAGTTGTG-3′ (forward) and 5′-GCCATGGATGAACTGAGGTA-3′ (reverse); β-actin (NM_031144), 5′-CCCTGTGCTGCTCACCGA-3′ (forward) and 5′-ACAGTGTGGGTGACCCCGTC-3′ (reverse). Data analyses were run with the PCR system software. The data were collected and analyzed using the comparative cycle threshold (ΔΔCq) method (24). Relative expression levels of MCP-1 and CCR2 were normalized to the expression of β-actin (25).
Western blot analysis
The spinal cord (L4-L6) of rats was removed after sacrifice, and the tissue was homogenized in lysis buffer (cat no. 38733; Sigma-Aldrich) containing phenylmethylsulfonyl fluoride and 0.02% protease inhibitor cocktail. Equivalent amounts of protein (30 µg) were separated using 10% SDS-PAGE, and transferred onto a polyvinylidene difluoride membrane. The membranes were blocked with 5% nonfat milk for 1 h at room temperature, and then incubated with primary antibodies against MCP-1 (cat no. AF-479-NA; goat anti-rat; dilution, 1:3,000; R&D Systems, Inc., Minneapolis, MN, USA) or β-actin (cat no. A5441; goat anti-rat polyclonal; dilution, 1:10,000; Sigma-Aldrich) overnight at 4°C. Subsequently, the membranes were incubated with horseradish peroxidase-conjugated rabbit anti-goat secondary antibody IgG (cat no. sc-2771; dilution, 1:5,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at 25°C. The bands were then visualized using an enhanced chemiluminescence kit (cat no. KC-430; Kangchen Biotech, Shanghai, China). Results were quantified using TotalLab Quant (version 11.5; NatureGene Corp, Medford, NJ, USA).
Intrathecal injection for determination of MCP-1 association with hyperalgesia
Intrathecal injection of morphine (in groups BM, BM+Ab and BM+IgG) or antibodies (in groups BM+Ab and B+Ab on day 15 after inoculation of Walker 256 cells) was performed as described previously (26), in order to determine whether MCP-1 was associated with hyperalgesia in MTBP rats. Briefly, rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (40 mg/kg), and an intrathecal catheter (PE-10 tube) was inserted into the L4 and L5 intervertebral space. Subsequently, morphine (20 µg/kg, twice a day), anti-MCP-1 neutralizing antibody (10 µg; R&D Systems, Inc.) or control goat IgG (10 µg; Santa Cruz Biotechnology, Inc.) was injected intrathecally at a total volume of 10 µl. The MCP-1 association with hyperalgesia was then investigated with the paw withdrawal threshold (PWT) and thermal withdrawal latency (TWL) tests.
PWT test
Mechanical allodynia was measured using Von Frey filaments (Stoelting Co., Wood Dale, IL, USA) as previously described (20,21) before cell injection and 3, 6, 9, 12, 15 and 18 days after cell injection. Briefly, the animals were placed in individual plastic boxes (20×25×15 cm) with a metal mesh floor and allowed to acclimatize for 30 min. Next, the PWT test was performed by stimulating the plantar surface with the Von Frey filaments, and these were held for 6–8 sec. Brisk withdrawal or paw flinching were considered as positive responses. PWT was assessed according to the ‘up-and-down’ method (27).
TWL test
Heat hypersensitivity was examined using a plantar tester (type 7370; UgoBasile, Varese, Italy), as previously described (28) before cell injection and 3, 6, 9, 12, 15 and 18 days after cell injection. Briefly, a radiant heat source was positioned beneath a glass floor and directed at the plantar surface of the hind paw. TWL was defined as the time (in sec) between the delivery of the thermal stimulus and withdrawal of the paw. Three measurements were obtained for each hind paw in each test session. The hind paw was tested alternately at 5-min intervals between consecutive tests. The mean latency of the three measurements per side was used.
Statistical analysis
Data are presented as the mean ± standard error of the mean. For RT-qPCR data, one-way analysis of variance (ANOVA), followed by Dunnett's test, was used to compare differences among the groups. For PWT and TWL tests, two-way ANOVA, followed by Bonferroni correction, was used for the comparison of groups. P<0.05 was considered to indicate statistically significant differences.
