Abstract
The aim of this study was to explore an effective method for the repair of cartilage defects using chitosan/glycerophosphate (C/GP) gel- and Matrigel-engineered human bone morphogenetic protein 7 (hBMP7)-expressing chondrocytes. Rabbit chondrocytes were obtained, cultured in vitro and transfected with an adenovirus containing hBMP7 and green fluorescent protein (Ad-hBMP7-GFP). The expression of hBMP7 in the transfected cells was tested by reverse transcription-polymerase chain reaction (RT-PCR) and western blotting. The phenotype of the transfected cells was evaluated by detecting the yields of collagen II and hyaluronic acid using RT-PCR and enzyme-linked immunosorbent assay (ELISA). The growth of chondrocytes in the C/GP gel and Matrigel was accessed by measuring the cell growth rate, hematoxylin and eosin (H&E) staining and observation under a scanning microscope. Twelve adult male New Zealand white rabbits were randomly divided into three groups. Two cartilage defects were created in the rabbits' knees by aseptic surgery. Group A (n=4) did not receive any treatment, group B (n=4) were treated with C/GP gel and Matrigel-engineered Ad-mock-GFP-transfected chondrocytes, and group C (n=4) were treated with C/GP gel and Matrigel-engineered Ad-hBMP7-GFP-transfected chondrocytes. Rabbits were sacrificed at 4 weeks after transplantation, and the repair effect was measured by the Wakitani scoring method. On the basis of the RT-PCR and western blot results, hBMP7 was efficiently overexpressed in the Ad-hBMP7-GFP-transfected chondrocytes. The ELISA results showed that the yields of collagen II and hyaluronic acid in Ad-hBMP7-GFP-transfected chondrocytes were significantly higher than those in Ad-mock-GFP-transfected chondrocytes. Chondrocytes have a better morphology and arrangement in a Matrigel scaffold than in C/GP, as assessed by H&E staining and scanning microscopy. According to the Wakitani score, Matrigel combined with Ad-hBMP7-GFP-transfected chondrocytes successfully promoted the repair of cartilage defects in rabbit knees.
Keywords: cartilage defect, bone morphogenetic protein 7, Matrigel scaffold
Introduction
As an inflammatory and degenerative rheumatic disease, cartilage defects are characterized by joint pain, locking phenomena and even severe disability (1). As a result of cartilage not readily self-healing, cartilage repair continues to be challenging for clinical doctors and scientists.
Tissue engineering technology has provided great opportunities for cartilage repair (2,3). For example, autologous chondrocyte transplantation (ACT) has been successfully tested in several animal experiments and clinical trials by expanding autologous chondrocytes in vitro and injecting them back into the defective cartilage site (4,5). However, some problems have been reported for this technique. Firstly, obtaining chondrocytes from the joint surface might introduce additional injury to the joint. Secondly, chondrocytes expanded in vitro readily undergo a process of dedifferentiation (6). The chondrocytes might suffer a reduction in the production of hyaluronic acid and type II collagen, which are two important components for cartilage regeneration. The loss of phenotypic traits in vitro might result in the loss of ability to regenerate stable and healthy cartilage in vivo (7).
Various efforts have been made to prevent chondrocytes from dedifferentiation in vitro and in vivo. Some gene therapies have achieved success in chondrocyte tissue culture and animal cartilage repair experiments. It has been reported that bone morphogenetic protein 2 (BMP2) treatment might help chondrocytes to maintain their phenotypes in vitro (8). Moreover, short-term exposure to fibroblast growth factor 2 (FGF-2) in vivo is also effective in stimulating cartilage repair or enlargement (9–12). Furthermore, 3-dimensional (3D) tissue culture exhibits huge advantages in maintaining the phenotype of chondrocytes. Different scaffold materials have been tested for cartilage repair in the last few years. Chitosan/glycerophosphate (C/GP) gel (13), Matrigel (14), collagen (15) and fibrin gel (16) have exhibited excellent performances in cartilage cell culture in vitro and cartilage repair in animal experiments.
Bone morphogenetic protein 7 (BMP7) is a member of the transforming growth factor (TGF)-β superfamily (17) that is crucial in the regulatory pathways for embryogenesis, tissue repair and regeneration (18). There is evidence that BMP7 is able to induce the differentiation of bone marrow-derived mesenchymal cells into chondrocytes (19) and might serve as an anabolic activator for chondrocytes in tissue culture (20). In addition, clinical studies have revealed that BMP7 is able to prevent the degeneration of cartilage during inflammatory arthritis (21,22). These observations suggest that BMP7 has great potential for use in cartilage repair and regeneration.
In the present study, gene therapies and 3D culture techniques were combined for use in the repair of cartilage defects. Two types of 3D culture gel-engineered Ad-hBMP7-GFP-transfected chondrocytes were prepared. By implanting the engineered gels into rabbit cartilage defects, their ability to improve cartilage repair in rabbit knees was evaluated and compared.
Materials and methods
Animals
A total of 12 adult male New Zealand White rabbits (50 weeks old; weight, 2.0–2.5 kg) and 12 female New Zealand White rabbits (6 weeks old, weight, 1.5–2.0 kg) were provided by the Laboratory Animal Center of Nantong University (Nantong, China). All animals were housed individually. Food and water were given ad libitum. The animals' general health and care conditions were monitored and recorded separately by the laboratory animal welfare officer. This study was approved by the laboratory animal ethics committee of Nantong University.
