Abstract
Forkhead box P3 (FOXP3), which is a transcription factor, has a primary role in the development and function of regulatory T cells, and thus contributes to homeostasis of the immune system. A previous study generated a cell-permeable fusion protein of mouse FOXP3 conjugated to a protein transduction domain (PTD-mFOXP3) that successfully blocked differentiation of type 17 T helper cells in vitro and alleviated experimental arthritis in mice. In the present study, the role of PTD-mFOXP3 in type 1 T helper (Th1) cell-mediated immunity was investigated and the possible mechanisms for its effects were explored. Under Th1 polarization conditions, cluster of differentiation 4+ T cells were treated with PTD-mFOXP3 and analyzed by flow cytometry in vitro, which revealed that PTD-mFOXP3 blocked Th1 differentiation in vitro. Mice models of delayed type hypersensitivity (DTH) reactions were generated by subcutaneous sensitization and challenge with ovalbumin (OVA) to the ears of mice. PTD-mFOXP3, which was administered via local subcutaneous injection, significantly reduced DTH-induced inflammation, including ear swelling (ear swelling, P<0.001; pinnae weight, P<0.05 or P<0.01 with 0.25 and 1.25 mg/kg PTD-mFOXP3, respectively), infiltration of T cells, and expression of interferon-γ at local inflammatory sites (mRNA level P<0.05) compared with the DTH group. The results of the present study demonstrated that PTD-mFOXP3 may attenuate DTH reactions by suppressing the infiltration and activity of Th1 cells.
Keywords: protein transduction domain, forkhead box P3, type 1 T helper cell, interferon-γ, delayed-type hypersensitivity
Introduction
Regulatory T cells (Tregs) are essential for maintaining self-tolerance and homeostasis of the immune system. In addition, the numbers and activity of Tregs are decreased in the development of a number of autoimmune diseases and Tregs may be used therapeutically for these disorders. Previously, van Amelsfort et al (1) demonstrated that cluster of differentiation (CD) 4+CD25+ Tregs are present and functional in patients with rheumatoid arthritis, with increased numbers of Tregs and Tregs with increased suppressive activity detected in the synovial fluid compared with the peripheral blood. Tregs were depleted in DBA/1 mice with collagen-induced arthritis by injection of an anti-CD25 monoclonal antibody, resulting in more severe disease, which could be reversed by adoptive transfer of CD4+ CD25+ Tregs (2). Forkhead box P3 (FOXP3), which is a transcription factor, has a primary role in Treg development and function (3,4). There have been numerous reports of the association between type 1 T helper (Th1) cells and Tregs (5–7). It has been demonstrated that interferon regulatory factor (IRF) −1, a pleiotropic transcription factor, is implicated in the regulation of Th1 development (5). Fragale et al (5) reported that IRF-1 negatively regulates CD4+CD25+ Treg development and function, by specifically repressing FOXP3 expression. Furthermore, it has been shown that Th1 effector molecules and transcription factors participate in the control of peripheral Treg generation (6). In the presence of interferon-γ (IFN-γ) Treg generation was inhibited; however, adaptive transfer of IFN-γ or signal transducer and activator of transcription 1 (STAT1) knockout T cells resulted in an increase in the quantity of Tregs (6). Ouaked et al (7) analyzed the effect of Th1 cytokines on human Treg differentiation at the gene expression and epigenetic levels, which revealed a mechanism by which the STAT1-activating cytokines interleukin-27 (IL-27) and IFN-γ increased transforming growth factor-β-induced FOXP3 expression.
The protein transduction domain (PTD), or cell-penetrating peptide, has been shown to efficiently deliver peptides, proteins, polyanionic oligonucleotides and other ‘cargo’ into cells for the diagnosis of disease or for disease therapy, in vitro and in vivo (8–12). To date, a number of PTDs have been found, containing sequences from the transactivator of transcription (TAT), herpes simplex virus protein VP22, Drosophila Antennapedia transcription factor (Antp) and biotinylated hepta-D-arginine (biotin R7) (13). The TAT sequence YGRKKRRQRRR, from human immunodeficiency virus-1, is a commonly used, powerful and safe delivery PTD that transduces proteins into cells. Numerous PTD fusion proteins have been generated, including the PTD-neuroglobin fusion protein, which is able to protect primary cortical neurons against hypoxia-induced injury (8).
A previous study performed by the present authors successfully generated a PTD-conjugated mouse FOXP3 protein (PTD-mFOXP3) that was able to convert CD4+CD25− T cells into Treg-like cells, block the type 17 T helper (Th17) cells differentiation program in vitro, and markedly delay disease incidence and alleviate the autoimmune symptoms of mice with collagen-induced arthritis (14). The present study aims to further investigate the role of PTD-mFOXP3 in the regulation of Th1 cell polarization and in Th1-mediated immunity in a murine model of delayed-type hypersensitivity (DTH).
Materials and methods
Experimental mice
A total of 90 male C57BL/6 (MHC haplotype; H-2b; age, 6–8 weeks; weight, 20±2 g) mice were purchased from the Comparative Medicine Centre of Yangzhou University (Yangzhou, China). Mice were housed in microisolator units at 22–24°C and 30–50% humidity, under a 12-h light-dark cycle with ad libitum access to food and water. All animal studies were approved by the Institutional Animal Care and Use Committee of Jiangsu University (SYXK 2013-0036; Zhenjiang, China) and conformed to the Science and Technology Department of Jiangsu Province (Jiangsu, China) Guide for the Use and Care of Laboratory Animals.