Results
MCP-1 and CCR2 expressions levels
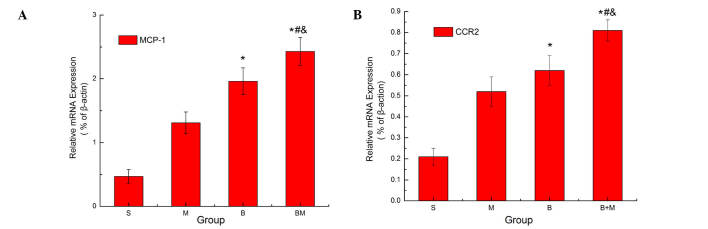
RT-qPCR was used to investigate the expression levels of MCP-1 and CCR2 in the rats at 18 days after Walker 256 cell injection. The results revealed that MCP-1 and CCR2 mRNA expression levels were significantly increased in the M, B and BM groups when compared with those in the S group (P<0.05; Fig. 1). In addition, compared with the M and B groups, MCP-1 and CCR2 expression levels were significantly higher in the BM group (P<0.05; Fig. 1), indicating that these were highly expressed in MTBP rats. Consistent with the RT-PCR results, western blot analysis revealed that MCP-1 protein expression was significantly higher in the BM group when compared with the S, M and B groups (P<0.05; Fig. 2).
Figure 1.
mRNA expression levels of (A) MCP-1 and (B) CCR2 (its receptor) in the spinal cord of MTBP rats, as determined by reverse transcription-quantitative polymerase chain reaction. The analysis revealed that MCP-1 and CCR2 expression levels increased in the spinal cord. β-actin served as a loading control. Values are presented as the mean ± standard error (n=6 in each group). *P<0.05 vs. S group; #P<0.05 vs. M group; &P<0.05 vs. B group. MCP-1, monocyte chemoattractant protein-1; CCR2, C-C chemokine receptor type 2; MTBP, morphine tolerance in bone cancer pain; S, sham; M, morphine; B, bone cancer pain; and BM, morphine tolerance and bone cancer pain.
Figure 2.
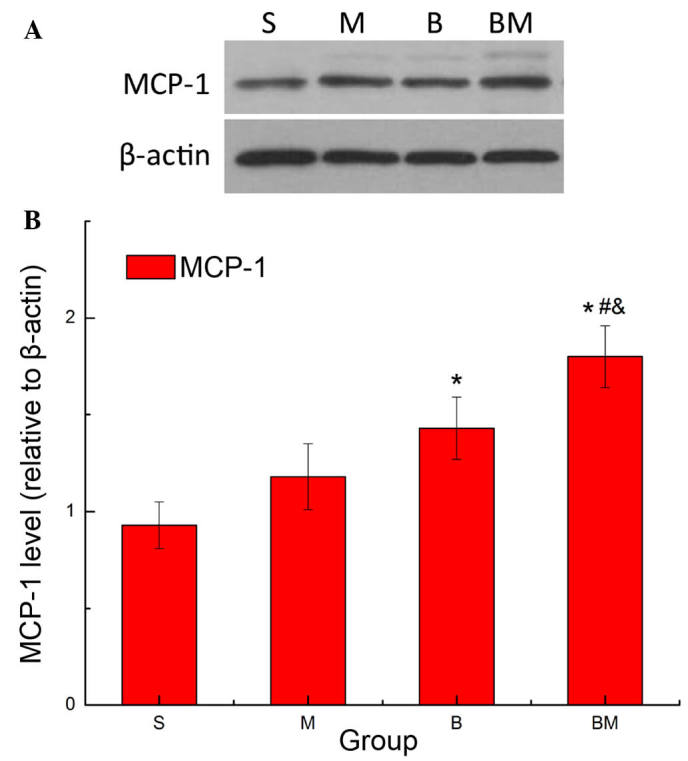
Protein expression of MCP-1 in the spinal cord of MTBP rats. (A) Representative western blot showing the MCP-1 protein expression in the spinal cord of MTBP rats. (B) Quantification of MCP-1 expression. Values are presented as the mean ± standard error (n=6 in each group). *P<0.05 vs. S group; #P<0.05 vs. M group; &P<0.05 vs. B group. MCP-1, monocyte chemoattractant protein-1; MTBP, morphine tolerance in bone cancer pain; S, sham; M, morphine; B, bone cancer pain; and BM, morphine tolerance and bone cancer pain.
