Abstract
Steroids are known to inhibit osteogenic differentiation and decrease bone formation in mesenchymal stem cells (MSCs), while concomitantly inducing steroid-induced avascular necrosis of the femoral head (SANFH). The aim of the present study was to evaluate the function of MSCs on differentiation in SANFH and investigate the pathobiological mechanisms underlying SANFH in a rabbit model. MSCs in the control, trauma-induced ANFH (TANFH) and SANFH groups were incubated with low-glucose complete Dulbeccos modified Eagles medium containing 10% fetal bovine serum. A number of adipocytes in the MSCs were stained with Sudan III and counted using a light microscope. The mRNA and protein expression levels of the adipose-specific 422 (AP2), peroxisome proliferator-activated receptor-γ (PPARγ), RUNX2, collagen type I (Col I) and miR-103 in the MSCs were determined using quantitative polymerase chain reaction and western blot analysis, respectively. In addition, the activities of osteocalcin (OC), alkaline phosphatase (ALP) and triglyceride (TG) in MSCs were analyzed using radioimmunoassay and determination kits. In the MSCs of the SANFH group, the mRNA and protein expression levels of AP2 and PPARγ were increased, while those of RUNX2 and Col I were reduced. Furthermore, the levels of OC and ALP activity in the MSCs of the SANFH group were decreased, and the activity of TG in the MSCs of the SANFH group was increased. In addition, the expression of miR-103 in the MSCs of the SANFH group was elevated. Following routine culture of the MSCs for 3 weeks, the number of adipocytes among the MSC population of the SANFH group was increased. Therefore, the results of the present study suggest that the osteogenic differentiation of MSCs in the SANFH was mitigated, while fat differentiation was promoted, which provides a novel explanation for the pathological changes associated with SANFH.
Keywords: marrow stromal cells, avascular necrosis of the femoral head, osteonecrosis
Introduction
Avascular necrosis of the femoral head (ANFH) is among the most common osteoarthritic diseases worldwide. Currently, ~30 million people suffer from ANFH globally, including ~4 million individuals in China (1). Particularly since the advent of hormone drugs and their wide application, ANFH incidence has gradually increased (2,3). Incomplete statistics from a recent survey demonstrated no significant gender or age differences in the incidence of ANFH; however, the incidence of ANFH in individuals with a history of hormone application, hip trauma, alcohol abuse or an associated disease was significantly increased (4,5). Steroid-induced ANFH (SANFH) is a type of ANFH caused by prolonged use of glucocorticoid (6).
Stem cells are undifferentiated or poorly-differentiated cells with self-replicative capacity and multi-differentiation potential (7). Under certain conditions, stem cells may be differentiated into various types of functional cells. Stem cells are immature cells and not fully differentiated, possessing the potential regenerative functions of multiple tissue types, and are known as ‘universal cells’ in the medical field (8). Mesenchymal stem cells (MSCs) are key members of the stem cell family, located in the mesoderm and ectoderm during the early development of cell differentiation (9). MSCs were originally identified in bone marrow and attracted increasing attention due to a number of features, including their multi-differentiation potential, hematopoietic support and promotion of stem cell implantation, immune regulation and self-replication (10). For instance, MSCs may be differentiated into the cells of various tissue types, including fat, bone, cartilage, muscle, tendon, ligament, nerve, liver, cardiac muscle and endothelial tissues, under specific induction conditions in vivo or in vitro. Following successive subculture and cryopreservation, stem cells continue to exhibit multi-differentiation potential and, thus, may function as ideal seed cells for the repair of the tissue and organ damage caused by aging and disease (11). Due to their wide range of sources, easy isolation and culture, strong differentiation potential and autologous transplantation, MSCs have gained increasing attention from researchers and are considered to be promising stem cell candidates for translation into clinical treatment (12).
As a result of the wide application of glucocorticoid in clinical treatment, glucocorticoid-induced ischemic ANFH has emerged as a primary cause of non-invasive ANFH in Chinese populations (13). Although there are numerous theories, the precise mechanism underlying the association between glucocorticoids and ANFH is not clear. According to a previous study (13), SANFH may be a disease associated with MSCs. Glucocorticoid serves a crucial regulatory function in the proliferation and differentiation of MSCs (13). Under certain conditions, hormones are able to induce MSC proliferation, differentiation and differentiation direction changes, potentially causing bone necrosis (14). The limited self-repair capacity associated with SANFH may be associated with the limited number and the weak proliferative activity of MSCs in femoral neck and femoral metaphyseal bone marrow of these patients (15). Detecting the number and the proliferative activity of MSCs in such patients is to seek evidence for the rationality of treating ANFH by autologous transplantation of bone MSCs (15). The aims of the present study were to observe the role of bone marrow stromal stem cells on cell differentiation in a rabbit model of SANFH and investigate the pathobiological mechanisms underlying the MSCs.