Primary cell culture and hBMP7 transfection
Rabbit articular knee cartilages were obtained from 6-week-old female New Zealand White rabbits. The cartilages were cut into three 1-mm cubes and digested with 100 µl 0.25% trypsin for 10 min at 37°C. The digested cartilages were centrifuged at 1,150 × g for 5 min at 20°C, and then the supernatant was discarded. The pellet was washed three times with F12-Dulbecco's modified Eagle's medium (DMEM; cat. no. E500003; Sangon Biotech Co., Ltd., Shanghai, China) and then incubated with 0.02% collagenase II and 0.004% deoxyribonuclease II at 4°C overnight. An 80-mesh stainless steel filter was used to remove undigested tissue. The filtered chondrocytes were centrifuged at 1,000 rpm for 5 min, and then washed twice with F12-DMEM medium. The cells were then seeded in tissue culture flasks with a density of 5×105/ml. Cell culture was maintained at 37°C in a humidified atmosphere containing 95% air and 5% CO2. The medium was changed once every 3 days. Cells were passaged when the culture reached 80–90% confluence. The third-passage cells were collected for the following transfection experiments. Briefly, the chondrocyte cells were seeded into 6-well cell culture plates at 2×105/ml, into which cover glasses had been placed beforehand. When the cells reached 80–90% confluence, the culture medium was changed to a serum-free medium and the culture was transfected with an adenovirus containing human green fluorescent protein (GFP)-labeled BMP7 (Ad-hBMP7-GFP; 106 PFU/ml) or an adenovirus containing GFP (Ad-mock-GFP; 106 PFU/ml), both of which were constructed and stored in our laboratory, at a multiplicity of infection (MOI, virus/cell) of 100, respectively. After 4 h incubation, the culture medium was changed to F12-DMEM complete medium and the cells were returned to the culture.
Reverse transcription polymerase chain reaction (RT-PCR)
RT-PCR was used to determine the mRNA expression levels of hBMP7 and collagen type II in the transfected cells. Total RNA was isolated from cells using an RNeasy mini kit (Qiagen, Inc., Valencia, CA, USA). Briefly, at 1 week after transfection, cells were harvested using 1 ml TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Homogenates were left at room temperature for 5 min to facilitate mRNA extraction and then centrifuged at 12,000 × g for 15 min. cDNA was reverse transcribed using a cDNA synthesis kit (Qiagen, Inc.) with 1 µg mRNA/20 µl reaction. RT-PCR analysis was carried out with 1 µg total RNA using a Qiagen OneStep RT-PCR kit (Qiagen, Inc.). The following primers were synthesized on the basis of the sequences reported in the Genbank database: hBMP7 forward, 5′-AACCTCGTGGAACATGACAAG-3′ and reverse, 5′-CTCCCCCTCACAGTAGTAGGC-3′ (product size 675 bp); collagen type II forward, 5′-GCACCCATGGACATTGGTGGC-3′ and reverse, 5′-GACACGGAGTAGCACCATTCG-3′ (product size 366 bp); glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward, 5′-CGACCACTTTGTCAAGCTCA-3′ and reverse, 5′-AGGGGAGATTCAGTGTGGTG-3′ (product size 226 bp). The reaction was carried out at 50°C for 30 min for cDNA synthesis, and at 95°C for 3 min for predenaturation. The samples were amplified for 22–26 cycles with denaturation at 94°C for 30 sec, annealing for 1 min at 65°C, extension at 72°C for 1 min and a final extension at 72°C for 10 min. RT-PCR amplification reactions were resolved on 2% agarose gels and the size of the amplified transcript confirmed by comparison with a standard DNA ladder (GelPilot 1 kb Plus Ladder; Qiagen, Inc.).
Western blot analysis
The western blotting method was used to detect hBMP7 protein in the transfected cells. Briefly, cells were collected at 1 week after transfection and then homogenized in a cell lysis buffer containing 50 mmol/l Tris, 10 mmol/l NaCl, 1% NP-40, 0.02% sodium azide and a protease inhibitor cocktail (Roche Diagnostics, Basel, Switzerland) at pH 7.4. Next, aliquots containing 50 µg protein were dissolved in Laemmli buffer and boiled at 95°C for 5 min, and the proteins were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. After being blocked with 5% fat-free milk, the target proteins were probed with anti-BMP7 and anti-β-actin antibodies (1:2,000; cat. nos. ab56023 and ab15263; Abcam, Cambridge, MA, USA), respectively. The bound antibodies were detected using horseradish peroxide-conjugated secondary antibodies (1:2,000; cat. no. ab6721; Abcam) and visualized using an enhanced chemiluminescent reagent. The relative levels of each blot to the control, β-actin were analyzed using ImageJ software, version 1.48 (National Institutes of Health, Bethesda, MD, USA).
Enzyme-linked immunosorbent assay (ELISA)
The amounts of hyaluronic acid and collagen type II in the cartilage cells were measured using ELISA kit for Hyaluronic Acid (cat. no. EKU08324; Biomatik, Wilmington, DE, USA) and ELISA kit for Cross Linked C-Telopeptide of Type II Collagen (CTXII; cat. no. EKU03505; Biomatik), respectively, according to the manufacturer's instructions. Colorimetric changes were evaluated using an ELISA plate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
3D cell cultures
Third-passage chondrocytes were collected for 3D culture. Briefly, cells were resuspended with C/GP and Matrigel solution, respectively, at a density of 5×107/ml. The C/GP hydrogel was prepared by mixing 2.0% (v/v) chitosan with 56% (w/w) β-glycerophosphate at the ratio of 7:1. For C/GP gel culture, the suspension of cells in C/GP solution was seeded into a 6-well plate directly, 1 ml per well. The plate was placed in a 37°C incubator for 10 min until it became a gel. A 4 ml aliquot of complete medium was added to each well and the plate was put back into the incubator. For Matrigel culture, firstly, 50 µl of 300 mg/l Matrigel-M199 medium solution was used to pre-coat the top chamber of a Boyden chamber. The precoated chamber was incubated at room temperature for 1 h. The suspension of cells in Matrigel solution was then seeded into the precoated chamber, 1 ml per well. The plate was placed into a 37°C cell incubator for 10 min until it became a gel. A 4 ml aliquot of complete medium was added to each well and the plate was put back into the incubator. The cultures were maintained for 14 days in the culture medium, at 37°C in a humidified 5% CO2/95% air atmosphere with medium replacement every 3 days.
