Abstract
The microenvironment of myocardium plays an important role in the fate and function of cardiomyocytes (CMs). Cardiovascular tissue engineering strategies commonly utilize stem cell sources in conjunction with microenvironmental cues that often include biochemical, electrical, spatial and biomechanical factors. Microenvironmental stimulation of CMs, in addition to the incorporation of intercellular interactions from non-CMs, results in the generation of engineered cardiac constructs. Current studies suggest that use of these factors when engineering cardiac constructs improve cardiac function when implanted in vivo. In this review, we summarize the approaches to modulate biochemical, electrical, biomechanical and spatial factors to induce CM differentiation and their subsequent organization for cardiac tissue engineering application.
Keywords: : biophysical, cardiomyocytes, mechanical, rigidity, stem cells, tissue engineering
Tissue engineering has emerged as a promising adjunct therapy to the treatment of cardiovascular disease (CVD). Tissue engineering employs principles from bioengineering and life sciences to generate tissue-like replacements to restore physiological function. In particular to cardiovascular tissue engineering, the purpose of the engineered myocardial tissue is to repair or regenerate myocardial tissue due to CVD, which affects 85.6 million American adults [1] and is associated with an overall rate of death of 222.9 per 100,000 Americans [1]. CVDs that can benefit from tissue engineering include myocardial infarction, congenital heart disease, complete heart block and atherosclerosis. These diseases have a detrimental effect on the myocardium's function to pump blood throughout the circulatory system. The myocardium itself relies on blood supply from vessels in order to maintain a steady flow of nutrients and oxygen to maintain normal pumping capacity. Currently, surgical interventions for treating CVDs include heart valve repair, bypass surgery and heart transplantation. Heart valve repair and bypass surgery primarily circumvent rather than repair the damaged tissues. The limitations of heart transplantation include the shortage of donor organs for transplantation, the limited durability of transplanted organs and the need to undergo immunosuppression regimen after transplantation. Therefore, tissue engineering is a promising approach to overcome these limitations by providing a new source of autologous myocardial tissue.
The myocardium is composed of four chambers, namely the left atrium, right atrium, left ventricle (LV), and right ventricle. The chambers are composed of multicellular populations that are exposed to a variety of environmental stimuli. At the cellular level, the myocardium is comprised of 30–40% cardiomyocytes (CMs) that are responsible for contractility of the myocardium, along with 60–70% non-CMs that include endothelial cells, vascular smooth muscle cells and fibroblasts [2–4]. Besides interacting with one another, the myocardial cells also respond to numerous microenvironmental stimuli such as biochemical, biomechanical and electrical stimulation. In order to generate CMs for cardiovascular tissue engineering applications, numerous stem and progenitor cells have been examined. In this review, we summarize recent advances in the generation of stem cell-derived CMs, with a focus on the role of microenvironmental factors in modulating CM differentiation for myocardial tissue engineering.
Properties of CMs
In the myocardium, native CMs can be distinguished from other cell types based on a unique set of cytoskeletal markers, including cardiac troponin-T, α-troponin, α-myosin heavy chain (α-MHC/MYH6), β-MHC (MYH7), atrial myosin light chain-2 (MLC2a), ventricular myosin light chain-2 (MLC2v) and α-actinin [5–7]. Transcriptional markers of cardiac phenotype include NKX2.5 and GATA4 [8,9]. Additionally, connexin-43 (Cx43) is a gap junctions expressed by CMs and other cells [10]. Electrophysiological mapping techniques such as microelectrode array, patch clamp, isometric force measurements, sharp electrode recordings and optical mapping are some of the methods to characterize the function of CMs [11–13]. Additionally, the function of mature CMs can be assessed using microscopy to reveal high mitochondrial numbers, organized sarcomeres and correct myofilament structure [14–16].
Sources for the generation of stem cell-derived CMs
Despite the fact that during mammalian development native fetal CMs replicate robustly, in the postnatal environment these cells become highly structured and enter a stable state of cell cycle senescence [17]. In order to derive CMs for tissue engineering, stem cells are an optimal cell source because of their ability to self-renew and give rise to CMs. The three major types of stem cells that have emerged as potential CM sources are cardiac stem cells, multipotent stem cells and pluripotent stem cells.
Resident cardiac stem & progenitor cells
Resident cardiac stem and progenitor cells that can differentiate into CMs have been identified and characterized by the expression of surface cell markers, c-kit (or CD117) [18–23], stem cell antigen-1 (Sca-1 or Ly6A) [24,25] and ATP-binding cassette transporter [26–28]. The c-kit+ cells are capable of self-renewal in both in vitro and in vivo settings, while maintaining the ability to differentiate into CM, endothelial and smooth muscle lineages [18,24,28–31]. However, recently it has been questioned whether c-kit+ cells are representative of cardiac progenitors [32,33]. By targeting the c-kit locus with several reporter genes in mice, Sultana et al. demonstrated that c-kit labeled cells in developing hearts were predominantly cardiac endothelial cells and not cardiac progenitors [34].
Multipotent stem cells
Multipotent stem and progenitor cells can be grouped into the two main categories of mesenchymal stem cells (MSCs) and mononuclear cells (MNCs). MSCs can be isolated from numerous parts of the body, including umbilical cord, bone marrow (BM), adipose tissue, tendon, skin, bone, thymus, muscle, brain, kidneys, lungs, spleen, liver, pancreas, synovial membrane and perivascular niches [35]. MSCs are multipotent, non-hematopoietic stem cells (HSCs) that can differentiate into mesenchymal and nonmesenchymal lineages. Mesenchymal lineages include osteoblasts, chondrocytes and adipocytes, while nonmesenchymal lineages include neurons, epithelium and hepatocytes [36]. MSC-derived CMs have been reported both in vitro [37,38] and in vivo in mice [39]. The advantages of utilizing MSC for cardiac regeneration include not only their multilineage differentiation potential, but also their immune-privileged features which may enable allogenic applications. However, a disadvantage of MSCs as the source of stem cell-derived CMs is the generally low efficiency of differentiation.
MNCs are the fraction of cells found in the BM or peripheral blood whose nuclei are rounded and lack granules in the cytoplasm. MNCs include HSCs as well as non-HSC populations. Human MNCs that are hematopoietic progenitors include lymphoid cells, macrophages and monocytes. Non-HSCs found within MNCs include embryonic-like stem cells, multipotent adult progenitor cells, hemangioblasts, endothelial progenitor cells and tissue committed stem cells [40]. Moreover, approximately 0.01–0.001% of human BM–MNC fractions represents MSCs [35]. MNCs can be isolated by density gradient centrifugation, a process that allows the separation of MNCs relatively quickly and easily [41]. The ease of isolation enables MNCs to be transplanted on the same day of harvesting [41]. However, like MSCs, a major limitation in MNCs is the limited efficiency of cardiac differentiation [42,43].
Pluripotent stem cells
More recently, another source of CMs has been identified from differentiated human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs). These hPSCs can differentiate into any specialized cell from the three lineages depending on exposure to specific chemical factors. In particular, hiPSCs have been determined to be more clinically relevant than hESCs, owing to the autologous source of donor cells that can then be reprogrammed into to a pluripotent state using genetic vectors. hPSCs often have distinct properties depending on derivation and maintenance. Their unique culture requirements, epigenetic features and gene expression mimics the dynamic development of pluripotency in the embryo [44]. The transcription factors OCT4, SOX2 and NANOG govern and define pluripotency based on their specific expression in pluripotent stem cells and embryos [44]. Numerous studies have derived CMs from hESCs (i.e., lines H7, and H13) [45,46], as well as from hiPSCs derived from blood cells and fibroblasts [45,46] for the purposes of tissue engineering [47].