Materials and methods
Animals
A total of 30 adult Japanese white rabbits (weight, 3.0±0.5 kg) were provided by the Experimental Animal Center of China University of Technology (Taipei, China). This study was approved by the Ethics Committee of 463 Hospital of PLA (Liaoning, China). Experimental animals were allocated at random into three groups (n=10 per group): Control, trauma-induced ANFH (TANFH) and SANFH groups. All experimental animals received a standard diet. The SANFH group rabbits were injected with 10 µg/kg lipopolysaccharide (Sigma-Aldrich, St. Louis, MO, USA) per day by intravenous injection for 2 days. Subsequently, 25 mg/kg dexamethasone (Sigma-Aldrich) was injected into the right gluteus medius muscle per day for 3 days. The control group rabbits were injected with an equal volume of normal saline by intravenous injection. Conventional dressing, and normal movement. All the experimental animals were administered 8×104 units gentamicin (Sigma-Aldrich) by gavage once a day for 4 weeks.
Cultivation and grouping of MSCs
Autologous primary MSCs were collected from 15–20 µl bone marrow extracted from each experimental animal using 5 ml α-minimum essential medium (Invitrogen Life Technologies, Carlsbad, CA, USA) with a 25-gauge needle by gradient centrifugation. Second-generation MSCs were plated into a 6-well plate and incubated with low-glucose complete Dulbeccos modified Eagles medium (DMEM; Invitrogen Life Technologies) containing 10% fetal bovine serum (FBS) with 100 U/ml penicillin/streptomycin (Invitrogen Life Technologies) in a humidified atmosphere at 37°C with 5% CO2. Cells were split when they reached 80% confluence and the third passage was used for the subsequent experiments.
Differentiation of MSCs into adipocytes at 3 weeks
All second-generation MSCs were placed into 6-well plate and incubated with low-glucose complete DMEM containing 10% FBS and 100 U/ml penicillin/streptomycin in a humidified atmosphere at 37°C with 5% CO2, for 3 weeks. The presence of adipocytes of MSCs was determined by Sudan III staining (Sangon Biotech Shanghai Co., Ltd., Shanghai, China), and positive cells were observed using inverted phase microscopy (1200-EX; JEOL Ltd., Tokyo, Japan).
Quantitative polymerase chain reaction (qPCR)
After routine culture for 1 week, total RNA was extracted using TRIzol according to the manufacturers instructions (Invitrogen Life Technologies). In accordance with the manufacturers instructions, the extracted RNA was reverse transcribed into cDNA using SuperScript III Reverse Transcriptase (Invitrogen Life Technologies). The mRNA expression levels of adipose-specific 422 (AP2), peroxisome proliferator-activated receptor-γ (PPARγ), RUNX2, collagen type I (Col I) and microRNA-103 (miR-103) were determined using qPCR. The qPCR cycling conditions were as follows: 95°C for 1 min, followed by 40 cycles of 95°C for 30 sec, 60°C for 45 sec and 72°C for 45 sec. The primer sequences used are listed in Table I.
Table I.
Polymerase chain reaction primers for AP2, PPARγ, RUNX2, Col I and miR-103.
| Primer | Sequence |
|---|---|
| AP2 | F, 5-TTACCCTGCTCACATCACTAG-3 |
| R, 5-TCTTGTCACTTGCTCATTGGG-3 | |
| PPARγ | F, 5-GATAGGTGTGATCTTAACTGTCGGAT-3 |
| R, 5-CGCTAACAGCTTCTCCTTCTCG-3 | |
| RUNX2 | F, 5-GGCTGTGGAGTTTGGTGTCTA-3 |
| R, 5-TCTGCTAAATTCTGCTTGGGT-3 | |
| Col I | F, 5-GAGAGAGCATGACCGATGGA-3 |
| R, 5-CGTGCTGTAGGTGAATCGAC-3 | |
| miR-103 | F, 5-TTCCCCTGTTTGGTGCTATGTTT-3 |
| R, 5-AGGTAAATTAAGAGGTATTATAGTTACAGTGCAAAAA-3 | |
| β-actin | F, 5-GTTGACATCCGTAAAGACC-3 |
| R, 5-GGAGCCAGGGCAGTAA-3 |
F, forward; R, reverse; AP2, adipose-specific 422; PPARγ, peroxisome proliferator-activated receptor-γ; Col I, collagen type I; miR-103, microRNA-103.