Induction of Th1 differentiation in vitro
CD4+ T cells were isolated from the spleens of C57BL/6 mice via negative selection using a Dynal Mouse CD4 Negative Isolation kit (cat. no., 11415D, Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's instructions. Isolated CD4+ T cells were seeded into 96-well plates at a density of 3×105 cells/well in RPMI 1640 medium supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), and Th1 differentiation was induced by stimulation for 4 days at 37°C in an atmosphere containing 5% CO2, with plate-bound anti-CD3 (5 µg/ml; dilution, 1:200; cat. no., 100,314; BioLegend, Inc., San Diego, CA, USA) and soluble anti-CD28 (2 µg/ml; dilution, 1:500; cat. no., 102,112; BioLegend, Inc.) antibodies, in the presence of 10 ng/ml IL-12 (cat. no., 210-12; PeproTech, Inc., Rocky Hill, NJ, USA), 10 µg/ml anti-IL-4 (cat no., 504,122; BioLegend, Inc) and 100 U/ml IL-2 (cat. no., 212-12; PeproTech, Inc.). Subsequently, various concentrations (320, 640 and 1,280 nM) of PTD-mFOXP3 or mFOXP3 (1,280 nM), prepared as previously described (14,15), were added. On day 4, cells were i) harvested for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assays or ii) stimulated for 4 h with phorbol 12-myristate 13-acetate (50 ng/ml) and ionomycin (1 mg/ml; both Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) and treated with GolgiStop (BD Pharmingen, San Diego, CA, USA) for intracellular cytokine analysis by flow cytometry.
Mice models of DTH
Mice were randomly divided into the following groups (n=6/group): i) Non-sensitized and ovalbumin (OVA)-challenged group, negative control (NC) group; ii) OVA-sensitized and OVA-challenged (DTH) group; iii) 1.25 mg/kg mFOXP3-treated group; iv) low-dose (0.25 mg/kg) PTD-mFOXP3-treated group; and v) high-dose (1.25 mg/kg) PTD-mFOXP3-treated DTH group. With the exception of the NC group, mice were immunized on day 0 via subcutaneous injection of OVA (250 µg) in phosphate-buffered saline (PBS), emulsified 1:1 in complete Freund's adjuvant (total volume, 200 µl). On day 7, the ear pinnae of the mice were measured with a Mitutoyo micrometer (Kawasaki, Japan), immediately followed by OVA challenge. For OVA challenge, 400 mg OVA in 20 µl PBS was intradermally injected into the right ear pinnae. The left ear pinnae received 20 µl sterile PBS alone (negative control). Pinnae were measured 24 h later and the difference in size was used as a measure of DTH. Specific ear swelling, including measurements of thickness and weight, was measured using a micrometer (Mitutoyo Corporation, Kawasaki, Japan) with a resolution of 0.1 mm and calculated by the following formula: (ME24 h-ME0 h)-(MC24 h-MC0 h), where ME was the measurement for the experimental ear and MC the measurement for the negative control ear. In the protein-treated groups, mFOXP3 or PTD-mFOXP3 was administered via subcutaneous injection in the local area of the right ear 2 h prior to OVA sensitization on day 0, 2 h prior to OVA challenge on day 7, and on days 1, 3 and 5. In the NC and DTH groups, mice were instead treated with PBS in the same volumes. Following the experiment the mice were sacrificed by CO2 inhalation, and their spleens, pinnae and their draining lymph nodes were harvested for further measurements. The weight of the pinnae was measured using analytical balance (resolution, 0.1 mg).
RT-qPCR
Total RNA from CD4+ T cells, which were isolated from spleens using TRIzol reagent (Thermo Fisher Scientific, Inc.), was reverse transcribed into cDNA using a reverse transcriptase, ReverTra Ace (Toyobo Co., Ltd., Osaka, Japan). qPCR was subsequently performed using SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd., Dalian, China), with a final volume of 20 µl containing 10 µl SYBR Green mix reagent, sense and antisense primers (0.4 µl, 10 mM), cDNA (1 µl) and DNA-free water (8.2 µl). A CFX96 real-time thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to detect and quantify transcripts encoding IFN-γ or T-bet as follows: 95°C pre-degeneration for 5 min, and 95°C degeneration for 15 sec, annealing at 58°C for 30 sec and extension at 72°C for 35 sec for 40 cycles, and a final extension at 72°C for 10 sec, terminated at 4°C. Total RNA was isolated from pinnae using the NucleoSpin RNA II kit (Machery-Nagel GmbH, Düren, Germany). Primer sequences are listed in Table I. Expression levels of mRNA were calculated based on the standard curve for absolute quantification in RT-qPCR. The PCR products of target genes were inserted into pMD18-T Vectors (cat. no. 6011; Takara Biotechnology Co., Ltd.) following amplification with PCR. Copy numbers were determined based on plasmid concentration and plasmid DNA was aliquoted serially into successive, 10-fold diminishing dilutions, from 1×108 to 1×103 specific copies, in sterile water. Plasmid DNA at each copy number dilution was subsequently amplified in the same plate under identical conditions. A standard curve was subsequently generated and used to calculate the quantity of target genes in the samples (16). Data were normalized to the housekeeping gene β-actin. Results are presented as the mean ± standard deviation of triplicate measurements.
Table I.
Primer sequences and conditions of IFN-γ and T-bet quantitative polymerase chain reaction.
| Gene | Primer sequence (5′-3′) | Amplicon size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| Mouse β-actin | Sense: TGGAATCCTGTGGCATCCATGAAAC | 349 | 58.0 |
| Antisense: TAAAACGCAGCTCAGTAACAGTCCG | |||
| Mouse IFN-γ | Sense: AAGCGTCATTGAATCACACC | 202 | 58.0 |
| Antisense: CGAATCAGCAGCGACTCCTTAG | |||
| Mouse T-bet | Sense: AGGGAACCGCTTATATGTCCAC | 194 | 59.5 |
| Antisense: GCTCTCCATCATTCACCTCCAC |
IFN-γ, interferon-γ.