In contrast to native CMs, pluripotent stem cell-derived CMs are associated with immature morphology and function, including disorganized myofibrils, reduced mitochondria, reduced force generation and different expressions of t-tubules and gap junctions [48,49]. Immature stem cell-derived CMs spontaneously beat and depend on glycolysis rather than fatty acid oxidation to produce ATP. Additionally, transplantation of nonentirely purified pluripotent stem cell-derived CMs carries a risk of teratoma formation [50,51]. Therefore, ongoing studies seek to thoroughly maturate stem cell-derived CMs using biochemical [52], electrical, spatial or mechanical factors to circumvent undifferentiated stem cells or immature CMs [53].
The CM microenvironment
Numerous cues in the extracellular microenvironment exert complex forces and interactions that ultimately become transduced into cellular cues that drive CM functional or phenotypic changes. For native CMs, the major microenvironmental cues include biochemical, electrical, spatial and biomechanical factors, along with intercellular interactions. Accordingly, these cues can be utilized to modulate the phenotype and function of CMs for in vitro engineering of myocardial tissue or in vivo myocardial repair. Below we review CM modulation by each of these microenvironmental factors (Table 1).
Table 1. . Summary of microenvironmental factors that modulate cardiomyocyte phenotype and function.
| Microenvironment | In vitro approach | Results | Ref. |
|---|---|---|---|
| Biochemical | Wnt | Differentiation of hPSCs to CMs (80–90% pure) | [54–57] |
| Notch | Differentiation of hPSCs to CMs | [58] | |
| BMP | CM/cardiac mesoderm specification | [59,60] | |
| FGF | – | [59] | |
| IGF-1 | CM and cardiac stem cell proliferation Antiapoptotic Prohypertrophic |
[61–64] | |
| Retinoic acid | Differentiation of hPSCs to CMs Antiapoptotic |
[65,66] | |
| |
Activin A |
– |
[60] |
| Electrical |
– |
Differentiation of hPSCs to CMs CM sarcomere organization CM electrophyiological maturation |
[67–70] |
| Spatial | Topographical patterning | hPSC–CM maturation | [71–73] |
| |
Chemical patterning |
CM alignment |
[71,74] |
| Biomechanical | Stiffness Stretch |
CM sarcomere organization Calcium dynamics CM alignment |
[75,76] |
BMP: Bone morphogenetic protein; CM: Cardiomyocyte; hPSC: human pluripotent stem cell.
Biochemical factors
Based on the participation of biochemical signaling pathways in the embryonic development of the myocardium, biochemical molecules and growth factors have been employed to promote stem cell differentiation into CMs. These pathways include Wnt [42,54–55], bone morphogenetic protein (BMP) [54,60], Notch [58], IGF-1 [61,62] and retinoic acid [65,66] signaling [77,78]. Temporal waves of canonical and noncanonical Wnt signaling play distinct roles during cardiac specification and morphogenesis [79]. The Wnt pathway is critical for inducing heart field formation, maintaining progenitor status and promoting myocardial differentiation [79]. In the canonical pathway, binding of Wnt ligands to the Frizzled-LRP5/6 receptor complex results in the uncoupling of β-catenin from a multiprotein degradation complex composed of adenomatous polyposis coli, axin and glycogen synthase kinase (GSK)-3 [78]. β-Catenin then translocates to the nucleus and activates target gene transcription [78]. Additionally, Wnt signaling also promotes BMP-4 and the noncanonical Wnt 11, which induces myocardial differentiation [79]. Notch signaling plays an essential role in atrioventricular (AV) canal development, ventricular myocardial development and outflow tract development [80]. Interaction of the Notch receptor with transmembrane ligand proteins (Serrate, Delta and Jagged) in a neighboring cell triggers the proteolytic cleavage of Notch [78]. Upon cleavage, Notch intracellular domain translocates to the nucleus to form a transcriptional complex with RBP-J to activate target genes [77,78]. BMP signaling also plays a fundamental role in myocardial chamber versus nonchamber determination [79]. Upon receptor activation, Smad proteins (Smad1/5/8) are phosphorylated and associated with a coactivator Smad (Smad 4), thereby enabling the resulting Smad complex to activate target genes in the nucleus [77]. IGF-1 regulates CM proliferation, differentiation, maturation and postnatal growth of the myocardium [81,82]. Retinoic acid, a metabolite of vitamin A/retinol, generally restricts the expression of heart field markers to limit the size of the heart field and signaling eventually gives rise to the atria [79].
These signaling pathways have been employed to induce differentiation of hPSCs into hPSC–CMs in vitro. In an early differentiation protocol, the biochemical signaling steps that mediate sequential induction of primitive-streak-like population, followed by cardiac mesoderm, and finally expansion of hPSC–CMs were employed to generate cardiovascular lineages [83]. Biochemical stimulation of BMP-4 and activin-A induced the formation of a primitive streak-like population. Sequential treatment with Wnt inhibitor DKK1 and vascular endothelial growth factor then induced a mesodermal progenitor fate. Finally, to support the expansion of cardiovascular lineages, basic FGF was added in the final stage of differentiation. More recently, refined protocols for hPSC–CM generation have led to improved differentiation yields up to 95% hPSC–CMs [56,57]. One protocol employs Wnt signaling activation using the small molecule GSK3B inhibitor, CHIR99021, followed by subsequent Wnt signaling inhibition using WNT-C59 [56]. Similarly, BMP-4, activin and insulin in serum-free conditions have been reported to produce and efficiency of 75% CMs [45]. These examples highlight the important role that biochemical signaling plays in inducing cardiac differentiation.
Although current differentiation procedures using biochemical molecules are efficient, the generated hPSC–CMs are often composed of heterogeneous CM subpopulations that include ventricular-like, atrial-like and nodal-like CMs. These stem cell-derived CMs possess phenotypes that are characterized by spontaneous beating and inadequate contractile machinery. Additionally, increasing the efficacy of these biochemical molecules requires taking advantage of delivery systems composed of various biomaterials such as matrices, films and microparticles. Accordingly, release kinetics, degradation and protein half-life should be considered when deciding on the delivery of hPSC–CMs or molecules to injured cardiac sites.
Electrical factors
The developing myocardium can be divided into the working myocardium, consisting of the atria and ventricles, as well as the electrical conduction system that includes the sinoatrial node, AV node, the AV bundle, the left and right bundle branches and the Purkinje fibers [84]. Myocardial contractility requires distinct electrical profiles that depend on sodium, potassium and calcium channels of CMs [84]. Since the myocardial contractility depends on the proper electrophysiology of CMs, electrical stimulation has been shown to improve the yield or maturity of stem cell-derived CMs. For instance, Tse et al. demonstrate that after application of electrical stimulation on 4-day-old embryoid bodies using electrodes at 6.6 V/cm, 1 Hz and 2 ms, the resultant hESC–CMs exhibited upregulation of cardiac specific genes (HCN1, MLC2V, SCN5A, SERCA, Kv4.3 and GATA4), promoted ventricular-like hESC-CM phenotype and significantly increased both spontaneous and caffeine-induced calcium flux [85]. Similarly, spontaneous contractility accompanied with an increase in reactive oxidative species (ROS) has been reported in 4–8 days old hESC-derived embryoid bodies suspended in low ionic content pulsing buffer after exposure to an electric field pulse of 1 V/mm for 1 or 90 s. The increase in ROS suggests that electrical stimulation plays a role in cardiac differentiation through intracellular generation of ROS [67]. Alternatively, the inclusion of conductive nanomaterials such as nanowires (nanoscale rods made of semiconducting materials) and nanotubes (molecular-scale tubes with high mechanical stiffness and strength) in CM cultures has been utilized in order to increase the electrical conductivity of CMs. Several studies have demonstrated that nanowires and nanotubes promote native and hPSC–CM electrophysiological maturation [68–70,86]. Tan et al. demonstrated that the addition of approximately 0.004% w/v of electrically conductive silicon nanowires in scaffold-free rat-neonatal cardiac spheroids formed electrically conductive networks [68]. Rat spheroids were stimulated at 15 V at 1 Hz to mimic the electrical pulses of native myocardium, which resulted in improvements in the contraction amplitude and synchronization [68]. Additionally, the study demonstrated significant improvement in both sarcomere length and Z-line width with the use of hiPSC–CM-derived spheroids and nanowires [68]. Although the expression of contractile proteins were seen after 7 days of culture with nanowires and maintained for 3 weeks, extended culture did not further improve hiPSC–CM maturation [68]. Nunes et al. employed electrical stimulation to hPSC–CMs cultured on biowires to improve cardiac maturation by enhancing myofibril ultrastructure, electrophysiological properties, calcium handling, along with elevating conduction velocity, compared with nonelectrically stimulated cells [69].