Association of MCP-1 with hyperalgesia
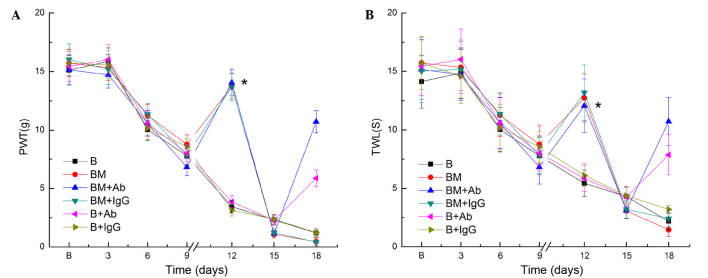
In the present study, an MTBP rat model was established, as observed by the results of PWL and TWL test. It was thus investigated whether MCP-1 was involved in hyperalgesia in MTBP rats by performing intrathecal injection of an anti-MCP-1 antibody on day 15 after Walker 256 cell inoculation. MTBP rats were subjected to PWT and TWL tests, and the results indicated significantly increased values on day 12 after continuous intrathecal administration of morphine for 3 days. However, PWT and TWL values were decreased on day 15, after the rats had received continuous intrathecal administration of morphine for a total of 6 days (i.e. day 18 after cell injection; Fig. 3). Furthermore, morphine tolerance was markedly reduced subsequent to the intrathecal administration of anti-MCP-1 antibodies (as was evident by the reduced tolerance to heat and filaments, but not after administration of control IgG (Fig. 3).
Figure 3.
Intrathecal injection of anti-MCP-1 neutralizing antibody attenuated mechanical allodynia in MTBP rats. (A) PWL and (B) TWL values were measured between days 1–18 after cell inoculation. Morphine was intrathecally injected between days 9 and 18 in all BM groups. A single intrathecal administration of anti-MCP-1 antibody (in groups BM+Ab and B+Ab), or control IgG (10 µg; in groups BM+IgG and B+IgG) was performed on day 15 after inoculation of Walker 256 cells. Values are presented as the mean ± standard error (n=8 rats in each group). ‘//’ in the x-axis indicates the time at which morphine administration was initiated. *P<0.05 vs. BM and BM+IgG groups. PWT, paw withdrawal threshold; TWL, thermal withdrawal latency; B, bone cancer pain group; BM, morphine tolerance and bone cancer pain group.
Discussion
Cancer-induced bone pain seriously impairs the quality of life of cancer patients; however, there are currently no effective therapies available for bone pain (29). In the present study, the mRNA expression levels of MCP-1 and its receptor CCR2 were found to be upregulated in the spinal cord of MTBP rats (BM group). In addition, the blockade of spinal MCP-1 with a neutralizing antibody attenuated mechanical and thermal allodynia in MTBP rats, as was evident from the decreased PWT and TWL values on day 15. These results suggest that MCP-1 may contribute to the initiation and maintenance of morphine tolerance in rats suffering from bone cancer pain.
MCP-1 is mainly expressed in the primary afferent neurons of the spinal cord, and is released from central terminals of primary afferents following nerve injury (15,30). The expression levels of MCP-1 have been demonstrated to be increased in the spinal cord in neuropathic pain (15,30). Consistent with the observations of these previous studies, the current study identified that the expression of MCP-1 increased in MTBP rats, suggesting that MCP-1 may contribute to the development of morphine tolerance in rats with cancer-induced bone pain. However, it remains unclear how MCP-1 contributes to neuropathic pain. Several studies have indicated that MCP-1 may act at multiple sites to facilitate pain induction, and the mechanisms underlying its action differed in various models of pain (31,32). In the present study, MCP-1 was found to contribute to the development of morphine tolerance in rats suffering from cancer-induced bone pain. It is speculated that morphine tolerance in these rats may have resulted from the increase of spinal MCP-1, which is likely to be released from dorsal root ganglion neurons at the dorsal horn of the spinal cord, or secreted from spinal cord neurons and glial cells. Further studies are required to identify the potential underlying mechanism in the future.
Accumulating evidence suggests that CCR2 serves an important role in neuropathic pain (8,17). This receptor is expressed in neuronal and glial cells in the spinal cord, and is upregulated in neuropathic pain (33). In the present study, CCR2 expression was found to be increased in the spinal cord of MTBP rats, suggesting that CCR2 served an important role in morphine tolerance development. In agreement with the present findings, previous studies have reported that CCR2 expression increased in the spinal cord of rats following the inoculation of osteolytic sarcoma cells into the humeri (34,35).