Flow cytometry
Purified CD4+ T cells from the spleen were treated with 1,280 nM mFOXP3 and various concentrations (320, 640, 1,280 nM) of PTD-mFOXP3 for 4 days. Subsequently, cells were harvested, stained with anti-CD4-fluorescein isothiocyanate (FITC) (dilution, 1:200; cat. no., 110,041; eBioscience, Inc., San Diego, CA, USA) and anti-IFN-γ-phycoerythrin (PE) (dilution, 1:100; cat. no., 127,311; eBioscience, Inc.), and analyzed by flow cytometry (BD FACSCalibur system; BD Biosciences, Franklin Lakes, NJ, USA). For the DTH model, cells from the pinnae draining lymph nodes were stained with anti-CD4-FITC, followed by fixation, permeabilization and staining with anti-FOXP3-PE.
Cell proliferation assay
Splenocytes (2×106/well) from each group were seeded into 48-well plates, followed by stimulation with or without OVA (50 µg/ml) for 72 h. MTT (5 mg/ml; 20 µl/well; cat. no., M2128; Sigma-Aldrich; Merck Millipore) was added to the culture 4 h prior to the end of the assay. The formazan formed in this reaction was dissolved with dimethylsulfoxide (150 µl/well). Absorbance intensity measured by a microplate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA) at 490 nm with a reference wavelength of 620 nm (17).
ELISA for IFN-γ concentration
Splenocytes (5×105/well) from each group were cultured in 48-well plates, followed by stimulation with or without OVA (50 µg/ml) for 72 h. Cells (2×105/well) isolated from pinnae draining lymph nodes after neck were cultured in 48-well plates, and cocultured with 20 µg/ml mitomycin C (MMC)-treated splenocytes (1×106/well; Kyowa Hakko Kirin Co. Ltd., Tokyo, Japan) and OVA (50 µg/ml) for 72 h. Supernatants were harvested and centrifuged at 350 × g at 4°C for 10 min. IFN-γ concentration was subsequently measured using a mouse IFN-γ ELISA Ready-SET-Go! kit (cat. no., 887,314; eBioscience, Inc.), according to the manufacturer's instructions.
Histopathological analysis
Pinnae were fixed with 10% neutral buffered formalin for 24 h, followed by decalcification for 2 days. Subsequently, tissue specimens were embedded in paraffin, cut into sections (thickness, 3–4 µm) and stained with hematoxylin and eosin (H&E) for routine histopathological analysis.
Statistical analysis
Data are presented as the mean ± standard deviation of triplicate readings for each treatment group and were calculated using Prism (version 5; GraphPad Software, Inc., La Jolla, CA, USA). Comparisons of the averages between experimental groups were analyzed using one-way analysis of variance. P<0.05 was considered to indicate a statistically significant difference.
Results
PTD-mFOXP3 inhibits IFN-γand T-bet production in vitro
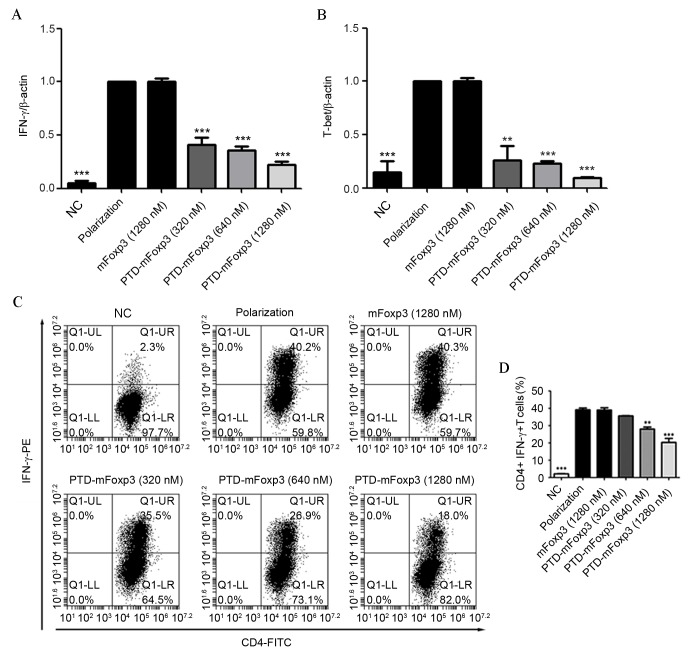
CD4+ T cells, isolated from the spleens of mice, were induced to differentiate into Th1 cells, and treated with mFOXP3 or PTD-mFOXP3. Results of this treatment were analyzed by RT-qPCR and flow cytometry (Fig. 1). It was observed that CD4+ T cells treated with all concentrations of PTD-mFOXP3 produced significantly less IFN-γ mRNA compared with the polarization group (P<0.001; Fig. 1A). In addition, as expected, PTD-mFOXP3 exposure significantly reduced the quantity of T-bet mRNA copies (P<0.01 at 320 nM, P<0.001 at 640 and 1,280 nM; Fig. 1B) and the percentage of IFN-γ-positive Th1 cells (P<0.01 at 640 nM, P<0.001 at 1,280 nM; Fig. 1C and D).
Figure 1.
PTD-mFOXP3 inhibits Th1 differentiation and IFN-γ production. Reverse transcription-quantitative polymerase chain reaction analysis of the mRNA expression levels of (A) IFN-γ and (B) T-bet in polarized CD4+ T cells. Data was normalized to the levels of the β-actin reference transcript. (C) Flow cytometry analysis of intracellular IFN-γ production by CD4+ T cells under Th1-polarizing conditions. (D) Percentage of CD4+IFN-γ+ T cells following PTD-mFOXP3 treatment. **P<0.01 and ***P<0.001 vs. the polarization group. NC, negative control group; IFN-γ, interferon-γ; mFOXP3, mouse forkhead box P3; PTD, protein transduction domain; PE, phycoerythrin; CD4, cluster of differentiation 4; FITC, fluorescein isothiocyanate.