A limitation of implantable electrode probes is the small contact areas of nanowires. Overcoming this limitation requires focusing on the electrode/myocardium interfaces and matching overall probe/myocardium feature sizes and mechanical properties. These studies underscore the importance of electrical stimulation as an important stimulus of cardiac phenotype and maturity.
Spatial factors
Biophysical cues are also important in directing the formation and development of the myocardium. Early cardiac development requires spatial interactions of inductive tissue across the germ layers of the embryo [87]. These spatial interactions are derived from the extracellular matrix (ECM), which are a milieu of extracellular proteins that provide structural support and can also modulate cell behavior and function. Furthermore, the adult myocardium is anisotropic and consists generally of parallel-aligned CMs to facilitate the propagation of electrical signals and mechanical contraction [88]. Accordingly, there have been numerous studies that use spatial patterning to mimic the cell-to-cell and cell-to-ECM interactions of CMs. Commonly used spatial patterning techniques for cardiac regeneration include topographic surface patterning and chemical surface patterning [89,90].
Topographic patterning relies on textured surfaces that provide biomimetic spatial guidance cues just as cells in vivo contact textured and not smooth surfaces [91]. Cell function is affected by topography depending on the topographical pattern. Anisotropic ridges and grooves often influence contact-guided cell alignment, whereas isotropic textures (with randomly or evenly distributed topographic features) affect global CM function [91]. These ridges and grooves are usually produced using micromachining or lithographic techniques [91,92]. Elongated cell shape and the directional organization of the cell cytoskeleton are often identified in response to anisotropic topographies such as ridges and grooves [71,74]. Cell orientation generally increases with increasing groove depth, but decreases with increasing groove width [91]. For example, neonatal rat CMs cultivated on microgrooved substrates for 7 days became highly elongated along the microgrooves, with more pronounced alignment evident with 1 µm compared with 4 µm periodicity [74]. Additionally, rat CMs maintained their contraction ability better when cultured on polystyrene and polyurethane nanogrooved substrates (450 nm in groove width and 100 or 350 nm in depth) [89]. Neonatal rat CMs were reported to form highly anisotropic cell arrays guided by the direction of the underlying nanoridges. Culturing CMs on PEG-based substrata with precisely controlled nanoscale also induced functional changes such as the conduction velocity and expression of Cx43 [93]. Additionally, hiPSC–CM organization and structural development was dependent on nanotopographical feature size, with improved CM development on grooves in the 700–1000 nm range [71].
Besides topographic patterning, spatial organization can be imparted onto substrates by the immobilization of biological molecules in controllable size and position through a chemical patterning process [91]. These biological molecules consist of ECM proteins such as collagen, laminin and fibronectin or peptides containing cell-binding domains such as arginine–glycine–aspartate [91]. This type of patterning relies on spatial patterns at the microscale or nanoscale to regulate cellular functions [91] such as differentiation, cell survival and proliferation [94]. These cellular responses are a result of mechanotransduction pathways mediated in part by integrin-ligand binding [91]. Salick et al. reported that micropatterned features with widths between 30 and 80 μm induced a notable increase in sarcomere alignment of hESC–CMs, relative to the long axis of the pattern direction [95]. These patterns were produced using high-resolution photolithography techniques and microcontact printing with areas ranging from 2500 to 160,000 μm2 [95]. Microcontact printing was developed for patterning molecules over large areas onto a variety of surfaces [94]. When multipotent cardiovascular progenitor cells were cultured on acrylamide gels with patterns of 20-μm thick with 20-μm spacing, they controllably differentiated into relatively pure populations of pacemaker-like or ventricular-like CMs [72]. The techniques for microcontact printing do not require special equipment and involve processing conditions that are not damaging to biomolecules [94]. However, microcontact printing is limited in pattern resolution due to diffusion of molecules during the printing process or deformation of stamps under applied pressure [94]. Alternatively to microcontact printing, hydrogels that degrade in response to UV light can be easily photopatterned in a multitude of shapes. For example, micropatterned protein-based hydrogels composed of methacrylated gelatin and a crosslinker containing o-nitrobenzyl ester groups were shown to improve alignment, and beating regularity of cultured neonatal rat CMs [73]. Various nanoprinting alternative techniques include electron-beam lithography, nanoimprinting, dip pen lithography, nanopipetting and nanoscale arginine–glycine–aspartate clustering using functional polymers are also used in chemically patterning to produce patterns as small as 10 nm [91].
Besides lithography and microcontact printing, electrospinning is another spatial patterning method that uses electrostatic forces to draw a homogeneous mixture of polymer and volatile solvent to produce small diameter nanoscale or microscale fibrous scaffolds [96–98]. The nanofibrous fibers ranging in diameter from tens to hundreds of nanometers mimic the ECM of native cardiac tissue [99,100]. Culturing native or stem cell-derived CMs onto electrospun substrates produce aligned cells that mimic the anisotropy of the native myocardium (Figure 1). Electrospun fibers are not only continuous but have high spatial interconnectivity and high porosity [99]. Native CM and stem cell-derived CM alignment has been reported with numerous electrospun materials, including polymethylglutarimide [101], polyurethane [102] and poly(ϵ-caprolactone)/gelatin composite [103] scaffolds. Generating fibrous scaffolds using electrospinning is relatively simple. For instance, Bursac et al. have shown that anisotropic polymer scaffolds prepared by leaching of aligned sucrose templates not only support aligned and interconnected rat cardiac cells, but also support continuous anisotropic impulse propagation [104]. Another technique known as cell electrospinning allows cell-laden composite living fibers containing primary CMs to be generated. The technique utilizes fine composite threads that encapsulate biosuspensions containing viable CMs, leading to high cellular infiltration into the scaffold [105,106]. Studies have demonstrated that cell electrospinning does not interfere with the integrity of the CMs, since the cells are able to preserve myofibrils and gap junctions within the electrospun fibers [105]. In summary, using spatial patterning techniques, patterning of CMs have shown to modulate their function and/or differentiation capacity from stem cells.
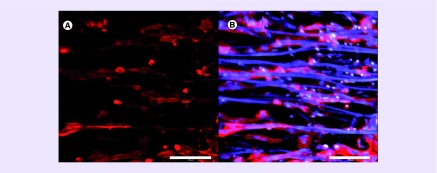
Figure 1. . Cardiomyocyte troponin-T-cell marker expression assay on an aligned anisotropic scaffold.
(A) Troponin-T immunostaining 72 h after seeding human induced pluripotent stem cell-derived cardiomyocytes. (B) Merged view of the human induced pluripotent stem cell-derived cardiomyocytes stained for troponin-T and Hoechst 33342 nuclear dye on parallel-aligned electrospun scaffolds that autofluorescence (in blue color).
Scale bar = 100 µm.
Biomechanical factors
Since cardiac tissue develops under dynamic conditions in the body, biomechanical stimuli are often used to mimic in vivo mechanical forces. The developing human heart experiences a continuously changing topography, native CM contraction and myocardial blood flow. These mechanical stimuli often affect native CM biochemical pathways and cellular processes influenced by intracellular tension and extracellular stress [107,108]. CMs respond to mechanical changes through integrin binding, receptor tyrosine kinase activation and cell membrane GTPase activation. Moreover, cellular mechanotransduction depends on cell–matrix interactions through focal adhesions, stress fibers and microtubules [109]. The two major methods of applying mechanical stimuli to cells are through substrate stiffness and stretching (Figure 2).