Western blot analysis for determination of AP2, PPARγ, RUNX2 and Col I protein expression levels
After routine culture for 1 week, total protein was extracted using a cell protein extraction kit (Beyotime Institute of Biotechnology, Nanjing, China). Bicinchoninic acid method was used to determine the total protein content, using a commercial kit according to the manufacturers instructions (Beyotime Institute of Biotechnology). Subsequently, 50-µg protein samples were separated by 12% SDS-polyacrylamide gel electrophoresis and transferred into nitrocellulose membranes. The membranes were then blocked and incubated with 5% defatted milk for 2 h at room temperature. Subsequently, the membranes were probed respectively with the following primary antibodies: Anti-AP2 (1:500; sc-184), anti-PPARγ (1:500; sc-6285), anti-RUNX2 (1:300; sc-10758), anti-Col I (1:500; sc-8566) and anti-β-actin (1:1,000; sc-7210) at 4°C overnight. All antibodies were purchased from Santa Cruz Biotechnology, Inc. (La Jolla, CA, USA). The membranes were washed with Tris-buffered saline with Tween 20, and incubated for 2 h with peroxidase-labeled goat anti-rabbit IgG (1:5,000; Santa Cruz Biotechnology, Inc.). Immunodetection was conducted using an enhanced chemiluminescence kit (Applygen Technologies, Inc., Beijing, China) and exposed on an X-ray film using an X-ray diffraction system (XPert Pro; Philips, Amsterdam, Netherlands).
Determination of osteocalcin (OC), alkaline phosphatase (ALP) and triglyceride (TG) content
After routine culture for 2 weeks, the content of OC in the media was determined by radioimmunoassay (Wuhan Elabscience Co., Ltd., Wuhan, China), as previously described (19). The contents of TG and ALP in the MSCs were detected using a TG and ALP determination kits, respectively (Beyotime Institute of Biotechnology, Haimen, China). The optical density was measured at 405 nm using an absorbance reader (BD Biosciences, San Jose, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation. The statistical significance of the differences between groups was analyzed using two-way analysis of variance. Statistical analysis was performed using SPSS software, version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Adipogenic differentiation of the MSCs
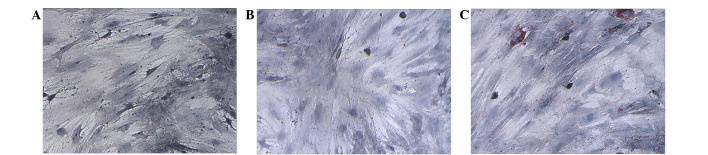
After routine culture of MSCs for 3 weeks, there was no marked adipocyte differentiation in the MSCs of the control and TANFH groups (Fig. 1A and B). However, adipocyte differentiation was evident in the MSCs of the SANFH group (Fig. 1C).
Figure 1.
Adipogenic differentiation of mesenchymal stem cells in the (A) control, (B) trauma-induced avascular necrosis of the femoral head (ANFH) and (C) steroid-induced ANFH groups (magnification, ×200).
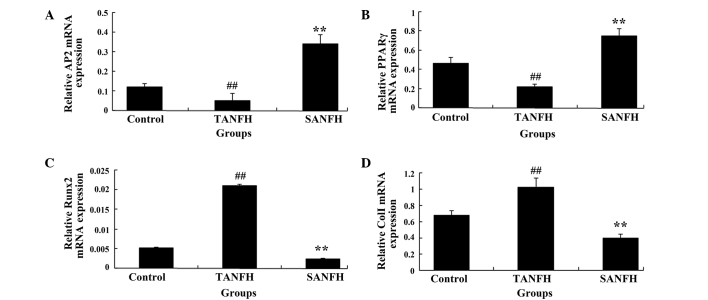
mRNA expression levels of AP2, PPARγ, RUNX2 and Col I in MSCs
After routine culture of MSCs for 1 week, the mRNA expression levels of AP2 and PPARγ in the TANFH group were significantly reduced (P<0.05; n=6) compared with the control group. In addition, the mRNA expression levels of AP2 and PPARγ in the SANFH group were significantly increased (P<0.05; n=6) compared with the TANFH group (Fig. 2A and B). However, the mRNA expression levels of RUNX2 and Col I were significantly increased (P<0.05; n=6), compared with control group. In addition, the mRNA expression levels of RUNX2 and Col I in the SANFH group were markedly reduced (P<0.05; n=6), compared with the TANFH group (Fig. 2C and D).
Figure 2.
Relative mRNA expression levels of (A) AP2, (B) PPARγ, (C) RUNX2 and (D) Col I in mesenchymal stem cells in the different groups (control group, TANFH group and SANFH group). ##P<0.05 vs. control group; **P<0.05 vs. TANFH group. AP2, adipose-specific 422; TANFH, trauma-induced avascular necrosis of the femoral head; SANFH, steroid-induced avascular necrosis of the femoral head; PPARγ, peroxisome proliferator-activated receptor-γ; Col I, collagen type I.