PTD-mFOXP3 suppresses DTH reactions in vivo
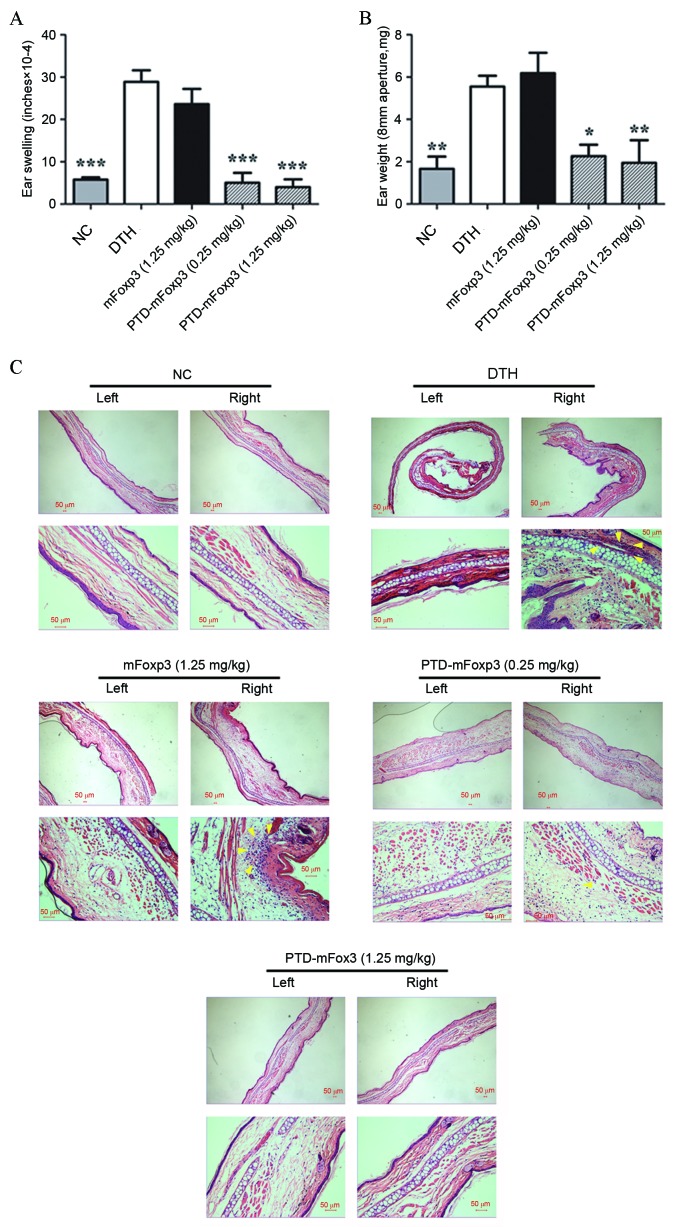
The effect of PTD-mFOXP3 on DTH reactions was investigated by measuring the pinnae swelling of mice sensitized and challenged with OVA (Fig. 2A and B). OVA challenge markedly increased the thickness of pinnae in OVA-sensitized mice compared with the nonsensitized NC group, indicating a successful DTH reaction induction. PTD-mFOXP3 exposure significantly suppressed pinnae swelling compared with the DTH group (P<0.001 at 0.25 mg/kg and 1.25 mg/kg PTD-mFOXP3, Fig. 2A; P<0.05 at 0.25 mg/kg PTD-mFOXP3, P<0.01 at 1.25 mg/kg PTD-mFOXP3, Fig. 2B). Histopathological examination with H&E stain revealed a marked infiltration of mononuclear cells into the subcutaneous tissues of the pinnae in the DTH and mFOXP3-treated groups; however, infiltration of mononuclear cells appeared to be diminished by PTD-mFOXP3 administration (Fig. 2C).
Figure 2.
Effect of PTD-mFOXP3 on the development of DTH in mice. DTH reactions in the ears of mice were induced by subcutaneous OVA-sensitization and challenge. (A) The change in thickness of ears following DTH. (B) The weight of pinnae aperture with diameter of 8 mm from each group following DTH. *P<0.05, **P<0.01 and ***P<0.001 vs. the DTH group. (C) Representative images of pathological sections of the left (control) and right (experimental) ear tissue from each group, stained with hematoxylin and eosin (upper panel magnification, ×100; lower panel magnification, ×400). Arrows indicate the infiltration of mononuclear cells. NC, negative control group; DTH, delayed type hypersensitivity; mFOXP3, mouse forkhead box P3; PTD, protein transduction domain; OVA, ovalbumin.