Scanning electron microscopy observation
Following double fixation with glutaraldehyde, dehydration with a graded ethanol series and coating with epoxy resin (Epon 812; Byxbio, Jiangsu, China), scanning electron microscopy was used to observe the morphology of Ad-mock-GFP- and Ad-hBMP7-GFP-transfected chondrocytes in Matrigel.
Animal model
A total of 12 adult male New Zealand White rabbits (50 weeks old; weight, 2.0–2.5 kg) were used to establish a cartilage defect rabbit model. Surgery was performed under general inhalation anesthesia and aseptic conditions. Briefly, the animals were anesthetized intravenously with 3% pentobarbital sodium (30 mg/kg). After sterilization with 1% povidone iodine, a medial incision was made into the joint cavity, the patella was pushed outward to expose the articular surface of the femoral trochlea and a drill was used to create two holes with diameter 4 mm and depth 5 mm at the articular surface of the femoral trochlear. The modeled rabbits were divided into three groups. Group A (n=4) did not receive any implantation treatment. In group B (n=4), two cartilage defects were implanted with C/GP gel-engineered Ad-mock-GFP-transfected chondrocytes and Matrigel-engineered Ad-mock-GFP-transfected chondrocytes, respectively. In group C (n=4), the two defects were implanted with C/GP gel-engineered Ad-hBMP7-GFP-transfected chondrocytes and Matrigel-engineered Ad-hBMP7-GFP-transfected chondrocytes, respectively. After 4 weeks, the animals were sacrificed by the injection of 10 ml air into the vein of the ear edge.
Hematoxylin and eosin (H&E) staining
Samples of cartilage tissue were fixed in 10% formalin solution, embedded in paraffin, and sectioned to 5 µm. The tissue sections were then stained with the H&E reagent according to standard protocols and observed using light microscopy.
General specimen observation
Rabbits in each group were sacrificed at 4 weeks after surgery to observe the defects. The tissue of the defect area and surrounding normal cartilage was removed for general observation. General specimen observation involved checking the smoothness, gloss and density of the articular cartilage surface and the condition of the joint with the surrounding normal cartilage.
Repair assessment
A histological score was given for the repaired cartilage tissue using the Wakitani method (23), which involves assessment of the cell types in the repaired tissue, matrix staining, surface smoothness, cartilage density and integration into the surrounding normal cartilage tissue.
Statistical analysis
The experimental data are presented as mean ± standard deviation. The statistical software package SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA) was used for data analysis. A t-test was adopted for comparison between groups, where P<0.05 was considered to indicate a statistically significant difference.
Results
Chondrocyte culture
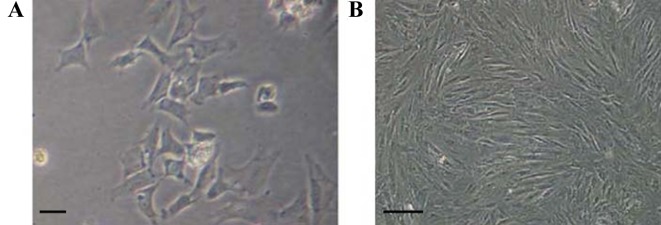
The rate of survival of the chondrocytes after isolation and plating was 90%. At 10 days after plating, cells reached nearly 90% confluence and started to exhibit the typical chondrocyte morphology with triangular or polygonal shape (Fig. 1A). Following the first passage, the cell growth rate markedly increased. The morphology of chondrocytes at the third generation is shown in Fig. 1B.
Figure 1.
Culture of rabbit chondrocytes. Chondrocytes at (A) day 10 after isolation (magnification, ×200) and (B) at the third passage (magnification, ×100). Scale bar, 100 µM.
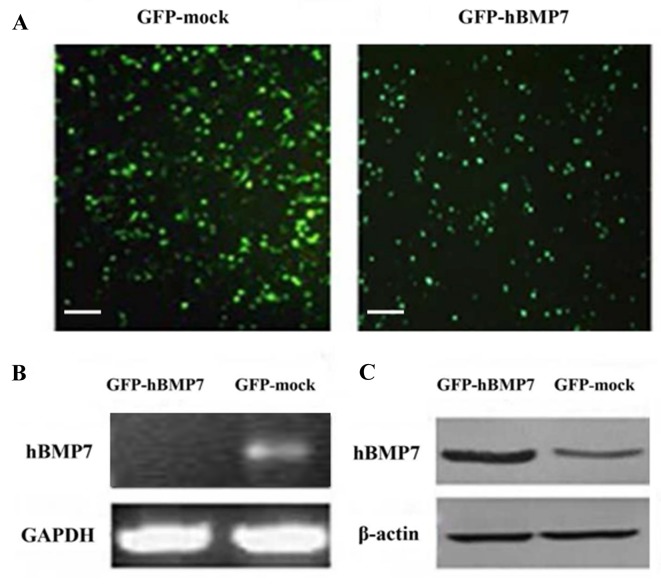
Ad-hBMP7-GFP transfection and expression of hBMP7 in transfected chondrocytes
To continuously express hBMP7 in chondrocytes, Ad-hBMP7-GFP and Ad-mock-GFP vectors were independently used to transfect chondrocytes (Fig. 2A). At 1 week after transfection, the expression of hBMP7 in the transfected chondrocytes was evaluated by RT-PCR and western blot analysis. As shown in Fig. 2B, RT-PCR analysis detected a thick hBMP7 target gene band of 470 bp in the Ad-hBMP7-GFP-transfected cells. Consistent with this, an hBMP7 protein band stronger than that in the mock-transfected cells was found by western blotting (Fig. 2C). These two results indicate that hBMP7 was successfully overexpressed in Ad-hBMP7-GFP-transfected cells.
Figure 2.