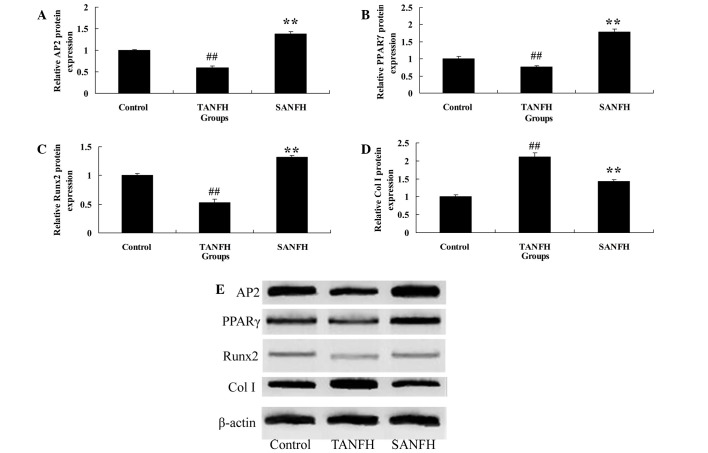
Protein expression levels of AP2, PPARγ, RUNX2 and Col I
The results of western blot analysis were consistent with those of the mRNA expression, showing a significant increase in the expression levels of AP2 and PPARγ protein in the SANFH group (P<0.05; n=6) compared with the TANFH group (Fig. 3A and B). In addition, the AP2 and PPARγ protein expression levels in the TANFH group were significantly reduced (P<0.05; n=6) compared with the control group (Fig. 3A and B). The protein expression level of RUNX2 in the SANFH group was reduced (P<0.05; n=6) compared with the TANFH group (Fig. 3C). Furthermore, the expression levels of RUNX2 protein in the TANFH group were promoted (P<0.05; n=6) compared with control group (Fig. 3C). In addition, the protein expression levels of Col I in the SANFH group were increased (P<0.05; n=6) compared with the TANFH group (Fig. 3D). The protein expression levels of Col I in the TANFH group were reduced (P<0.05; n=6) compared with the control group (Fig. 3D).
Figure 3.
Relative protein expression levels of (A) AP2, (B) PPARγ, (C) RUNX2 and (D) Col I in mesenchymal stem cells in the different groups. (E) Western blot analysis demonstrating the expression levels of the various proteins, with β-actin used as a loading control. ##P<0.05 vs. control group; **P<0.05 vs. TANFH group. AP2, adipose-specific 422; TANFH, trauma-induced avascular necrosis of the femoral head; SANFH, steroid-induced avascular necrosis of the femoral head; PPARγ, peroxisome proliferator-activated receptor-γ; Col I, collagen type I.
Content of OC in the media, TG content and ALP activity in the cells
Following routine culture of the MSCs for 2 weeks, the OC content and ALP activity values in the TANFH group were significantly increased (P<0.05; n=6) compared with the control group. By contrast, the OC content and ALP activity values in the SANFH group were significantly reduced (P<0.05; n=6), compared with the TANFH group (Fig. 4A and B). In addition, the quantity of TG in the TANFH group was significantly reduced (P<0.05; n=6) compared with the control group. However, the amount of TG in the SANFH group was significantly increased (P<0.05, n=6) compared with the TANFH group (Fig. 4C).
Figure 4.