PTD-mFOXP3 alleviates the severity of DTH by upregulating Tregs
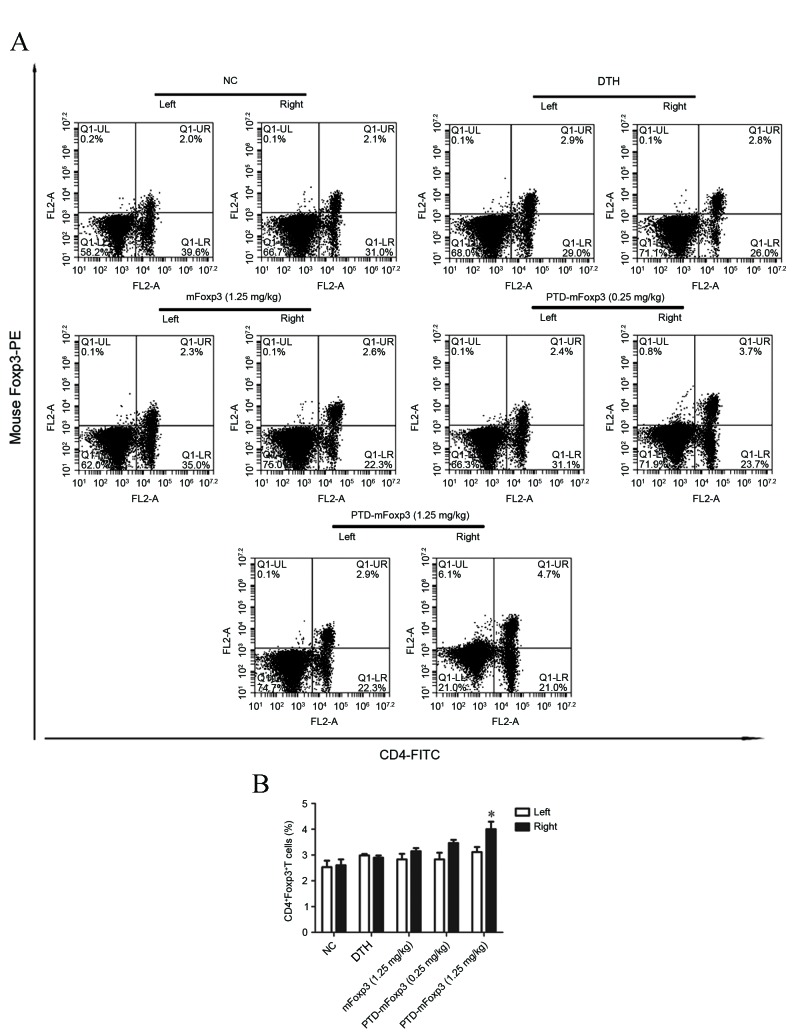
To characterize the types of inflammatory cells affected by PTD-mFOXP3, the expression of IFN-γ and T-bet in the pinnae was measured by RT-qPCR. This identified that IFN-γ and T-bet mRNA expression levels were upregulated in the DTH and mFOXP3-treated groups compared with the NC group, while PTD-mFOXP3 significantly suppressed the mRNA expression levels of IFN-γ and T-bet compared with the DTH group (P<0.05, Fig. 3A; P<0.001, Fig. 3B). Lymphocytes from the pinnae draining lymph nodes were stimulated with OVA and MMC-treated splenocytes to induce IFN-γ protein expression, which was measured by ELISA. The results determined that PTD-mFOXP3 significantly suppressed IFN-γ protein expression compared with the DTH group (0.25 mg/kg, P<0.01; 1.25 mg/kg, P<0.001) in pinnae lymphocytes (Fig. 3C). Then, the number of Th1 (CD4+IFN-γ+) and Treg (CD4+FOXP3+) cells was measured by flow cytometry. There was no significant difference in the quantity if Th1 cells between different groups (data not shown). However, the percentage of Tregs was significantly increased in mice treated with PTD-mFOXP3 (1.25 mg/kg) compared with the DTH group (P<0.05; Fig. 4).
Figure 3.
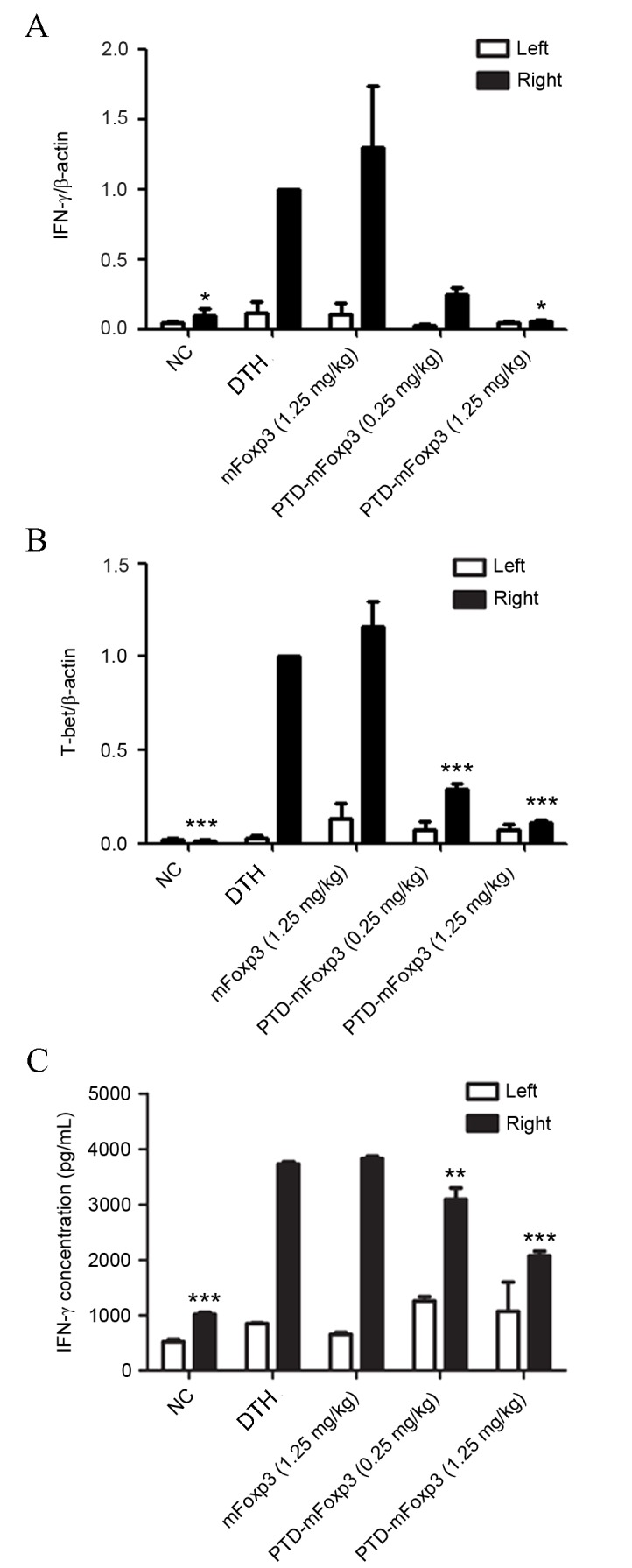
Effect of PTD-mFOXP3 on the production of IFN-γ by the pinna. Left (control) and right (experimental) ear pinnae were harvested and the expression levels of (A) IFN-γ and (B) T-bet were assessed by reverse transcription-quantitative polymerase chain reaction analysis. Data was normalized to the levels of the β-actin reference transcript. (C) IFN-γ concentration, as measured by ELISA, in lymphocytes isolated from pinnae draining lymph nodes that were stimulated for 72 h with OVA and MMC-treated splenocytes. *P<0.05, **P<0.01 and ***P<0.001 vs. the DTH group. NC, negative control group; DTH, delayed type hypersensitivity; IFN-γ, interferon-γ; mFOXP3, mouse forkhead box P3; PTD, protein transduction domain.