Transfection and overexpression of hBMP7 in chondrocytes. (A) Chondrocytes transfected by Ad-mock-GFP (left panel) and transfected by Ad-hBMP7-GFP (right panel). Scale bar, 100 µm. (B) Reverse transcription-polymerase chain reaction analysis of hBMP7 in Ad-mock-GFP- and Ad-hBMP7-GFP-transfected chondrocytes. (C) Western blot analysis of hBMP7 in Ad-mock-GFP- and Ad-hBMP7-GFP-transfected chondrocytes. hBMP7, human bone morphogenetic protein 7; Ad, adenovirus; GFP, green fluorescent protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
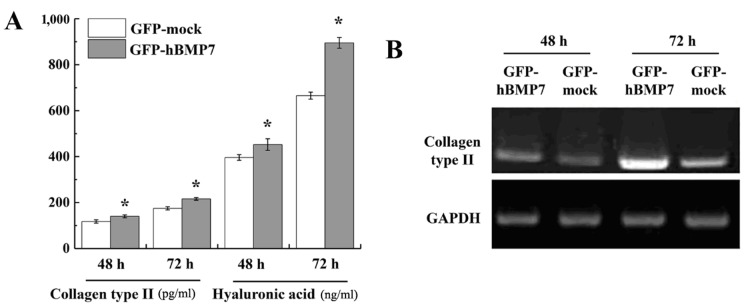
Collagen II and hyaluronic acid production in transfected chondrocytes
Articular cartilage is mainly composed of a large amount of extracellular components secreted by chondrocytes (6). Collagen type II and hyaluronic acid are two important and well-studied components in those secretions (4). Therefore, to evaluate the phenotype of transfected chondrocytes, the yields of collagen type II and hyaluronic acid were investigated by ELISA. As shown in Fig. 3A, the production of collagen type II and hyaluronic acid from Ad-hBMP7-GFP-transfected cells was significantly higher than that in Ad-mock-GFP-transfected cells (P<0.05). RT-PCR analysis demonstrated that collagen type II gene expression was notably increased in Ad-hBMP7-GFP-transfected cells compared with Ad-mock-GFP-transfected cells (Fig. 3B).
Figure 3.
Yield of collagen type II and hyaluronic acid in transfected chondrocytes. (A) Enzyme-linked immunosorbent assay analysis of the yield of collagen type II (pg/ml) and hyaluronic acid (ng/ml) in transfected chondrocytes. Results are presented as mean ± standard deviation (n=3). *P<0.05 vs. the GFP-mock group. (B) Detection of the mRNA level of collagen type II in transfected chondrocytes as evaluated by reverse transcription-polymerase chain reaction. hBMP7, human bone morphogenetic protein 7; GFP, green fluorescent protein.
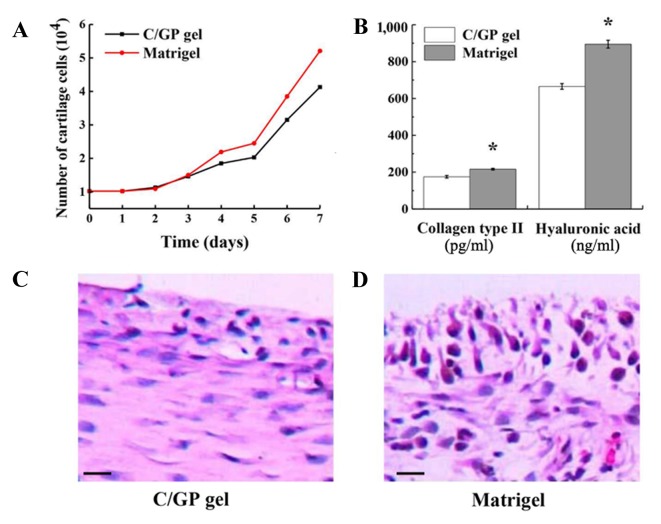
Growth of chondrocytes in the 3D culture system
3D culture systems have been widely adopted in tissue culture as they have huge advantages in maintaining phenotypes in vitro. As two commonly used 3D culture materials, chitosan (C/GP gel) and Matrigel have displayed great performances in cancer and stem cell research (24). In the present study, these two gels were compared in the culture of chondrocytes in vitro. No significant difference in growth rate was observed between those two scaffolds, although the Matrigel exhibited a slightly faster growth rate than the C/GP gel with a 4.2-day cell doubling time compared with a 5.0-day cell doubling time (Fig. 4A). However, on the basis of the significant difference in the yield of collagen type II and hyaluronic acid (P<0.01; Fig. 4B), Matrigel is more helpful in restoring the phenotype of chondrocytes. Furthermore, with H&E staining, the morphology and organization of the chondrocytes in Matrigel appeared much better (Fig. 4C). In Matrigel, the majority of the chondrocytes were tightly packed, integrated into layers and surrounded by the extracellular matrix secreted by chondrocytes. By contrast, the chondrocyte proliferation in the C/GP gel was relatively weak. Most of the cartilage cells were loosely arranged and a reduced quantity of extracellular matrix was secreted. The greatest disadvantage of the C/GP gel was that the chondrocytes were not integrated into the layers. In conclusion, Matrigel is superior to C/GP gel for the culture of chondrocytes in vitro.
Figure 4.
Chondrocyte growth in 3D culture systems. (A) Growth curve of chondrocytes in C/GP gel and Matrigel 3D culture systems. (B) Yield of collagen type II and hyaluronic acid from chondrocytes in C/GP gel and Matrigel. Results are presented as mean ± standard deviation (n=3). *P<0.05 vs. the C/GP gel group. Hematoxylin and eosin staining of chondrocytes in (C) C/GP gel and (D) Matrigel culture systems. Scale bar, 50 µm. C/GP, chitosan/glycerophosphate; 3D, 3-dimensional.