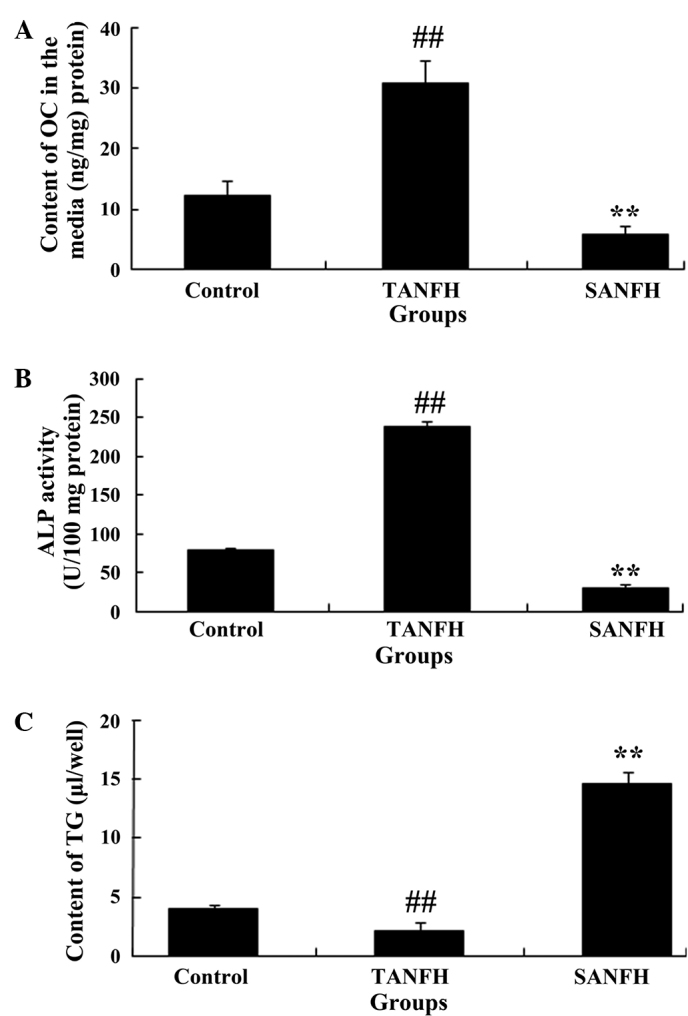
(A) Content of OC in the media, (B) TG content, and (C) ALP activity values in mesenchymal stem cells in the different groups (control group, TANFH group and SANFH group). ##P<0.05 vs. control group; **P<0.05 vs. TANFH group. OC, osteocalcin; TANFH, trauma-induced avascular necrosis of the femoral head; SANFH, steroid-induced avascular necrosis of the femoral head; ALP, alkaline phosphatase; TG, triglyceride.
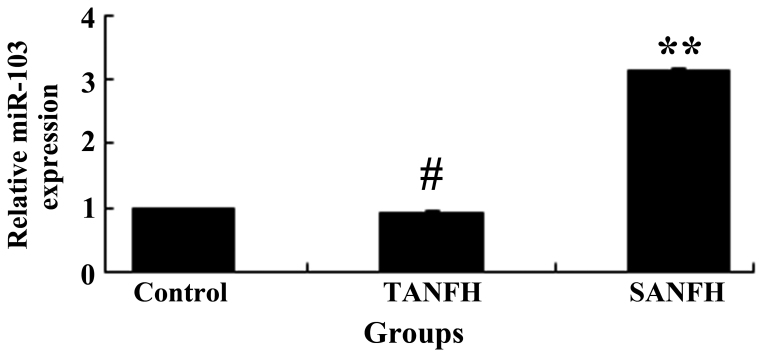
Expression levels of miR-103
Following routine culture of MSCs for 1 week, the miR-103 expression levels of the MSCs were evaluated using qPCR analysis. The miR-103 expression levels of the MSCs in the SANFH group were increased compared with the TANFH group (P<0.05; n=6), and the difference was statistically significant. Furthermore, the miR-103 expression levels of MSCs in the TANFH group were comparable to the control group (P>0.05; n=6), with no statistically significant differences observed (Fig. 5).
Figure 5.
Expression of miR-103. **P<0.05 vs. TANFH group. miR-103, microRNA-103; TANFH, trauma-induced avascular necrosis of the femoral head; SANFH, steroid-induced avascular necrosis of the femoral head.
Discussion
ANFH, also known as aseptic necrosis of the femoral head and avascular necrosis, involves the blockage of the blood supply to the femoral head, and is among the most common and intractable chronic diseases encountered in clinical orthopedics (16). ANFH has increased in incidence from being a rare disease into a common one (17,18). Risk factors, including hip injury, long-term use of hormone drugs and alcoholism, may lead to blockage of the femoral head blood supply, which prevents the femoral bone tissue from receiving normal nutrition. As a result, necrosis of the bone tissue cells, bone marrow cells and fat cells in the femoral head may occur, which directly causes ANFH (19).
Stem cell self-replication and differentiation is primarily determined by the state of the cell itself and a range of micro-environmental factors, including cyclins adjusting the cell cycle and cyclin-dependent kinase, gene transcription factors and the cytoplasm factor that impact the asymmetric cell division (8). Micro-environmental factors include the interactions between stem cells and surrounding cells, extracellular matrix and a variety of soluble factors. MSCs are pluripotent stem cells, which are able to differentiate into osteoblasts, adipocytes, fibroblasts and chondrocytes (20). With the increasing development of stem cell technology, researchers have attempted to utilize the osteogenesis and vascularization of MSCs, which function directly at the lesion site of ANFH, to aid in the treatment of early ANFH (7).
In China, glucocorticoid administration is the primary cause of non-traumatic ANFH and, according to statistics, accounts for ~50% of cases (21). At present, the exact pathological mechanism underlying SANFH is not fully known; however, numerous studies have indicated that a reduced number and the weakened activity of MSCs in the femoral neck and proximal femur may be among the underlying causes of SANFH (22–24). Due to the reduction in the number or the activity level of MSCs, their osteogenetic capacity decreases, and the lesion of necrotic bone cannot be effectively repaired, leading to the collapse of the femoral head (25). In the present study, following the routine culture of MSCs for 3 weeks, the number of adipocytes was found to be clearly detectable in the MSCs of the SANFH group compared with control and TANFH groups.