Figure 4.
Effect of PTD-mFOXP3 on the percentage of CD4+Foxp3+ T cells from pinnae draining lymph nodes. Lymphocytes were obtained from the left (control) and right (experimental) pinnae draining lymph nodes and analyzed by flow cytometry for CD4+ FOXP3+ T cells. (A) Representative dot plots showing the distribution of CD4+FOXP3+ T cells. (B) Percentage of CD4+FOXP3+ T cells in each group. *P<0.05 vs. the DTH group. NC, negative control group; DTH, delayed type hypersensitivity; IFN-γ, interferon-γ; mFOXP3, mouse forkhead box P3; PTD, protein transduction domain; PE, phycoerythrin; CD4, cluster of differentiation 4; FITC, fluorescein isothiocyanate; OVA, ovalbumin.
Differential effects of PTD-mFOXP3 on T cell functionality
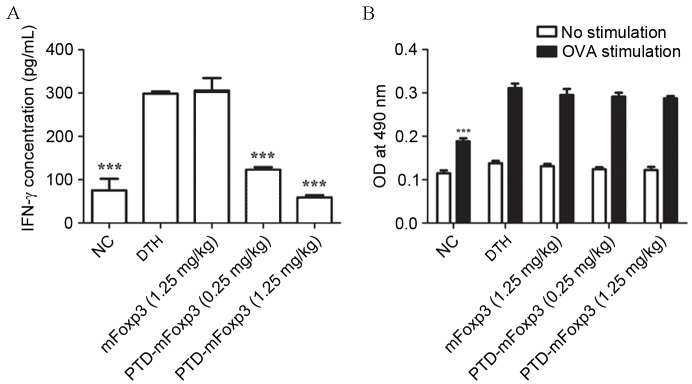
Cytokines expressed by T cells serve an essential role in dictating antigen-specific humoral and cell-mediated immune reactions. To investigate the influence of PTD-mFOXP3 on the functionality of T cells in DTH reactions, splenocytes harvested from the different groups were stimulated with OVA for 72 h to induce IFN-γ production. IFN-γ production was markedly increased in the DTH group compared with the NC group (Fig. 5A), demonstrating successful induction of antigen-specific T cell reactivity. Consistent with the reduced ear swelling observed previously, PTD-mFOXP3 (0.25 and 1.25 mg/kg) significantly suppressed OVA-induced IFN-γ production compared with the DTH group (P<0.001; Fig. 5A). The cell proliferation of OVA-stimulated splenocytes was then examined using the MTT assay. The results of this assay showed that splenocyte viability was unaltered in mice treated with PTD-mFOXP3 (Fig. 5B).
Figure 5.
Differential effects of PTD-mFOXP3 on T cell functionality. Splenocytes isolated from each group were cultured in the presence or absence of OVA (50 µg/ml) for 72 h. (A) Expression of IFN-γ of splenocytes was measured by ELISA. (B) Viability of splenocytes was determined by MTT assay. ***P<0.001 vs. the DTH group. NC, negative control group; DTH, delayed type hypersensitivity group; IFN-γ, interferon-γ; mFOXP3, mouse forkhead box P3; PTD, protein transduction domain; OVA, ovalbumin; OD, optical density.
Discussion
Th1 cells, polarized from CD4+ T cells, contribute to the anti-tumor response and the elimination of intracellular pathogens, such as Mycobacterium tuberculosis, Human cytomegalovirus and Trypanosoma cruzi (18–22). However, Th1 cells have important roles in the pathogenesis of numerous diseases, including transplant rejection, autoimmune diseases and hypersensitivity (23–27). Conversely, Tregs have a critical role in maintaining immune homeostasis, controlling autoimmune diseases, and inhibiting excessive immune responses against microbes and allergens. It has previously been demonstrated that Tregs are able to downregulate Th1 function, reverse pre-established autoimmune pathology and may be used in the treatment of autoimmune diseases (28,29). Unfortunately, the adoptive transfer of Tregs may convert them Th1-like Treg or Th17 in the local microenvironment of autoimmune disease and loss of the function of Tregs (30,31). Cyclosporine and FK506 are immunosuppressants that target Th1 cells and can be used to prevent transplantation rejection and reduce the effects of autoimmune diseases; however, they have severe hepatotoxicity, nephrotoxicity and neurotoxicity and are unable to expand the numbers of Treg (32). Therefore, it is important to develop a novel method to treat autoimmune diseases and prevent transplant rejection.
The transcription factor, FOXP3, has a primary role in the development and function of Tregs, thus contributing to immune homeostasis. A previous study reported that PTD-mFOXP3 was able to block Th17 differentiation in vitro and downregulate T cell IL-17 production, through modulating levels of RAR-related orphan receptor γt (RORγt) (14). Furthermore, PTD-mFOXP3 decreased IL-2 and IFN-γ expression in activated CD4+CD25− T cells (14). In addition, another study found that a fusion protein of PTD-hFOXP3 had a similar function (15). IFN-γ is a key cytokine expressed by Th1 cells. The present study hypothesized that PTD-mFOXP3 was able to block Th1 differentiation and cell function. The results of the present study demonstrated that PTD-mFOXP3 inhibits Th1 cell generation, and downregulates the expression of Th1-associated cytokines (IFN-γ) and transcripts (T-bet). Th1 cells serve a primary role in triggering the antigen-specific immune response in DTH reactions. Therefore, it was hypothesized that PTD-mFOXP3 may inhibit DTH reactions. This hypothesis was substantiated by the results of the present study, which demonstrated that OVA-induced ear swelling was suppressed by the administration of PTD-mFOXP3. In addition, T cell infiltration and IFN-γ expression in the pinnae was attenuated by PTD-mFOXP3 treatment. Furthermore, OVA-induced IFN-γ production was also suppressed by PTD-mFOXP3 exposure.