Growth of transfected chondrocytes in Matrigel scaffold
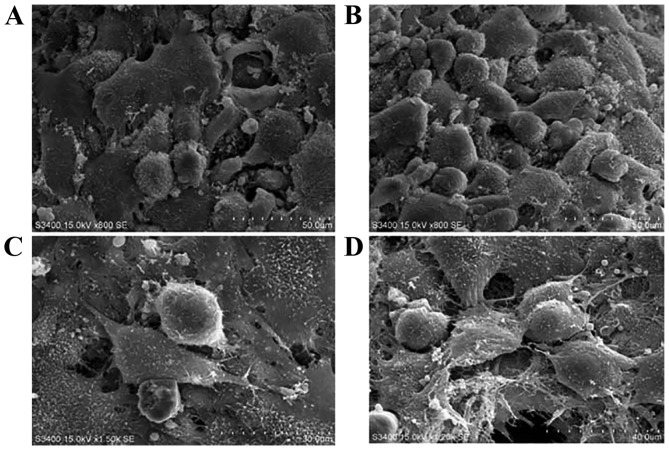
Scanning electron microscopy was used to observe the morphology of Ad-mock-GFP- and Ad-hBMP7-GFP-transfected chondrocytes in Matrigel (Fig. 5). The two types of cell showed a spherical shape and gathered along the fibers within the extracellular matrix. However, in comparison with the Ad-mock-GFP-transfected chondrocytes, a greater amount of extracellular secretion was observed in the Ad-hBMP7-GFP-transfected chondrocytes (Fig. 4). This observation suggests that hBMP7 is able to promote chondrocyte secretion, which might be helpful for cartilage regeneration in vivo.
Figure 5.
Scanning electron microscopy imaging of chondrocytes in Matrigel. (A) Ad-mock-transfected chondrocytes and (B) Ad-hBMP7-transfected chondrocytes in Matrigel scaffold (magnification, ×1,500). (C) Ad-mock-transfected chondrocytes and (D) Ad-hBMP7-transfected chondrocytes in Matrigel scaffold (magnification, ×3,000). hBMP7, human bone morphogenetic protein 7; Ad, adenovirus.
Repair assessment of the different 3D culture systems
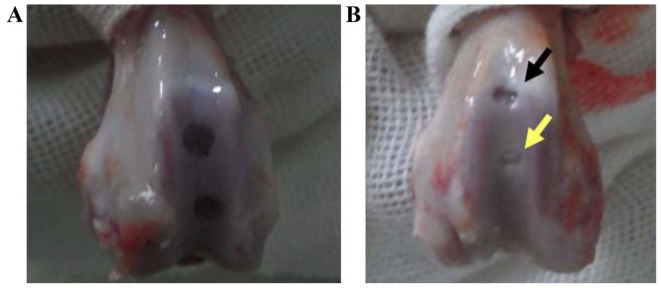
To determine which scaffold is better for cartilage repair, C/GP gel and Matrigel with Ad-mock-GFP-transfected chondrocytes were implanted into rabbit knees, respectively, as described Materials and methods. At 4 weeks after implantation, the repair was assessed by the Wakitani method. As a control group, the rabbits in group A did not receive any implantation treatment. After 4 weeks, the defects in group A remained evident and filled with blood clots (Fig. 6A). In group B, the defects were treated with C/GP gel and Matrigel scaffold with Ad-mock-GFP-transfected chondrocytes. Overall, group B presented better repair than group A. However, significant differences between the C/GP gel site and Matrigel site in group B were observed (Fig. 6B). In the Matrigel site, ≤3/4 of normal cartilage was found and the surface looked relatively flat and smooth. However, in the C/GP gel site, only 1/3 of the cartilage defect was repaired and the cartilage surface did not appear to be flat or smooth. According to Wakitani scoring (Fig. 7), Matrigel performed best with a score of 3.14±0.88 followed by C/GP gel with a score of 3.94±0.91 and the control group with a score of 8.16±0.86. In conclusion, both C/GP gel and matrigel scaffold helped to repair the cartilage defect. However, Matrigel scaffold provided significantly improved results than did C/GP gel, with a Wakitani score of 3.14 vs. 3.94 (P<0.05). Therefore, Matrigel scaffold was used to perform a further experiment.
Figure 6.
Repair of rabbit knee cartilage by implantation of 3D culture scaffolds. (A) Repair result of group A without any treatment. (B) Repair result of group B treated with two different 3D culture scaffolds containing Ad-mock-GFP-transfected chondrocytes. C/GP gel (black arrow), Matrigel (yellow arrow). 3D, 3-dimensional; Ad, adenovirus; C/GP, chitosan/glycerophosphate.
Figure 7.
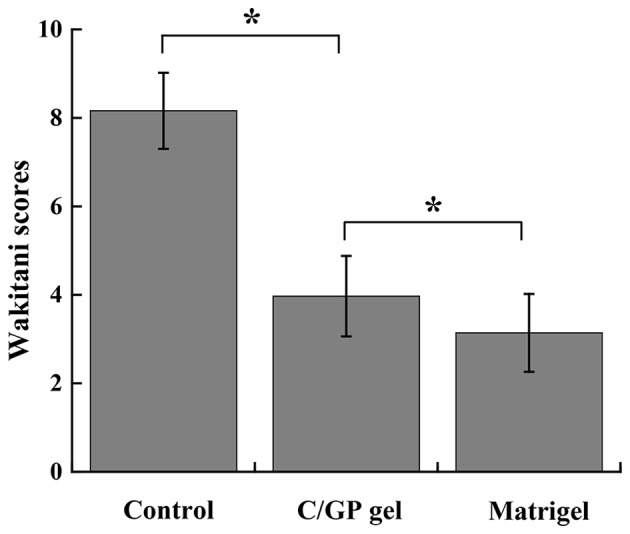
Repair assessment of different 3D culture scaffolds by the Wakitani scoring method. Results are presented as mean ± standard deviation (n=4). *P<0.05 as indicated. 3D, 3-dimensional; C/GP, chitosan/glycerophosphate.