AP2 is a specific indicator in the late stages of adipocyte differentiation and is expressed only during the differentiation process of fat cells (26). PPARγ is an adipogenic transcription factor involved in the induction of adipocyte differentiation. The development of ANFH is closely associated with the elevated expression of PPARγ. Furthermore, the downregulation or suppression of PPARγ in MSCs is able to inhibit steroid-induced adipogenic differentiation, and thus may be effective in the prevention of ANFH. PPARγ is a key transcription factor in mammalian adipogenesis (27). By contrast, RUNX2 and Col I are studied most extensively as crucial factors in the osteogenic process; the expression of RUNX2 and Col I promotes the osteogenic differentiation of MSCs. The appearance of Col I is a key feature of osteoblast differentiation, representing the differentiation degree of stromal stem cells into osteoblasts (28). It has been observed that mice with the homozygous deletion of these genes lack functional osteoblasts. In the present study, we identified a significant increase in the mRNA and protein expression levels of AP2 and PPARγ in the SANFH group. By contrast, the mRNA and protein expression levels of RUNX2 and Col I in the SANFH group were reduced significantly. These differences in expression were consistent with those observed in the in control and TANFH cells.
OC is a K-dependent calcium-binding protein synthesized and secreted by osteoblasts, and a type of non-collagen acidic glycoprotein. The osteoblasts expressing OC can be completely induced and converted to fat cells (29). In addition, ALP is able to hydrolyze organic phosphate to release inorganic phosphate, thus forming hydroxyapatite, which is a necessary enzyme in the osteogenesis process. ALP expression indicates a state of osteogenesis, which is the beginning of the osteoblast differentiation, and positively correlates with osteoblast differentiation and maturation (30). In the present study, the OC content and ALP activity values in SANFH cells were significantly reduced, and the quantity of TG in the SANFH cells was significantly increased, compared with the control and TANFH cells.
MicroRNA-103 (miR-103) is a class of non-coding RNA nucleotide with small molecules that are able to regulate the expression of the target gene transcription, and serves a crucial function in physiological processes, including body growth, cell reproduction, metabolism and apoptosis (31). Certain studies have observed that miR-103 is highly upregulated in senescent MSCs (32,33). Preadipocytes are able to increase the expression levels of the adipocyte marker genes, PPARγ and AP2, by upregulating miR-103. In the current study, the miR-103 expression levels of the MSCs in the SANFH group were higher comparable with those in the control and TANFH groups, with statistically significant differences observed. These results indicate that miR-103 may activate the differentiation of the MSCs into adipocytes in the SANFH group via the upregulation of PPARγ and AP2. The expression levels of miR-103 in MSCs may, therefore, require further investigation in future studies.
In conclusion, the results of the present study indicated that the number of adipocytes in the MSCs of the SANFH group was increased compared with the control and TANFH cells. These results further confirm that the contents of AP2, PPARγ, RUNX2 and Col I are different under different pathogenic factors associated with ANFH. In the MSCs of the SANFH group, the mRNA and protein expression levels of AP2 and PPARγ were increased, while those of RUNX2 and Col I were reduced. The levels of OC and ALP activity in the MSCs of the SANFH group were decreased, and the activity of TG in the MSCs of the SANFH group was increased. Furthermore, the expression of miR-103 in the MSCs of the SANFH group was promoted. The study suggests that the MSCs in the SANFH group exhibited reduced osteogenic differentiation and promoted fat differentiation, which provides a novel explanation for the pathological changes associated with SANFH.
Acknowledgements
This study was supported by a grant from the Liaoning Province Natural Science Foundation of China (no. 201202234).