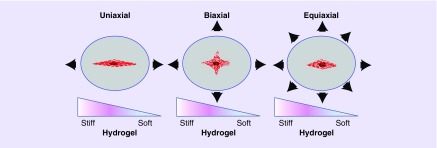
Figure 2. . Biomechanical stretching.
Illustrations of uniaxial, biaxial and equiaxial stretch.
The stiffness of the ECM regulates biological processes such as motility, cell fate, morphology and gene expression [110–112]. Accordingly, Young et al. reported impaired native CM maturation via decreased myofibril formation and contractility on substrates that were softer or stiffer than the mature CM niche (∼10 kPa) [111]. Similarly, neonatal rat CMs cultured on polyacrylamide substrates of stiffness comparable to the native adult rat myocardium (22–50 kPa) were reported to be optimal as the native CMs had superior elongation (aspect ratio >4.3), high contractile force development (0.52–1.60 mN/mm2) and well-developed striations [113]. Additionally, Rodriguez et al. reported greater twitch force, greater intracellular calcium during contraction and greater sarcomeric length in neonatal rat CMs on stiffer PDMS microposts [75]. In contrast, Jacot et al. demonstrated that neonatal rat ventricular CMs plated onto 10 kPa polyacrylamide collagen coated substrates developed aligned sarcomeres, greater mechanical force and larger calcium transients, when compared with stiffer substrates which generated cells with unaligned sarcomeres [76]. Stiffness also plays an important role in directing cardiac differentiation of cardiac progenitors [114], adult stem cells [115] and pluripotent stem cells [116].
Native CMs are also subjected to biomechanical stretch in vivo and often have to adapt to tissue stresses due to myocardial pressure and volume changes. Accordingly, biomechanical stretch plays an important role in the growth, differentiation and development of stem cell-derived CMs [107,108]. Different biochemical pathways might be activated depending on two major variables including the frequency; applied in a dynamic or static manner; and the direction of stretch applied (i.e., uniaxial, biaxial or equiaxial) (Figure 2). For instance uniaxial cyclic stretch-induced realignment of hESC–CMs was found to be driven by mechanosensitive TRPV4 channels which was able to induce Akt phosphorylation [117]. The hESC-derived CMs that were stretched cyclically at 1.0 Hz for 2 weeks at 10% strain were more mature than hESC-derived CMs on unstretched control scaffolds. The stretched hESC-derived CMs displayed increased cardiac α-MHC, α-actinin, GATA-4 and Nkx2.5 mRNA [118]. Likewise, when neonatal rat CMs were exposed to biaxial cyclic stretch at 10% elongation, there was a stretch induced increase in the CM focal adhesion kinase (FAK) Y397 phosphorylation and FAK activation [119]. Equiaxial cyclic stretch at 10–25% stretch has also been shown to increase the percentage of hESC-CM with organized sarcomere structure and increased αMHC, βMHC and atrial natriuretic factor mRNA after 24 h [120]. A 20% equiaxial static stretch has also been reported to activate MAPKs of neonatal rat ventricular CMs [121]. Equiaxial mechanical stretch activated ERK1/2, p38 and JNK through β1integrin and FAK-independent and -dependent mechanisms [121]. Mechanical conditioning can improve the functional properties of engineered cardiac tissues. However, force specifications such as the magnitude, frequency, continuous or intermittent, duty cycle that mimic the myocardium are difficult to reproduce consistently.
Intercellular interactions
Besides biochemical, biophysical and electrical stimuli, another important interaction is derived from cell–cell communication with non-CMs. The myocardium depends on blood supply from vessels composed of vascular cells such as endothelial cells, pericytes, vascular smooth muscle cells and fibroblasts. These vascular lineages also aid in the normal function of the myocardium. CM interactions with vascular lineages and other cell types such as telocytes play an important in the maintenance of the myocardium. Cardiac telocytes extend long, slender processes, which are used to embrace the myocardial progenitors and form a 3D network throughout the myocardium [122,123]. The connections between telocytes and CMs have been reported to comprise of dot junctions with nanocontacts or asymmetric junctions [124]. While native CMs are aligned in myocardium tissue in sheets called laminae, fibroblasts are dispersed in the extracellular matrix that surrounds these CMs and contribute to electromechanical signaling through gap junctions such as Cx43 and Cx45 [125]. These electromechanical coupled signals ultimately affect contractility of the myocardium. Native CM secretion of factors such as ET-1, basic FGF, urocortin, adenosine and the enzyme heme oxygenase regulates myocardial requirements for oxygen and nutrients with the blood flow by varying vascular tone [126].
Endothelial cells make up the inner lining of vessels, while pericytes and vascular smooth muscle cells surround and stabilize the endothelial tubes. Endothelial cells located in the smaller vessels or capillaries in particular interact with CMs and connect them to vasculature through the Cx43 gap junction [125]. Therefore, it is important to consider the incorporation of these cells when engineering CMs. For instance, cocultured hPSC–CMs, hPSC–ECs and amniotic human MSCs embedded in hydrogels exhibited an increase in contractility and survival when compared with hydrogels containing hPSC–CMs alone [127].
Endothelial cell–cell interactions modulate the contractile state of native CMs through paracrine interactions. Nitric oxide in the heart affects the initiation of ventricular relaxation, while endothelin-1 causes CM constriction as well as the release of signaling molecules such as prostaglandin I2 and nitric oxide [128]. Endothelial cells also promote CM survival by expressing neuregulin, which protects against anthracycline-, H2O2- and adrenergic receptor-induced CM death [128]. Additionally, the interaction between endothelial cells and ESC cells has been shown to facilitate ESC differentiation into ESC–CMs [129]. For example, EphrinB4, which is expressed in endothelial cells provide a niche to direct ESC cell differentiation into ESC–CMs [129]. Intercellular interactions are important, however, it is important that the initial distribution of cells within engineered constructs after seeding is related to the distribution of myocardium.
In summary, intercellular communication with other cell types plays an important role in the maintenance of hPSC–CM function as well as induction of hPSC–CM lineage.
Applying microenvironmental cues & intercellular interactions for cardiac tissue engineering
Toward the goal of engineering myocardial tissue constructs for treatment of heart failure, modulation of microenvironmental factors and intercellular interactions have been employed to improve the function and maturation of CMs. Below we provide examples in which microenvironmental factors were employed to construct myocardial tissue constructs for preclinical testing in the ischemic myocardium.
Electrical stimulation
Electric stimulation of cardiac tissue engineered models has been reported to affect CM function. Zhou et al. demonstrated that electric field stimulation of primary CMs cultured in single-walled carbon nanotube/gelatin (SWNT/gelatin) composite scaffolds induced synchronized contractility [130]. SWNT/gelatin showed that the scaffolds were highly microporous structure and a well-developed network in which coil SWNTs were uniformly distributed. The scaffolds were seeded with ventricular cardiac cells from 1-day-old neonatal Sprague–Dawley rats under static conditions for 3 days, following 5 days of electrical field stimulation [130]. These composite scaffolds were then implanted into Sprague–Dawley rats with large myocardial infarct. Four weeks after implantation, there was evidence of integration with the host myocardium, based on migration of the transplanted CMs and integration of the SWNTs with the host myocardium [130]. The engineered myocardial tissue also inhibited further pathological deterioration of infarcted heart [130]. In a related technology, the addition of gold nanowires within the macropores of alginate scaffolds connected nonconducting pore walls that increased electrical signal propagation throughout engineered myocardial tissue [131]. Incorporation of gold nanowires within alginate scaffolds seeded with rat neonatal CMs and fibroblasts resulted in higher levels of the proteins involved in muscle contraction and electrical coupling such as Cx43 [131]. In another example, Barash et al. used a cultivation system that combined electrical stimulation with perfusion of thick, functional engineered myocardial tissue [132]. The custom-made electrical stimulator was integrated by inserting two carbon rod electrodes into a perfusion bioreactor that had several neonatal rat cardiac cell constructs. The study revealed that cultivation for only 4 days under perfusion and continuous electrical stimulus (74.4 mA/cm2, 2 ms, bipolar, 1 Hz) promoted cell elongation and striation, and enhanced the expression level of cx43 gap junctional protein [132].