Repair assessment of Ad-hBMP7-GFP-transfected chondrocytes in Matrigel scaffold
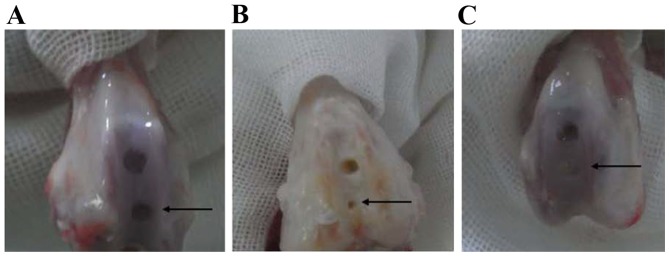
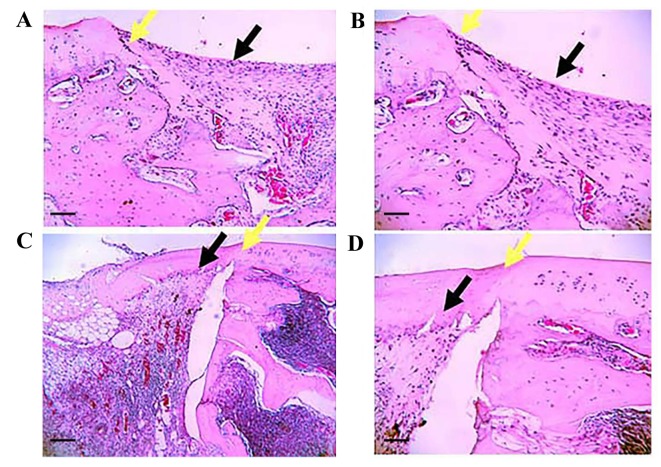
Matrigel scaffold-engineered Ad-hBMP7-GFP- or Ad-mock-GFP-transfected chondrocytes were implanted into rabbit knees. No rabbits died following surgery and all 12 animals exhibited normal feeding and activity within 2 weeks. At 4 weeks after implantation, cartilage repair was assessed by the Wakitani scoring method and H&E staining. As shown in Fig. 8, the defects in group A were clearly visible as the clear boundary line between the defects and normal cartilage tissue. In group B, the defects were treated with Matrigel containing Ad-mock-GFP-transfected chondrocytes. The defects were partially repaired; however, the cartilage surface was not smooth or flat like the healthy cartilage. The color and texture remained different from the surrounding tissue. In group C, the defects were almost completely healed following the treatment with Matrigel scaffolds containing Ad-hBMP7-GFP-transfected chondrocytes. In particular, the cartilage surface looked flat and smooth, and it was difficult to see the boundary line between the defects and normal cartilage tissue. Most notably, no inflammatory cell infiltration was found in the defect. According to the Wakitani scoring method, group C had a score of 3.06±0.88 that was significantly lower than that of group B (3.81±0.91) (Fig. 9). H&E staining results further confirmed that regeneration of cartilage tissue in group C significantly outperformed that in group B (Fig. 10). The newly generated cartilage tissue filled all the defective area in group C. However, the repair in group B was limited, with some defects not being filled with regenerated cartilage.
Figure 8.
Repair of rabbit knee cartilage of different groups. Repair results of (A) untreated cartilage defect, (B) cartilage defect treated with Matrigel scaffold-engineered Ad-mock-GFP-transfected chondrocytes and (C) cartilage defect treated with Matrigel scaffold-engineered Ad-hBMP7-GFP-transfected chondrocytes; Matrigel site (black arrow). Ad, adenovirus; GFP, green fluorescent protein; hBMP7, human bone morphogenetic protein 7.
Figure 9.
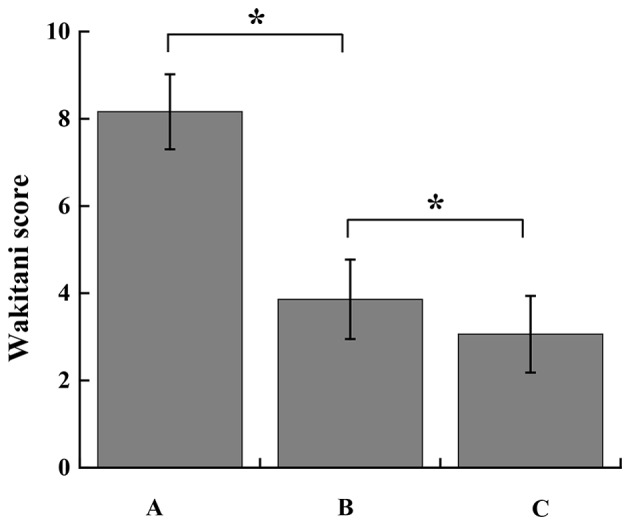
Repair assessment of the groups by the Wakitani scoring method. Results are presented as mean ± standard deviation (n=4). *P<0.05 as indicated. Group A, untreated cartilage defect; group B, cartilage defect treated with Matrigel scaffold-engineered Ad-mock-GFP-transfected chondrocytes; group C, cartilage defect treated with Matrigel scaffold-engineered Ad-hBMP7-GFP-transfected chondrocytes. Ad, adenovirus; GFP, green fluorescent protein; hBMP7, human bone morphogenetic protein 7.
Figure 10.
Repair of rabbit knee cartilage assessed by hematoxylin and eosin staining. Rabbit knee cartilage specimens from (A) group C (magnification, ×120), (B) group C (magnification, ×300); (C) group B (magnification, ×120) and (D) group B (magnification, ×300). Group B knees were treated with Matrigel scaffold-engineered Ad-mock-GFP-transfected chondrocytes, and group C knees were treated with Matrigel scaffold-engineered Ad-hBMP7-GFP-transfected chondrocytes. (A and C) Scale bar, 100 µm; (B and D) scale bar, 250 µm. Black arrows, site of transplantation; yellow arrows, edge of transplantation site. Ad, adenovirus; GFP, green fluorescent protein; hBMP7, human bone morphogenetic protein 7.