In conclusion, PTD-mFOXP3 downregulates the development and function of Th1 cells. Therefore, PTD-mFOXP3 has the potential to be developed as a treatment for DTH reactions and other diseases associated with the Th1 response. Future investigation into the mechanism by which PTD-mFOXP3 exerts its effects on Th1 cells is required.
Acknowledgements
The present study was supported by the following grants: The National Natural Science Foundation of China (grant nos. 81273202, 31200676, 31400773, 81172834 and 81671541), the Natural Science Foundation of the Jiangsu Higher Education Institution of China (grant no. 14KJB320001), Senior Talents Scientific Research Foundation (Jiangsu University, Jiangsu, China; grant no. 14JDG042) Jiangsu Province Postdoctoral Research Foundation of China (grant no. 1402170C), Clinical Medicine, Science & Technology Project (Jiangsu, China; grant no. BL2013024), Program of Innovative Research Team of Jiangsu Province, Project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Key Academic Program Development of Jiangsu University (grant no. 1291270019).
Glossary
Abbreviations
- DTH
delayed-type hypersensitivity
- Treg
regulatory T cell
- FOXP3
forkhead box P3
- PTD
protein transduction domain
References
- 1.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: Differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–2785. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 2.Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, Snijders A, Offringa R, de Vries RR, Toes RE. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–1460. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 3.Lowther DE, Hafler DA. Regulatory T cells in the central nervous system. Immunol Rev. 2012;248:156–169. doi: 10.1111/j.1600-065X.2012.01130.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol. 2011;23:424–430. doi: 10.1016/j.smim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Fragale A, Gabriele L, Stellacci E, Borghi P, Perrotti E, Ilari R, Lanciotti A, Remoli AL, Venditti M, Belardelli F, Battistini A. IFN regulatory factor-1 negatively regulates CD4+ CD25+ regulatory T cell differentiation by repressing Foxp3 expression. J Immunol. 2008;181:1673–1682. doi: 10.4049/jimmunol.181.3.1673. [DOI] [PubMed] [Google Scholar]
- 6.Caretto D, Katzman SD, Villarino AV, Gallo E, Abbas AK. Cutting edge: The Th1 response inhibits the generation of peripheral regulatory T cells. J Immunol. 2010;184:30–34. doi: 10.4049/jimmunol.0903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouaked N, Mantel PY, Bassin C, Burgler S, Siegmund K, Akdis CA, Schmidt-Weber CB. Regulation of the foxp3 gene by the Th1 cytokines: The role of IL-27-induced STAT1. J Immunol. 2009;182:1041–1049. doi: 10.4049/jimmunol.182.2.1041. [DOI] [PubMed] [Google Scholar]
- 8.Zhou G, Shan P, Hu X, Zheng X, Zhou S. Neuroprotective effect of TAT PTD-Ngb fusion protein on primary cortical neurons against hypoxia-induced apoptosis. Neurol Sci. 2013;34:1771–1778. doi: 10.1007/s10072-013-1333-9. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Thompson JD, Chan WK. A cell-penetrating peptide suppresses the hypoxia inducible factor-1 function by binding to the helix-loop-helix domain of the aryl hydrocarbon receptor nuclear translocator. Chem Biol Interact. 2013;203:401–411. doi: 10.1016/j.cbi.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim KS, Cha MJ, Kim JK, Park EJ, Chae JW, Rhim T, Hwang KC, Kim YH. Protective effects of protein transduction domain-metallothionein fusion proteins against hypoxia- and oxidative stress-induced apoptosis in an ischemia/reperfusion rat model. J Control Release. 2013;169:306–312. doi: 10.1016/j.jconrel.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Pan J, Su Y, Hou X, He H, Liu S, Wu J, Rao P. Protective effect of recombinant protein SOD-TAT on radiation-induced lung injury in mice. Life Sci. 2012;91:89–93. doi: 10.1016/j.lfs.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Wadia J, Eguchi A, Dowdy SF. DNA delivery into mammalian cells using bacteriophage λ displaying the TAT transduction domain. Cold Spring Harb Protoc. 20132013 doi: 10.1101/pdb.prot072660. pii: pdb.prot072660. [DOI] [PubMed] [Google Scholar]
- 13.Rothbard JB, Garlington S, Lin Q, Kirschberg T, Kreider E, McGrane PL, Wender PA, Khavari PA. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat Med. 2000;6:1253–1257. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Ji B, Sun M, Wu W, Huang L, Sun A, Zong Y, Xia S, Shi L, Qian H, et al. Cell-penetrable mouse forkhead box protein 3 alleviates experimental arthritis in mice by up-regulating regulatory T cells. Clin Exp Immunol. 2015;181:87–99. doi: 10.1111/cei.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Xu X, Lin X, Tian Y, Ji B, Xia S, Xu S, Yin Q, Zhang M, Jiao Z, et al. PTD-hFOXP3 protein acts as an immune regulator to convert human CD4(+)CD25(−) T cells to regulatory T-like cells. J Cell Biochem. 2012;113:3797–3809. doi: 10.1002/jcb.24255. [DOI] [PubMed] [Google Scholar]