Biophysical regulation
There are several reported models that utilize biophysical cues. For instance, when cardiosphere-derived cells were combined with nanotopographically defined hydrogels, early cardiomyogenesis was dramatically enhanced [133]. It was reported that only highly anisotropic grooves (400-nm wide) and ridges (400-nm wide and 500-nm high) nanofeatures enhanced cardiac differentiation. The study used ultraviolet assisted capillary nanomolding techniques to produce biomimetic nanostructures of PEG-based hydrogels [133]. When the patches were transplanted in a rat myocardial infarction model there was an enhanced retention of transplanted cells and integration with the host tissue [133]. Similarly, Liau et al. utilized soft lithography to reproducibly generate cardiac tissues with controllable size and 3D architecture [134]. Alignment cues in the fibrin-based hydrogels were developed using photolithographic micromolding techniques [134]. The 3D topographical cues in the fibrin-based hydrogel matrix generated anisotropic 3D cardiac tissue patches with dense, uniformly aligned, highly differentiated and electromechanically coupled murine embryonic stem cell-derived CMs [134]. The patches generated rapid action potential conduction with velocities between 22 and 25 cm/s, and contractile forces of up to 2 mN. Lin et al. also reported CMs cultured on gelatin-coated aligned nondegradable polyacrylonitrile electrospun nanofibers (320 nm in diameter) demonstrated alignment, better beating frequency and amplitude than CMs on randomly oriented nanofibers [135]. When the electrospun nanofibrous cardiac patches were implanted in a rat myocardial infarction model, aligned patches cocultivated with ECs demonstrated improved left ventricle ejection fraction compared with the random patches cocultivated with ECs. Additionally, 1 day after myocardial infarction, transplanted cells seeded on aligned electrospun patches were found to protect a greater number of host CMs against apoptosis [135].
Mechanical stretch
Besides electrical and biophysical cues, application of cyclic mechanical stretch to engineered myocardial tissue has been shown to improve CM alignment along the stretch axis, contractile function and gene expression of phenotypic CM markers [136]. Anisotropic stretch-conditioned fibrin scaffolds seeded with a physiologically relevant population of neonatal rat heart cells (39% CMs, 15% SMA+, <0.5% CD31+) or a CM-depleted population (consisting of 1.8% CMs, 11.5% SMA+, <0.5% CD31+) were reported to ameliorate left ventricular remodeling in immunocompetent rats. Transplantation of patches containing CMs was associated with a reduction in infarct size and LV wall thinning to a greater extent than non-CM patches. Additionally, donor cell invasion and restoration of complete cardiac function was present only in the hearts that received a patch containing both CMs and non-CMs [137]. Moreover, bioreactors are often used to biomechanically stimulate the complex environment of innate CMs. Lux et al. applied cyclic stretch on their decellularized porcine small intestinal submucosa patches, perfused biological vascularized matrix patches as well as a 3D construct containing neonatal rat heart cells [136].
Cell–cell interactions
Intercellular and intracellular interactions have been shown to influence the performance of implanted cells. In one study engineered heart tissues (EHTs) were created from neonatal rat hearts and growth supplements embedded in collagen and Matrigel [138]. The EHTs were reconstituted in circular molds and subjected to mechanical strain. The study used multiloop EHTs that consisted of longitudinally oriented and interconnected muscle strands that contracted spontaneously at 1–2 Hz [138]. Additionally, blood vessels containing donor endothelial cells and smooth muscle cells were found within the EHTs 4 weeks after implanting the multiloop EHTs into male Wistar rats that had large myocardial infarcts [138]. Therefore, the CMs had multicellular interactions with both endothelial and smooth muscle cells. However, the recipient's CMs did not integrate into the EHTs nor did the donor's CMs integrate into the recipient's myocardium. Functionally, the EHTs prevented further dilation, induced systolic wall thickening of infarcted myocardial segments and improved fractional area shortening of infarcted hearts compared with controls [138]. Engineered heart muscle containing 70–95% pure hESC–CMs were similarly reported to enhance sarcomere alignment and increased cx43 expression at 220 days after transplantation after implantation in a chronic rat myocardial infarction model [139]. The study also reported cell death was limited to the first 2 weeks following implantation with no significant cell loss afterward ≤85 days [139]. Therefore, implantation of EHTs made from hESC–CMs therefore, lead to long-term engraftment of implanted cells in a chronic myocardial infarction model. Recently, Wendel et al. developed fibrin gel aligned cardiac patches containing hiPSC–CMs and pericytes [140]. When engrafted in rats with acute infarcts, not only did the CMs survive and proliferate, but the patches also reduced the infarct size and improved the cardiac function [140]. The inclusion of vasculature comprised of endothelial and perivascular cells is another approach that would circumvent cell death within the patch and encourage integration with the host when implanted. For instance, Kedem et al. reported fully vascularized patches 7 days post implantation onto infarcted rat hearts when mature vasculature from the omentum was incorporated in an alginate cardiac patches [141]. The patches, consisting of neonatal cardiac cells, showed electrical integration into the host myocardium 28 days post implantation [141].
Conclusion
Engineering cardiac tissue that mimics native myocardium tissue depends on modulating several microenvironmental cues, including biochemical, electrical, spatial and biomechanical factors, along with intercellular interactions. The implementation of these different microenviromental cues can influence CM differentiation, maturation, organization and electrophysiology, in order to improve the engineered cardiac tissue's functionality and ultimately myocardial function.
Future perspective
Cardiovascular tissue engineering relies on the generation of functional cardiac constructs that can be integrated within the body as treatment for CVDs. However, there are challenges to overcome in order to ensure proper integration of the constructs into the myocardium. CM cell survival in vivo, maturation and organization are the major hurdles to overcome when developing constructs. In order to promote cell homing, engraftment and retention at the treatment site, studies now modify the surface of stem cells. For instance, interactions that typically do not exist between MSCs and the endothelium can be stimulated. Prevascularization of constructs might improve cell survival by providing cells that are not located in the constructs periphery oxygen and nutrients through blood vessels [142]. Incorporation of electronics in cardiac constructs may also allow online monitoring and reporting of engineered-tissue performance [143]. Additionally, new techniques are constantly emerging that take advantage of precise cell organization within constructs such as 3D bioprinting. Despite the challenges, the generation of functional cardiac constructs represents a promising supportive therapy in postsurgical heart recovery therapy.
Executive summary.
Background
Cardiovascular tissue engineering employs the use of stem cells, microenviromental cues and intercellular interactions to generate functional tissue constructs.
Cardiomyocyte (CM) sources include resident cardiac stem cells, adult stem cells and pluripotent stem cells.
Microenvironmental cues
Biochemical molecules and growth factors have been employed to promote stem cell differentiation into CMs. These molecules are present in biochemical signaling pathways that regulate embryonic development of the myocardium.
The extracellular matrix, which is a scaffolding structure of extracellular proteins in which cells reside, modulate cell function in part through spatial factors. Spatial factors rely on the use of spatial patterning to mimic the cell-to-cell and cell-to-extracellular matrix interactions of CMs.
Biomechanical stimulation of CMs is accomplished by altering substrate stiffness and stretching CMs.
Intercellular interactions between CMs and non-CMs occur through electromechanical signaling and paracrine signaling.
Applications for cardiac tissue engineering
Microenvironmental stimulation of CMs affect CM proliferation, maturation, organization, survival and electrophysiological characteristics.