Discussion
Cartilage repair and regeneration is a major challenge associated with joint diseases, particularly for large-scale cartilage defects. Autologous chondrocyte transplantation is a promising method for cartilage repair, even though the implanted chondrocytes may undergo a loss of phenotype. As important members of the TGF-β superfamily, BMP proteins including BMP2, BMP6, BMP9, BMP12 and BMP13 have been shown to be helpful in maintaining the characteristics of chondrocytes in vitro (25–27). In the present study, it was found that hBMP7 is able to promote the secretion of collagen type II and hyaluronic acid from chondrocytes and is very helpful for cartilage regeneration. A recent study demonstrated that locally produced BMP7 is a prerequisite for postnatal synovial joint homeostasis and may be involved in osteoarthritic changes in BMP7-knockout mice (28). Another study showed that BMP7 has a protective function at the site of joint inflammation (29).
The US Food and Drug Administration has approved the application of recombinant human BMP7 in a clinical trial for the treatment of bone-related diseases (30). However, due to the high price and short half-life in vivo, continuation of this trial is challenging. The development of a safe and feasible gene therapy that guarantees the stable expression of the target cytokine gene in human cells is essential. In the present study, hBMP7 was successfully overexpressed in chondrocytes by an adenovirus-based transfection technique. Previously, adenovirus-based transfection systems have been used to regulate the immune response by inducing target gene expression (31,32). Such systems have been proposed as effective and safe gene therapies for humans. Previous observations together with the findings of the present study indicate that adenovirus-based transfection techniques may provide a new low-cost, long-term treatment for cartilage repair in which a suitable amount of target proteins can be produced in vivo.
In addition to specific cytokines, the effectiveness of a 3D culture scaffold also sheds light on cartilage repair (13,16,24,33). In the present study, Matrigel significantly outperformed C/GP gel in chondrocyte culture by slightly increasing the growth rate of chondrocytes, markedly improving the cell morphology and providing a higher yield of collagen type II and hyaluronic acid. These results are consistent with the strong performance of Matrigel with other cell lines, including cancer stem-like cells and mesenchymal stem cells (14,34).
In conclusion, in the present study, a specific cytokine and 3D culture system were combined together by growing Ad-hBMP7-GFP-transfected chondrocytes with Matrigel. Notably, following the transplantation of the Matrigel scaffold containing Ad-hBMP7-GFP-transfected chondrocytes into cartilage defects in rabbit knees, the cartilage defects were almost healed within 4 weeks. This positive result lays a good foundation for the treatment of cartilage repair in the future.
Glossary
Abbreviations
- ACT
autologous chondrocyte transplantation
- BMP7
bone morphogenetic protein 7
- C/GP
chitosan/glycerophosphate
- FGF-2
fibroblast growth factor 2
- TGF-β
transforming growth factor β
References
- 1.Widuchowski W, Lukasik P, Kwiatkowski G, Faltus R, Szyluk K, Widuchowski J, Koczy B. Isolated full thickness chondral injuries. Prevalance and outcome of treatment. A retrospective study of 5233 knee arthroscopies. Acta Chir Orthop Traumatol Cech. 2008;75:382–386. [PubMed] [Google Scholar]
- 2.Luyten FP. A scientific basis for the biologic regeneration of synovial joints. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:167–169. doi: 10.1016/S1079-2104(97)90109-8. [DOI] [PubMed] [Google Scholar]
- 3.Recommendations for the medical management of osteoarthritis of the hip and knee, corp-author. 2000 update. American College of rheumatology subcommittee on osteoarthritis guidelines. Arthritis Rheum. 2000;43:1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 4.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 5.Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 7.Dell'Accio F, De Bari C, Luyten FP. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum. 2001;44:1608–1619. doi: 10.1002/1529-0131(200107)44:7<1608::AID-ART284>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 8.Rui YF, Du L, Wang Y, Wang Y, Lui PP, Tang TT, Chan KM, Dai KR. Bone morphogenetic protein 2 promotes transforming growth factor β3-induced chondrogenesis of human osteoarthritic synovium-derived stem cells. Chin Med J (Engl) 2010;123:3040–3048. [PubMed] [Google Scholar]
- 9.Otsuka Y, Mizuta H, Takagi K, Iyama K, Yoshitake Y, Nishikawa K, Suzuki F, Hiraki Y. Requirement of fibroblast growth factor signaling for regeneration of epiphyseal morphology in rabbit full-thickness defects of articular cartilage. Dev Growth Differ. 1997;39:143–56. doi: 10.1046/j.1440-169X.1997.t01-1-00003.x. [DOI] [PubMed] [Google Scholar]
- 10.Shida J, Jingushi S, Izumi T, Iwaki A, Sugioka Y. Basic fibroblast growth factor stimulates articular cartilage enlargement in young rats in vivo. J Orthop Res. 1996;14:265–272. doi: 10.1002/jor.1100140215. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi H, Kurokawa T, Hanada K, Hiyama Y, Tamura M, Ogata E, Matsumoto T. Stimulation of fracture repair by recombinant human basic fibroblast growth factor in normal and streptozotocin-diabetic rats. Endocrinology. 1994;135:774–781. doi: 10.1210/en.135.2.774. [DOI] [PubMed] [Google Scholar]