References
- 1.Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, Liu B, Yu X. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50:325–330. doi: 10.1016/j.bone.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Dulin JN, Karoly ED, Wang Y, Strobel HW, Grill RJ. Licofelone modulates neuroinflammation and attenuates mechanical hypersensitivity in the chronic phase of spinal cord injury. J Neurosci. 2013;33:652–664. doi: 10.1523/JNEUROSCI.6128-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang YG, Jiang DM, Quan ZX, Ou YS. Insulin with chondroitinase ABC treats the rat model of acute spinal cord injury. J Int Med Res. 2009;37:1097–1107. doi: 10.1177/147323000903700414. [DOI] [PubMed] [Google Scholar]
- 4.Molaei S, Roudkenar MH, Amiri F, Harati MD, Bahadori M, Jaleh F, Jalili MA, Roushandeh A Mohammadi. Down-regulation of the autophagy gene, ATG7, protects bone marrow-derived mesenchymal stem cells from stressful conditions. Blood Res. 2015;50:80–86. doi: 10.5045/br.2015.50.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi J, Jo M, Lee E, Oh YK, Choi D. The role of autophagy in human endometrium. Biol Reprod. 2012;86:70. doi: 10.1095/biolreprod.111.096206. [DOI] [PubMed] [Google Scholar]
- 6.Allavena G, Carrarelli P, Del Bello B, Luisi S, Petraglia F, Maellaro E. Autophagy is upregulated in ovarian endometriosis: A possible interplay with p53 and heme oxygenase-1. Fertil Steril. 2015;103:1244–1251. doi: 10.1016/j.fertnstert.2015.02.007. e1241. 10.1016/j.fertnstert.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Jeong J, Shin K, Lee SB, Lee DR, Kwon H. Patient-tailored application for Duchene muscular dystrophy on mdx mice based induced mesenchymal stem cells. Exp Mol Pathol. 2014;97:253–258. doi: 10.1016/j.yexmp.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Ulrich D, Muralitharan R, Gargett CE. Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin Biol Ther. 2013;13:1387–1400. doi: 10.1517/14712598.2013.826187. [DOI] [PubMed] [Google Scholar]
- 9.Alfaro MP, Young PP. Lessons from genetically altered mesenchymal stem cells (MSCs): Candidates for improved MSC-directed myocardial repair. Cell Transplant. 2012;21:1065–1074. doi: 10.3727/096368911X612477. [DOI] [PubMed] [Google Scholar]
- 10.Hansson M, Tonning A, Frandsen U, Petri A, Rajagopal J, Englund MC, Heller RS, Håkansson J, Fleckner J, Sköld HN, et al. Artifactual insulin release from differentiated embryonic stem cells. Diabetes. 2004;53:2603–2609. doi: 10.2337/diabetes.53.10.2603. [DOI] [PubMed] [Google Scholar]
- 11.Zeighami S, Hadjibabaie M, Ashouri A, Sarayani A, Khoee SH, Mousavi S, Radfar M, Ghavamzadeh A. Assessment of cyclosporine serum concentrations on the incidence of acute graft versus host disease post hematopoietic stem cell transplantation. Iran J Pharm Res. 2014;13:305–312. [PMC free article] [PubMed] [Google Scholar]
- 12.Mahdavishahri N, Matin M Moghatam, Fereidoni M, Yarjanli Z, Rad SA Banihashem, Ahmadi S Khajeh. In vitro Assay of Human Gingival Scaffold in Differentiation of Rat's Bone Marrow Mesenchymal Stem Cells to Keratinocystes. Iran J Basic Med Sci. 2012;15:1185–1190. [PMC free article] [PubMed] [Google Scholar]
- 13.Hu N, Feng C, Jiang Y, Miao Q, Liu H. Regulative Effect of Mir-205 on Osteogenic Differentiation of Bone Mesenchymal Stem Cells (BMSCs): Possible Role of SATB2/Runx2 and ERK/MAPK Pathway. Int J Mol Sci. 2015;16:10491–10506. doi: 10.3390/ijms160510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang RL, Yuan Y, Tu J, Zou GM, Li Q. Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation. Cell Death Dis. 2014;5:e1187. doi: 10.1038/cddis.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glynn ER, Londono AS, Zinn SA, Hoagland TA, Govoni KE. Culture conditions for equine bone marrow mesenchymal stem cells and expression of key transcription factors during their differentiation into osteoblasts. J Anim Sci Biotechnol. 2013;4:40. doi: 10.1186/2049-1891-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domitrović R, Rashed K, Cvijanović O, Vladimir-Knežević S, Škoda M, Višnić A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem Biol Interact. 2015;230:21–29. doi: 10.1016/j.cbi.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Wang D, Wang G, Liu M, Sun L, Zong W, Jiang H, Zhang H, Li H, Gong J, Sun S. A novel animal model of osteonecrosis of the femoral head induced using a magnetic resonance imaging-guided argon-helium cryotherapy system. Exp Ther Med. 2014;7:1525–1528. doi: 10.3892/etm.2014.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Li J, Liu M, Zhao G, Hao L, Li Y. Inhibition of peroxisome proliferator-activated receptor-γ in steroid-induced adipogenic differentiation of the bone marrow mesenchymal stem cells of rabbit using small interference RNA. Chin Med J (Engl) 2014;127:130–136. [PubMed] [Google Scholar]