Microenvironmental stimulation of CMs and incorporation of intercellular interactions from non-CMs are used to generate cardiac constructs that can be implanted into the diseased myocardium.
Challenges
CM cell survival in vivo, maturation and organization.
Footnotes
Financial & competing interests disclosure
This work was supported in part by grants to NF Huang from the US NIH (R00HL098688, R01HL127113 and R21EB020235), a Merit Review Award (1I01BX002310) from the Department of Veterans Affairs Biomedical Laboratory Research and Development service, the Stanford Chemistry Engineering & Medicine for Human Health, the Stanford Cardiovascular Institute and a McCormick Gabilan fellowship. M Wanjare was supported by a diversity supplement throughthe US NIH (R01HL127113). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: a report from the American Heart Association. Circulation. 2015;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am. J. Physiol. Heart Circ. Physiol. 2007;293(3):H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 3.Zak R. Development and proliferative capacity of cardiac muscle cells. Circ. Res. 1974;35(Suppl. 2):17–26. [PubMed] [Google Scholar]
- 4.Nag A. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1979;28(109):41–61. [PubMed] [Google Scholar]
- 5.Mccall SJ, Nassar R, Malouf NN, et al. Development and cardiac contractility: cardiac troponin T isoforms and cytosolic calcium in rabbit. Pediatr. Res. 2006;60(3):276–281. doi: 10.1203/01.pdr.0000233004.95404.1f. [DOI] [PubMed] [Google Scholar]
- 6.Edwards JG, Ghaleh B. Divergence of beta-myosin heavy chain (betaMHC) expression in fetal rat cardiomyocytes in vitro and adult rat heart in vivo . Biochem. Biophys. Res. Commun. 1997;230(2):340–343. doi: 10.1006/bbrc.1996.5963. [DOI] [PubMed] [Google Scholar]
- 7.Eikemo H, Moltzau LR, Hussain RI, et al. CaMKII in addition to MLCK contributes to phosphorylation of regulatory light chain in cardiomyocytes. Biochem. Biophys. Res. Commun. 2016;471(1):219–225. doi: 10.1016/j.bbrc.2016.01.132. [DOI] [PubMed] [Google Scholar]
- 8.Kasahara H, Ueyama T, Wakimoto H, et al. Nkx2.5 homeoprotein regulates expression of gap junction protein connexin 43 and sarcomere organization in postnatal cardiomyocytes. J. Mol. Cell. Cardiol. 2003;35(3):243–256. doi: 10.1016/s0022-2828(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 9.Vidyasekar P, Shyamsunder P, Santhakumar R, Arun R, Verma RS. A simplified protocol for the isolation and culture of cardiomyocytes and progenitor cells from neonatal mouse ventricles. Eur. J. Cell Biol. 2015;94(10):444–452. doi: 10.1016/j.ejcb.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Den Haan AD, Veldkamp MW, Bakker D, et al. Organ explant culture of neonatal rat ventricles: a new model to study gene and cell therapy. PLoS ONE. 2013;8(3):e59290. doi: 10.1371/journal.pone.0059290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian QH, Oberhofer M, Ruppenthal S, et al. Optical measurement of action potential in adult ventricular myocytes. Biophys. J. 2011;100(3):292–292. [Google Scholar]
- 12.Trantidou T, Terracciano CM, Kontziampasis D, Humphrey EJ, Prodromakis T. Biorealistic cardiac cell culture platforms with integrated monitoring of extracellular action potentials. Sci. Rep. 2015;5:11067. doi: 10.1038/srep11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SA, Lee SR, Tung L, Yue DT. Optical mapping of optogenetically shaped cardiac action potentials. Sci. Rep. 2014;4:6125. doi: 10.1038/srep06125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folmes CD, Dzeja PP, Nelson TJ, Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 11(5):596–606. doi: 10.1016/j.stem.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung S, Dzeja PP, Faustino RS, Perez-Terzic C, Behfar A, Terzic A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat. Clin. Pract. Cardiovasc. Med. 2007;4(Suppl. 1):S60–S67. doi: 10.1038/ncpcardio0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan K, Furst DO, Wobus AM. Modulation of sarcomere organization during embryonic stem cell-derived cardiomyocyte differentiation. Eur. J. Cell Biol. 1999;78(11):813–823. doi: 10.1016/S0171-9335(99)80032-6. [DOI] [PubMed] [Google Scholar]
- 17.Soonpaa MH, Field LJ. Assessment of cardiomyocyte DNA synthesis during hypertrophy in adult mice. Am. J. Physiol. 1994;266(4 Pt 2):H1439–H1445. doi: 10.1152/ajpheart.1994.266.4.H1439. [DOI] [PubMed] [Google Scholar]
- 18.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 19.Khan M, Koch WJ. c-kit+ cardiac stem cells: spontaneous creation or a perplexing reality. Circ. Res. 2016;118(5):783–785. doi: 10.1161/CIRCRESAHA.115.308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira-Martins J, Ogorek B, Cappetta D, et al. Cardiomyogenesis in the developing heart is regulated by C-kit-positive cardiac stem cells. Circ. Res. 2012;110(5):701–715. doi: 10.1161/CIRCRESAHA.111.259507. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Torella D, Ellison GM, Mendez-Ferrer S, Ibanez B, Nadal-Ginard B. Resident human cardiac stem cells: role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat. Clin. Pract. Cardiovasc. Med. 2006;3(Suppl. 1):S8–S13. doi: 10.1038/ncpcardio0409. [DOI] [PubMed] [Google Scholar]
- 22.Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121(18):1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tallini YN, Greene KS, Craven M, et al. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc. Natl Acad. Sci. USA. 2009;106(6):1808–1813. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Chen H, Feng B, et al. Isolation and characterization of a Sca-1+/CD31- progenitor cell lineage derived from mouse heart tissue. BMC Biotechnol. 2014;14(1):75. doi: 10.1186/1472-6750-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl Acad. Sci. USA. 2003;100(21):12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin CM, Meeson AP, Robertson SM, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev. Biol. 2004;265(1):262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 27.Meissner K, Heydrich B, Jedlitschky G, et al. The ATP-binding cassette transporter ABCG2 (BCRP), a marker for side population stem cells, is expressed in human heart. J. Histochem. Cytochem. 2006;54(2):215–221. doi: 10.1369/jhc.5A6750.2005. [DOI] [PubMed] [Google Scholar]
- 28.Doyle MJ, Maher TJ, Li Q, Garry M, Sorrentino BP, Martin CM. Abcg2 labeled cells contribute to different cell populations in the embryonic and adult heart. Stem Cells Dev. 2016;25(3):277–284. doi: 10.1089/scd.2015.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maher TJ, Ren Y, Li Q, et al. ATP-binding cassette transporter Abcg2 lineage contributes to the cardiac vasculature after oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2014;306(12):H1610–H1618. doi: 10.1152/ajpheart.00638.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc. Natl Acad. Sci. USA. 2007;104(35):14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosoda T, D'amario D, Cabral-Da-Silva MC, et al. Clonality of mouse and human cardiomyogenesis in vivo . Proc. Natl Acad. Sci. USA. 2009;106(40):17169–17174. doi: 10.1073/pnas.0903089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatzistergos KE, Takeuchi LM, Saur D, et al. cKit+ cardiac progenitors of neural crest origin. Proc. Natl Acad. Sci. USA. 2015;112(42):13051–13056. doi: 10.1073/pnas.1517201112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Berlo JH, Kanisicak O, Maillet M, et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509(7500):337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sultana N, Zhang L, Yan J, et al. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat. Commun. 2015;6:8701. doi: 10.1038/ncomms9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Can A. Searching for in vivo traces of mesenchymal stem cells and their ancestors. In: Turksen K, editor. Adult and Embryonic Stem Cells. Humana Press; Totowa, NJ, USA: 2012. pp. 11–24. [Google Scholar]