- 16.Lee C, Kim J, Shin SG, Hwang S. Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J Biotechnol. 2006;123:273–280. doi: 10.1016/j.jbiotec.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Zhao XQ, Wang TX, Liu W, Wang CD, Wang D, Shang T, Shen LH, Ren L. Multifunctional Au@IPN-pNIPAAm nanogels for cancer cell imaging and combined chemo-photothermal treatment. J Mater Chem. 2011;21:7240–7247. doi: 10.1039/c1jm10277j. [DOI] [Google Scholar]
- 18.González FB, Villar SR, Fernández Bussy R, Martin GH, Pérol L, Manarin R, Spinelli SV, Pilon C, Cohen JL, Bottasso OA, et al. Immunoendocrine dysbalance during uncontrolled T. cruzi infection is associated with the acquisition of a Th-1-like phenotype by Foxp3(+) T cells. Brain Behav Immun. 2015;45:219–232. doi: 10.1016/j.bbi.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wunsch M, Zhang W, Hanson J, Caspell R, Karulin AY, Recks MS, Kuerten S, Sundararaman S, Lehmann PV. Characterization of the HCMV-Specific CD4 T cell responses that are associated with protective immunity. Viruses. 2015;7:4414–4437. doi: 10.3390/v7082828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaCasse CJ, Janikashvili N, Larmonier CB, Alizadeh D, Hanke N, Kartchner J, Situ E, Centuori S, Har-Noy M, Bonnotte B, et al. Th-1 lymphocytes induce dendritic cell tumor killing activity by an IFN-γ-dependent mechanism. J Immunol. 2011;187:6310–6317. doi: 10.4049/jimmunol.1101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janikashvili N, LaCasse CJ, Larmonier C, Trad M, Herrell A, Bustamante S, Bonnotte B, Har-Noy M, Larmonier N, Katsanis E. Allogeneic effector/memory Th-1 cells impair FoxP3+ regulatory T lymphocytes and synergize with chaperone-rich cell lysate vaccine to treat leukemia. Blood. 2011;117:1555–1564. doi: 10.1182/blood-2010-06-288621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeyanathan M, McCormick S, Lai R, Afkhami S, Shaler CR, Horvath CN, Damjanovic D, Zganiacz A, Barra N, Ashkar A, et al. Pulmonary M. tuberculosis infection delays Th1 immunity via immunoadaptor DAP12-regulated IRAK-M and IL-10 expression in antigen-presenting cells. Mucosal Immunol. 2014;7:670–683. doi: 10.1038/mi.2013.86. [DOI] [PubMed] [Google Scholar]
- 23.Guidry TV, Hunter RL, Jr, Actor JK. Mycobacterial glycolipid trehalose 6,6′-dimycolate-induced hypersensitive granulomas: Contribution of CD4+ lymphocytes. Microbiology. 2007;153:3360–3369. doi: 10.1099/mic.0.2007/010850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rau CS, Lin MW, Wu SC, Wu YC, Lu TH, Tzeng SL, Chen YC, Wu CJ, Hsieh CH. Regulatory and effector helper T-cell profile after nerve xenografting in the toll-like receptor-deficient mice. Int J Med Sci. 2015;12:650–654. doi: 10.7150/ijms.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu X, Cao W, Ma H, Feng J, Li X, Zhang X. Acitretin exerted a greater influence on T-helper (Th)1 and Th17 than on Th2 cells in treatment of psoriasis vulgaris. J Dermatol. 2012;39:916–921. doi: 10.1111/j.1346-8138.2012.01637.x. [DOI] [PubMed] [Google Scholar]
- 26.Skurkovich S, Skurkovich B. Anticytokine therapy, especially anti-interferon-gamma, as a pathogenetic treatment in TH-1 autoimmune diseases. Ann N Y Acad Sci. 2005;1051:684–700. doi: 10.1196/annals.1361.113. [DOI] [PubMed] [Google Scholar]
- 27.Inukai Y, Momobayashi A, Sugawara N, Aso Y. Changes in expression of T-helper (Th) 1- and Th2-associated chemokine receptors on peripheral blood lymphocytes and plasma concentrations of their ligands, interferon-inducible protein-10 and thymus and activation-regulated chemokine, after antithyroid drug administration in hyperthyroid patients with Graves' disease. Eur J Endocrinol. 2007;156:623–630. doi: 10.1530/EJE-07-0019. [DOI] [PubMed] [Google Scholar]
- 28.Olguin JE, Fernández J, Salinas N, Juárez I, Rodriguez-Sosa M, Campuzano J, Castellanos C, Saavedra R. Adoptive transfer of CD4(+)Foxp3(+) regulatory T cells to C57BL/6J mice during acute infection with Toxoplasma gondii down modulates the exacerbated Th1 immune response. Microbes Infect. 2015;17:586–595. doi: 10.1016/j.micinf.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Mahne AE, Klementowicz JE, Chou A, Nguyen V, Tang Q. Therapeutic regulatory T cells subvert effector T cell function in inflamed islets to halt autoimmune diabetes. J Immunol. 2015;194:3147–3155. doi: 10.4049/jimmunol.1402739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deknuydt F, Bioley G, Valmori D, Ayyoub M. IL-1beta and IL-2 convert human Treg into T(H)17 cells. Clin Immunol. 2009;131:298–307. doi: 10.1016/j.clim.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Feng T, Cao AT, Weaver CT, Elson CO, Cong Y. Interleukin-12 converts Foxp3+ regulatory T cells to interferon-gamma-producing Foxp3+ T cells that inhibit colitis. Gastroenterology. 2011;140:2031–2043. doi: 10.1053/j.gastro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schreiber TH, Wolf D, Tsai MS, Chirinos J, Deyev VV, Gonzalez L, Malek TR, Levy RB, Podack ER. Therapeutic Treg expansion in mice by TNFRSF25 prevents allergic lung inflammation. J Clin Invest. 2010;120:3629–3640. doi: 10.1172/JCI42933. [DOI] [PMC free article] [PubMed] [Google Scholar]