Several studies have demonstrated that MCP-1 is implicated in pain facilitation through the activation of spinal microglia in animal models of neuropathic pain (10,15,17). Activated microglia releases a variety of modulators, which contribute to central sensitization; and thereby, enhance pain states (35). It has also been reported that MCP-1 directly functions on spinal cord neurons and contributes to the central sensitization of pain induced by nerve injury (11,33). In the present study, MCP-1 and CCR2 expression levels were upregulated in MTBP rats. It is likely that MCP-1 may affect CCR2 expression on microglia, leading to the activation of microglia in the spinal dorsal horn, and eventually central sensitization. Since glial cells and neurons in the spinal cord express MCP-1 and CCR2, it is possible that MCP-1/CCR2 signaling may be involved in morphine tolerance observed in bone cancer pain via crosstalk between these neurons and glial cells. However, the current study did not identify which type of cells upregulated the expression of MCP-1 and CCR2. Further studies are required to examine the spinal cord cell types that express MCP-1 and its receptor CCR2 in MTBP rats.
In conclusion, the present study revealed that the expression levels of MCP-1 and CCR2 were increased in MTBP rats. In addition, the blockade of MCP-1 significantly attenuated mechanical allodynia in MTBP rats, suggesting that spinal MCP-1 and its receptor CCR2 may contribute to morphine tolerance development in MTBP rats with cancer-induced bone pain through the induction of central sensitization.
Acknowledgements
The present study was supported by grants from the National Natural Science Fund of China (no. 81471134), and the Natural Science Fund of Shandong Province (no. ZR2014HL036).
References
- 1.Zoëga S, Fridriksdottir N, Sigurdardottir V, Gunnarsdottir S. Pain and other symptoms and their relationship to quality of life in cancer patients on opioids. Qual Life Res. 2013;22:1273–1280. doi: 10.1007/s11136-012-0264-x. [DOI] [PubMed] [Google Scholar]
- 2.Kane CM, Hoskin P, Bennett MI. Cancer induced bone pain. BMJ. 2015;350:h315. doi: 10.1136/bmj.h315. [DOI] [PubMed] [Google Scholar]
- 3.Falk S, Dickenson AH. Pain and nociception: Mechanisms of cancer-induced bone pain. J Clin Oncol. 2014;32:1647–1654. doi: 10.1200/JCO.2013.51.7219. [DOI] [PubMed] [Google Scholar]
- 4.Falk S, Schwab SD, Frøsig-Jørgensen M, Clausen RP, Dickenson AH, Heegaard AM. P2X7 receptor-mediated analgesia in cancer-induced bone pain. Neuroscience. 2015;291:93–105. doi: 10.1016/j.neuroscience.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Bannister K. Opioid-induced hyperalgesia: Where are we now? Curr Opin Support Palliat Care. 2015;9:116–121. doi: 10.1097/SPC.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 6.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: A current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Ma W, Chabot JG, Quirion R. Morphological evidence for the involvement of microglial p38 activation in CGRP-associated development of morphine antinociceptive tolerance. Peptides. 2010;31:2179–2184. doi: 10.1016/j.peptides.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Pevida M, González-Rodríguez S, Lastra A, García-Suárez O, Hidalgo A, Menéndez L, Baamonde A. Involvement of spinal chemokine CCL2 in the hyperalgesia evoked by bone cancer in mice: A role for astroglia and microglia. Cell Mol Neurobiol. 2014;34:143–156. doi: 10.1007/s10571-013-9995-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin D, Yang JP, Hu JH, Wang LN, Zuo JL. MCP-1 stimulates spinal microglia via PI3K/Akt pathway in bone cancer pain. Brain Res. 2015;1599:158–167. doi: 10.1016/j.brainres.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- 11.Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knerlich-Lukoschus F, Juraschek M, Blömer U, Lucius R, Mehdorn HM, Held-Feindt J. Force-dependent development of neuropathic central pain and time-related CCL2/CCR2 expression after graded spinal cord contusion injuries of the rat. J Neurotrauma. 2008;25:427–448. doi: 10.1089/neu.2007.0431. [DOI] [PubMed] [Google Scholar]