- 36.Da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 37.Planat-Benard V, Menard C, Andre M, et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ. Res. 2004;94(2):223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 38.Xu WR, Zhang XR, Qian H, et al. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro . Exp. Biol. Med. 2004;229(7):623–631. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 39.Fraser JK, Schreiber R, Strem B, et al. Plasticity of human adipose stem cells toward endothelial cells and cardiomyocytes. Nat. Clin. Pract. Cardiovasc. Med. 2006;3(Suppl. 1):S33–S37. doi: 10.1038/ncpcardio0444. [DOI] [PubMed] [Google Scholar]
- 40.Cuende N, Rico L, Herrera C. Concise review: bone marrow mononuclear cells for the treatment of ischemic syndromes: medicinal product or cell transplantation? Stem Cells Transl. Med. 2012;1(5):403–408. doi: 10.5966/sctm.2011-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dawn B, Abdel-Latif A, Sanganalmath SK, Flaherty MP, Zuba-Surma EK. Cardiac repair with adult bone marrow-derived cells: the clinical evidence. Antioxid. Redox Signal. 2009;11(8):1865–1882. doi: 10.1089/ars.2009.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flaherty MP, Abdel-Latif A, Li Q, et al. Noncanonical Wnt11 signaling is sufficient to induce cardiomyogenic differentiation in unfractionated bone marrow mononuclear cells. Circulation. 2008;117(17):2241–2252. doi: 10.1161/CIRCULATIONAHA.107.741066. [DOI] [PubMed] [Google Scholar]
- 43.Yang HS, Bhang SH, Kim IK, et al. In situ cardiomyogenic differentiation of implanted bone marrow mononuclear cells by local delivery of transforming growth factor-beta1. Cell Transplant. 2012;21(1):299–312. doi: 10.3727/096368911X580527. [DOI] [PubMed] [Google Scholar]
- 44.De Los Angeles A, Ferrari F, Xi R, et al. Hallmarks of pluripotency. Nature. 2015;525(7570):469–478. doi: 10.1038/nature15515. [DOI] [PubMed] [Google Scholar]
- 45.Jha R, Xu RH, Xu C. Efficient differentiation of cardiomyocytes from human pluripotent stem cells with growth factors. Methods Mol. Biol. 2015;1299:115–131. doi: 10.1007/978-1-4939-2572-8_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Wilson GF, Soerens AG, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009;104(4):e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doherty KR, Talbert DR, Trusk PB, Moran DM, Shell SA, Bacus S. Structural and functional screening in human induced-pluripotent stem cell-derived cardiomyocytes accurately identifies cardiotoxicity of multiple drug types. Toxicol. Appl. Pharmacol. 2015;285(1):51–60. doi: 10.1016/j.taap.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Feinberg AW, Ripplinger CM, Van Der Meer P, et al. Functional differences in engineered myocardium from embryonic stem cell-derived versus neonatal cardiomyocytes. Stem Cell Rep. 2013;1(5):387–396. doi: 10.1016/j.stemcr.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22(14):1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolossov E, Bostani T, Roell W, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J. Exp. Med. 2006;203(10):2315–2327. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nussbaum J, Minami E, Laflamme MA, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21(7):1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]; • This publication characterizes the formation of teratomas as a result of embryonic stem cell implantation into the myocardium.
- 52.Drawnel FM, Boccardo S, Prummer M, et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014;9(3):810–821. doi: 10.1016/j.celrep.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 53.Ribeiro AJ, Ang YS, Fu JD, et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl Acad. Sci. USA. 2015;112(41):12705–12710. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This publication discusses the role of rigidity and spatial patterning of human pluripotent stem cell–cardiomyocytes (hPSC–CMs) to enhance cellular maturity.
- 54.Kadari A, Mekala S, Wagner N, et al. Robust generation of cardiomyocytes from human iPS cells requires precise modulation of BMP and WNT signaling. Stem Cell Rev. 2015;11(4):560–569. doi: 10.1007/s12015-014-9564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lian X, Hsiao C, Wilson G, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl Acad. Sci. USA. 2012;109(27):E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This publication describes a frequently used protocol using chemical factors to reproducibly generate hPSC–CMs.
- 56.Burridge PW, Matsa E, Shukla P, et al. Chemically defined generation of human cardiomyocytes. Nat. Methods. 2014;11(8):855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This publication describes a simple and reproducible chemical approach to generate hPSC–CMs.
- 57.Lian X, Zhang J, Azarin SM, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 2013;8(1):162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen VC, Stull R, Joo D, Cheng X, Keller G. Notch signaling respecifies the hemangioblast to a cardiac fate. Nat. Biotech. 2008;26(10):1168–1178. doi: 10.1038/nbt.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tirosh-Finkel L, Zeisel A, Brodt-Ivenshitz M, et al. BMP-mediated inhibition of FGF signaling promotes cardiomyocyte differentiation of anterior heart field progenitors. Development. 2010;137(18):2989–3000. doi: 10.1242/dev.051649. [DOI] [PubMed] [Google Scholar]
- 60.Kattman SJ, Witty AD, Gagliardi M, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8(2):228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 61.Mcdevitt TC, Laflamme MA, Murry CE. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. J. Mol. Cell. Cardiol. 2005;39(6):865–873. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li P, Cavallero S, Gu Y, et al. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development. 2011;138(9):1795–1805. doi: 10.1242/dev.054338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torella D, Rota M, Nurzynska D, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ. Res. 2004;94(4):514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 64.Lee WL, Chen JW, Ting CT, Lin SJ, Wang PH. Changes of the insulin-like growth factor I system during acute myocardial infarction: implications on left ventricular remodeling. J. Clin. Endocrinol. Metab. 1999;84(5):1575–1581. doi: 10.1210/jcem.84.5.5676. [DOI] [PubMed] [Google Scholar]
- 65.Wobus AM, Kaomei G, Shan J, et al. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 1997;29(6):1525–1539. doi: 10.1006/jmcc.1997.0433. [DOI] [PubMed] [Google Scholar]
- 66.Rohwedel J, Guan K, Wobus AM. Induction of cellular differentiation by retinoic acid in vitro . Cells Tissues Organs. 1999;165(3–4):190–202. doi: 10.1159/000016699. [DOI] [PubMed] [Google Scholar]
- 67.Serena E, Figallo E, Tandon N, et al. Electrical stimulation of human embryonic stem cells: cardiac differentiation and the generation of reactive oxygen species. Exp. Cell Res. 2009;315(20):3611–3619. doi: 10.1016/j.yexcr.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan Y, Richards D, Xu R, et al. Silicon nanowire-induced maturation of cardiomyocytes derived from human induced pluripotent stem cells. Nano Lett. 2015;15(5):2765–2772. doi: 10.1021/nl502227a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nunes SS, Miklas JW, Liu J, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat. Methods. 2013;10(8):781–787. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miklas JW, Nunes SS, Zhang B, Radisic M. Design and fabrication of biological wires. Methods Mol. Biol. 2014;1181:157–165. doi: 10.1007/978-1-4939-1047-2_14. [DOI] [PubMed] [Google Scholar]