- 12.Chuma H, Mizuta H, Kudo S, Takagi K, Hiraki Y. One day exposure to FGF-2 was sufficient for the regenerative repair of full-thickness defects of articular cartilage in rabbits. Osteoarthritis Cartilage. 2004;12:834–842. doi: 10.1016/j.joca.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Guo CA, Liu XG, Huo JZ, Jiang C, Wen XJ, Chen ZR. Novel gene-modified-tissue engineering of cartilage using stable transforming growth factor-beta1-transfected mesenchymal stem cells grown on chitosan scaffolds. J Biosci Bioeng. 2007;103:547–556. doi: 10.1263/jbb.103.547. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura S, Wakitani S, Kimura T, Maeda A, Caplan AI, Shino K, Ochi T. Articular cartilage repair. Rabbit experiments with a collagen gel-biomatrix and chondrocytes cultured in it. Acta Orthop Scand. 1998;69:56–62. doi: 10.3109/17453679809002358. [DOI] [PubMed] [Google Scholar]
- 16.Xu JW, Zaporojan V, Peretti GM, Roses RE, Morse KB, Roy AK, Mesa JM, Randolph MA, Bonassar LJ, Yaremchuk MJ. Injectable tissue-engineered cartilage with different chondrocyte sources. Plast Reconstr Surg. 2004;113:1361–1371. doi: 10.1097/01.PRS.0000111594.52661.29. [DOI] [PubMed] [Google Scholar]
- 17.Cullinane DM, Lietman SA, Inoue N, Deitz LW, Chao EY. The effect of recombinant human osteogenic protein-1 (bone morphogenetic protein-7) impregnation on allografts in a canine intercalary bone defect. J Orthop Res. 2002;20:1240–1245. doi: 10.1016/S0736-0266(02)00056-6. [DOI] [PubMed] [Google Scholar]
- 18.Mason JM, Breitbart AS, Barcia M, Porti D, Pergolizzi RG, Grande DA. Cartilage and bone regeneration using gene-enhanced tissue engineering. Clin Orthop Relat Res. 2000:S171–S178. doi: 10.1097/00003086-200010001-00023. (379 Suppl) [DOI] [PubMed] [Google Scholar]
- 19.Bai X, Li G, Zhao C, Duan H, Qu F. BMP7 induces the differentiation of bone marrow-derived mesenchymal cells into chondrocytes. Med Biol Eng Comput. 2011;49:687–692. doi: 10.1007/s11517-010-0729-4. [DOI] [PubMed] [Google Scholar]
- 20.Stöve J, Schneider-Wald B, Scharf HP, Schwarz ML. Bone morphogenetic protein 7 (bmp-7) stimulates proteoglycan synthesis in human osteoarthritic chondrocytes in vitro. Biomed Pharmacother. 2006;60:639–643. doi: 10.1016/j.biopha.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi M, Muneta T, Ju YJ, Mochizuki T, Sekiya I. Weekly intra-articular injections of bone morphogenetic protein-7 inhibits osteoarthritis progression. Arthritis Res Ther. 2008;10:R118. doi: 10.1186/ar2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi T, Muneta T, Tsuji K, Sekiya I. BMP-7 inhibits cartilage degeneration through suppression of inflammation in rat zymosan-induced arthritis. Cell Tissue Res. 2011;344:321–332. doi: 10.1007/s00441-011-1154-1. [DOI] [PubMed] [Google Scholar]
- 23.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Kievit FM, Florczyk SJ, Leung MC, Veiseh O, Park JO, Disis ML, Zhang M. Chitosan-alginate 3D scaffolds as a mimic of the glioma tumor microenvironment. Biomaterials. 2010;31:5903–5910. doi: 10.1016/j.biomaterials.2010.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gooch KJ, Blunk T, Courter DL, Sieminski AL, Vunjak-Novakovic G, Freed LE. Bone morphogenetic proteins-2-12 and −13 modulate in vitro development of engineered cartilage. Tissue Eng. 2002;8:591–601. doi: 10.1089/107632702760240517. [DOI] [PubMed] [Google Scholar]
- 26.Blunk T, Sieminski AL, Appel B, Croft C, Courter DL, Chieh JJ, Goepferich A, Khurana JS, Gooch KJ. Bone morphogenetic protein 9: A potent modulator of cartilage development in vitro. Growth Factors. 2003;21:71–77. doi: 10.1080/0897719031000148822. [DOI] [PubMed] [Google Scholar]
- 27.Bobacz K, Gruber R, Soleiman A, Erlacher L, Smolen JS, Graninger WB. Expression of bone morphogenetic protein 6 in healthy and osteoarthritic human articular chondrocytes and stimulation of matrix synthesis in vitro. Arthritis Rheum. 2003;48:2501–2508. doi: 10.1002/art.11248. [DOI] [PubMed] [Google Scholar]
- 28.Abula K, Muneta T, Miyatake K, Yamada J, Matsukura Y, Inoue M, Sekiya I, Graf D, Economides AN, Rosen V, Tsuji K. Elimination of BMP7 from the developing limb mesenchyme leads to articular cartilage degeneration and synovial inflammation with increased age. FEBS Lett. 2015;589:1240–1248. doi: 10.1016/j.febslet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C, Li Y, Tang W, Kamiya N, Kim H. Lactoferrin activates BMP7 gene expression through the mitogen-activated protein kinase ERK pathway in articular cartilage. Biochem Biophys Res Commun. 2013;431:31–35. doi: 10.1016/j.bbrc.2012.12.111. [DOI] [PubMed] [Google Scholar]
- 30.White AP, et al. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop. 2007;31:735–741. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thacker EE, Nakayama M, Smith BF, Bird RC, Muminova Z, Strong TV, Timares L, Korokhov N, O'Neill AM, de Gruijl TD, et al. A genetically engineered adenovirus vector targeted to CD40 mediates transduction of canine dendritic cells and promotes antigen-specific immune responses in vivo. Vaccine. 2009;27:7116–7124. doi: 10.1016/j.vaccine.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vorburger SA, Hunt KK. Adenoviral gene therapy. Oncologist. 2002:46–59. doi: 10.1634/theoncologist.7-1-46. [DOI] [PubMed] [Google Scholar]
- 33.Hunziker EB. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 34.Yeung TM, Gandhi SC, Wilding JL, Muschel R, Bodmer WF. Cancer stem cells from colorectal cancer-derived cell lines. Proc Natl Acad Sci USA. 2010;107:3722–3727. doi: 10.1073/pnas.0915135107. [DOI] [PMC free article] [PubMed] [Google Scholar]