- 13.Jeon SM, Sung JK, Cho HJ. Expression of monocyte chemoattractant protein-1 and its induction by tumor necrosis factor receptor 1 in sensory neurons in the ventral rhizotomy model of neuropathic pain. Neuroscience. 2011;190:354–366. doi: 10.1016/j.neuroscience.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 14.Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci USA. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain. 2009;13:263–272. doi: 10.1016/j.ejpain.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Peters CM, Eisenach JC. Contribution of the chemokine (C-C motif) ligand 2 (CCL2) to mechanical hypersensitivity after surgical incision in rats. Anesthesiology. 2010;112:1250–1258. doi: 10.1097/ALN.0b013e3181d3d978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu JH, Yang JP, Liu L, Li CF, Wang LN, Ji FH, Cheng H. Involvement of CX3CR1 in bone cancer pain through the activation of microglia p38 MAPK pathway in the spinal cord. Brain Res. 2012;1465:1–9. doi: 10.1016/j.brainres.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Lan LS, Ping YJ, Na WL, Miao J, Cheng QQ, Ni MZ, Lei L, Fang LC, Guang RC, Jin Z, Wei L. Down-regulation of Toll-like receptor 4 gene expression by short interfering RNA attenuates bone cancer pain in a rat model. Mol Pain. 2010;6:2. doi: 10.1186/1744-8069-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Yang J, Wang L, Jiang M, Qiu Q, Ma Z, Liu L, Li C, Ren C, Zhou J, Li W. Tibia tumor-induced cancer pain involves spinal p38 mitogen-activated protein kinase activation via TLR4-dependent mechanisms. Brain Res. 2010;1346:213–223. doi: 10.1016/j.brainres.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Wang LN, Yao M, Yang JP, Peng J, Peng Y, Li CF, Zhang YB, Ji FH, Cheng H, Xu QN, et al. Cancer-induced bone pain sequentially activates the ERK/MAPK pathway in different cell types in the rat spinal cord. Mol Pain. 2011;7:48. doi: 10.1186/1744-8069-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang LN, Yang JP, Zhan Y, Ji FH, Wang XY, Zuo JL, Xu QN. Minocycline-induced reduction of brain-derived neurotrophic factor expression in relation to cancer-induced bone pain in rats. J Neurosci Res. 2012;90:672–681. doi: 10.1002/jnr.22788. [DOI] [PubMed] [Google Scholar]
- 23.Sandhir R, Gregory E, He YY, Berman NE. Upregulation of inflammatory mediators in a model of chronic pain after spinal cord injury. Neurochem Res. 2011;36:856–862. doi: 10.1007/s11064-011-0414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luger NM, Mach DB, Sevcik MA, Mantyh PW. Bone cancer pain: From model to mechanism to therapy. J Pain Symptom Manage. 2005;29:S32–S46. doi: 10.1016/j.jpainsymman.2005.01.008. (Suppl 5) [DOI] [PubMed] [Google Scholar]
- 27.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 28.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 29.Hang LH, Luo H, Li SN, Shu WW, Chen Z, Chen YF, Yuan JF, Shi LL, Shao DH. Involvement of spinal Bv8/Prokineticin 2 in a rat model of cancer-induced bone pain. Basic Clin Pharmacol Toxicol. 2015;117:180–185. doi: 10.1111/bcpt.12386. [DOI] [PubMed] [Google Scholar]
- 30.Van Steenwinckel J, Reaux-Le Goazigo A, Pommier B, Mauborgne A, Dansereau MA, Kitabgi P, Sarret P, Pohl M, Mélik Parsadaniantz S. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J Neurosci. 2011;31:5865–5875. doi: 10.1523/JNEUROSCI.5986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung H, Bhangoo S, Banisadr G, Freitag C, Ren D, White FA, Miller RJ. Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain. J Neurosci. 2009;29:8051–8062. doi: 10.1523/JNEUROSCI.0485-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu JH, Zheng XY, Yang JP, Wang LN, Ji FH. Involvement of spinal monocyte chemoattractant protein-1 (MCP-1) in cancer-induced bone pain in rats. Neurosci Lett. 2012;517:60–63. doi: 10.1016/j.neulet.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Hu JH, Wu MY, Tao M, Yang JP. Changes in protein expression and distribution of spinal CCR2 in a rat model of bone cancer pain. Brain Res. 2013;1509:1–7. doi: 10.1016/j.brainres.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Vit JP, Ohara PT, Tien DA, Fike JR, Eikmeier L, Beitz A, Wilcox GL, Jasmin L. The analgesic effect of low dose focal irradiation in a mouse model of bone cancer is associated with spinal changes in neuro-mediators of nociception. Pain. 2006;120:188–201. doi: 10.1016/j.pain.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 35.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]