- 19.Yin L, Li YB, Wang YS. Dexamethasone-induced adipogenesis in primary marrow stromal cell cultures: Mechanism of steroid-induced osteonecrosis. Chin Med J (Engl) 2006;119:581–588. [PubMed] [Google Scholar]
- 20.Abnosi MH, Mehranjani M Solemani, Momeni H, Shojafar E, Barati M. Induction of Apoptosis in the Rat Bone Marrow Mesenchymal Stem Cells Following Sodium Arsenite Treatment with the Dose Lesser than that Used for Treatment of Malignant Patient. Iran J Basic Med Sci. 2012;15:900–906. [PMC free article] [PubMed] [Google Scholar]
- 21.Takata M, Nakagomi T, Kashiwamura S, NakanoDoi A, Saino O, Nakagomi N, Okamura H, Mimura O, Taguchi A, Matsuyama T. Glucocorticoid-induced TNF receptor-triggered T cells are key modulators for survival/death of neural stem/progenitor cells induced by ischemic stroke. Cell Death Differ. 2012;19:756–767. doi: 10.1038/cdd.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin M, Luo Y, Meng XB, Wang M, Wang HW, Song SY, Ye JX, Pan RL, Yao F, Wu P, et al. Myricitrin attenuates endothelial cell apoptosis to prevent atherosclerosis: An insight into PI3K/Akt activation and STAT3 signaling pathways. Vascul Pharmacol. 2015;70:23–34. doi: 10.1016/j.vph.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Jiang N, Qin CH, Tan CX, Wen SF, Ma YF, Dong F, Diao XC, Zhang P, Yu B. A retrospective analysis of 140 patients with giant cell tumor in the extremity: A multicenter study based on four hospitals in South China. Cancer Epidemiol. 2013;37:294–299. doi: 10.1016/j.canep.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Li DX, Zhang J, Zhang Y, Zhao PW, Yang LM. Inhibitory effect of berberine on human skin squamous cell carcinoma A431 cells. Genet Mol Res. 2015;14:10553–10568. doi: 10.4238/2015.September.8.17. [DOI] [PubMed] [Google Scholar]
- 25.Peffer ME, Chandran UR, Luthra S, Volonte D, Galbiati F, Garabedian MJ, Monaghan AP, DeFranco DB. Caveolin-1 regulates genomic action of the glucocorticoid receptor in neural stem cells. Mol Cell Biol. 2014;34:2611–2623. doi: 10.1128/MCB.01121-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes Y, Ojeda M, Araya D, Dueñas F, Fernández MS, Peralta OA. Isolation and multilineage differentiation of bone marrow mesenchymal stem cells from abattoir-derived bovine fetuses. BMC Vet Res. 2013;9:133. doi: 10.1186/1746-6148-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun J, Wang Y, Li Y, Zhao G. Downregulation of PPARγ by miR-548d-5p suppresses the adipogenic differentiation of human bone marrow mesenchymal stem cells and enhances their osteogenic potential. J Transl Med. 2014;12:168. doi: 10.1186/1479-5876-12-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fensky F, Reichert JC, Traube A, Rackwitz L, Siebenlist S, Noth U. Chondrogenic predifferentiation of human mesenchymal stem cells in collagen type I hydrogels. Biomed Tech. Berl. 2014 doi: 10.1515/bmt-2013-0076. [DOI] [PubMed] [Google Scholar]
- 29.Qiu X, Jin X, Shao Z, Zhao X. 17β-estradiol induces the proliferation of hematopoietic stem cells by promoting the osteogenic differentiation of mesenchymal stem cells. Tohoku J Exp Med. 2014;233:141–148. doi: 10.1620/tjem.233.141. [DOI] [PubMed] [Google Scholar]
- 30.Bose R, Moors M, Tofighi R, Cascante A, Hermanson O, Ceccatelli S. Glucocorticoids induce long-lasting effects in neural stem cells resulting in senescence-related alterations. Cell Death Dis. 2010;1:e92. doi: 10.1038/cddis.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo JK, Kim CH, Jung HY, Lee DR, Kim JK. Discovery and characterization of miRNA during cellular senescence in bone marrow-derived human mesenchymal stem cells. Exp Gerontol. 2014;58:139–145. doi: 10.1016/j.exger.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q, Qin R, Fang Y, Li H. Berberine Sensitizes Human Ovarian Cancer Cells to Cisplatin Through miR-93/PTEN/Akt Signaling Pathway. Cell Physiol Biochem. 2015;36:956–965. doi: 10.1159/000430270. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Ji Q, Ye N, Sui H, Zhou L, Zhu H, Fan Z, Cai J, Li Q. Berberine Inhibits Invasion and Metastasis of Colorectal Cancer Cells via COX-2/PGE2 Mediated JAK2/STAT3 Signaling Pathway. PLoS One. 2015;10:e0123478. doi: 10.1371/journal.pone.0123478. [DOI] [PMC free article] [PubMed] [Google Scholar]