- 71.Carson D, Hnilova M, Yang X, et al. Nanotopography-induced structural anisotropy and sarcomere development in human cardiomyocytes derived from induced pluripotent stem cells. ACS Appl. Mater. Interfaces. 2016;8(34):21923–21932. doi: 10.1021/acsami.5b11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Birket MJ, Ribeiro MC, Verkerk AO, et al. Expansion and patterning of cardiovascular progenitors derived from human pluripotent stem cells. Nat. Biotechnol. 2015;33(9):970–979. doi: 10.1038/nbt.3271. [DOI] [PubMed] [Google Scholar]
- 73.Tsang KM, Annabi N, Ercole F, et al. Facile one-step micropatterning using photodegradable methacrylated gelatin hydrogels for improved cardiomyocyte organization and alignment. Adv. Funct. Mater. 2015;25(6):977–986. doi: 10.1002/adfm.201403124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heidi Au HT, Cui B, Chu ZE, Veres T, Radisic M. Cell culture chips for simultaneous application of topographical and electrical cues enhance phenotype of cardiomyocytes. Lab Chip. 2009;9(4):564–575. doi: 10.1039/b810034a. [DOI] [PubMed] [Google Scholar]
- 75.Rodriguez AG, Han SJ, Regnier M, Sniadecki NJ. Substrate stiffness increases twitch power of neonatal cardiomyocytes in correlation with changes in myofibril structure and intracellular calcium. Biophys. J. 2011;101(10):2455–2464. doi: 10.1016/j.bpj.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jacot JG, Mcculloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys. J. 2008;95(7):3479–3487. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parikh A, Wu J, Blanton RM, Tzanakakis ES. Signaling pathways and gene regulatory networks in cardiomyocyte differentiation. Tissue Eng. Part B Rev. 2015;21(4):377–392. doi: 10.1089/ten.teb.2014.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rochais F, Mesbah K, Kelly RG. Signaling pathways controlling second heart field development. Circ. Res. 2009;104(8):933–942. doi: 10.1161/CIRCRESAHA.109.194464. [DOI] [PubMed] [Google Scholar]
- 79.Willis M, Homeister JW, Stone JR. Cellular and Molecular Pathobiology of Cardiovascular Disease. Academic Press; Cambridge, MA, USA: 2014. [Google Scholar]
- 80.Niessen K, Karsan A. Notch signaling in cardiac development. Circ. Res. 2008;102(10):1169–1181. doi: 10.1161/CIRCRESAHA.108.174318. [DOI] [PubMed] [Google Scholar]
- 81.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101(6):660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ito H, Hiroe M, Hirata Y, et al. Insulin-like growth factor-I induces hypertrophy with enhanced expression of muscle specific genes in cultured rat cardiomyocytes. Circulation. 1993;87(5):1715–1721. doi: 10.1161/01.cir.87.5.1715. [DOI] [PubMed] [Google Scholar]
- 83.Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 84.Van Den Heuvel NH, Van Veen TA, Lim B, Jonsson MK. Lessons from the heart: mirroring electrophysiological characteristics during cardiac development to in vitro differentiation of stem cell derived cardiomyocytes. J. Mol. Cell. Cardiol. 2014;67(0):12–25. doi: 10.1016/j.yjmcc.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 85.Chan YC, Ting S, Lee YK, et al. Electrical stimulation promotes maturation of cardiomyocytes derived from human embryonic stem cells. J. Cardiovasc. Transl. Res. 2013;6(6):989–999. doi: 10.1007/s12265-013-9510-z. [DOI] [PubMed] [Google Scholar]
- 86.Thavandiran N, Dubois N, Mikryukov A, et al. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc. Natl Acad. Sci. USA. 2013;110(49):E4698–E4707. doi: 10.1073/pnas.1311120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerecht-Nir S, Radisic M, Park H, et al. Biophysical regulation during cardiac development and application to tissue engineering. Int. J. Dev. Biol. 2006;50(2–3):233–243. doi: 10.1387/ijdb.052041sg. [DOI] [PubMed] [Google Scholar]
- 88.Valderrabano M. Influence of anisotropic conduction properties in the propagation of the cardiac action potential. Prog. Biophys. Mol. Biol. 2007;94(1–2):144–168. doi: 10.1016/j.pbiomolbio.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang PY, Yu J, Lin JH, Tsai WB. Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates. Acta Biomater. 2011;7(9):3285–3293. doi: 10.1016/j.actbio.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 90.Sia J, Yu P, Srivastava D, Li S. Effect of biophysical cues on reprogramming to cardiomyocytes. Biomaterials. 2016;103:1–11. doi: 10.1016/j.biomaterials.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 91.Lim JY, Donahue HJ. Cell sensing and response to micro- and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng. 2007;13(8):1879–1891. doi: 10.1089/ten.2006.0154. [DOI] [PubMed] [Google Scholar]
- 92.Nikkhah M, Edalat F, Manoucheri S, Khademhosseini A. Engineering microscale topographies to control the cell-substrate interface. Biomaterials. 2012;33(21):5230–5246. doi: 10.1016/j.biomaterials.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim DH, Lipke EA, Kim P, et al. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc. Natl Acad. Sci. USA. 2010;107(2):565–570. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martínez E, Pla-Roca M, Samitier J. Micro/nanopatterning of proteins using a nanoimprint-based contact printing technique. Methods Mol. Biol. 2012;811:79–87. doi: 10.1007/978-1-61779-388-2_5. [DOI] [PubMed] [Google Scholar]
- 95.Salick MR, Napiwocki BN, Sha J, et al. Micropattern width dependent sarcomere development in human ESC-derived cardiomyocytes. Biomaterials. 2014;35(15):4454–4464. doi: 10.1016/j.biomaterials.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kai D, Jin G, Prabhakaran MP, Ramakrishna S. Electrospun synthetic and natural nanofibers for regenerative medicine and stem cells. Biotechnol. J. 2013;8(1):59–72. doi: 10.1002/biot.201200249. [DOI] [PubMed] [Google Scholar]
- 97.Castaño O, Eltohamy M, Kim H-W. Electrospinning technology in tissue regeneration. Methods Mol. Biol. 2012;811:127–140. doi: 10.1007/978-1-61779-388-2_9. [DOI] [PubMed] [Google Scholar]
- 98.Corda S, Samuel JL, Rappaport L. Extracellular matrix and growth factors during heart growth. Heart Fail. Rev. 2000;5(2):119–130. doi: 10.1023/A:1009806403194. [DOI] [PubMed] [Google Scholar]
- 99.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011;6(1):13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carver W, Burgess M, Jyring R, et al. Extracellular matrix expression, organization, and interaction with heart myocytes during development and disease. In: Figulla HR, Kandolf R, McManus B, editors. Idiopathic Dilated Cardiomyopathy: Cellular and Molecular Mechanisms, Clinical Consequences. Springer; Berlin, Germany: 1993. pp. 97–108. [Google Scholar]
- 101.Orlova Y, Magome N, Liu L, Chen Y, Agladze K. Electrospun nanofibers as a tool for architecture control in engineered cardiac tissue. Biomaterials. 2011;32(24):5615–5624. doi: 10.1016/j.biomaterials.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 102.Parrag IC, Zandstra PW, Woodhouse KA. Fiber alignment and coculture with fibroblasts improves the differentiated phenotype of murine embryonic stem cell-derived cardiomyocytes for cardiac tissue engineering. Biotechnol. Bioeng. 2012;109(3):813–822. doi: 10.1002/bit.23353. [DOI] [PubMed] [Google Scholar]
- 103.Kai D, Prabhakaran MP, Jin G, Ramakrishna S. Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2011;98(2):379–386. doi: 10.1002/jbm.b.31862. [DOI] [PubMed] [Google Scholar]
- 104.Bursac N, Loo Y, Leong K, Tung L. Novel anisotropic engineered cardiac tissues: studies of electrical propagation. Biochem. Biophys. Res. Commun. 2007;361(4):847–853. doi: 10.1016/j.bbrc.2007.07.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ehler E, Jayasinghe SN. Cell electrospinning cardiac patches for tissue engineering the heart. Analyst. 2014;139(18):4449–4452. doi: 10.1039/c4an00766b. [DOI] [PubMed] [Google Scholar]
- 106.Townsend-Nicholson A, Jayasinghe SN. Cell electrospinning: a unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules. 2006;7(12):3364–3369. doi: 10.1021/bm060649h. [DOI] [PubMed] [Google Scholar]
- 107.Happe CL, Engler AJ. Mechanical forces reshape differentiation cues that guide cardiomyogenesis. Circ. Res. 2016;118(2):296–310. doi: 10.1161/CIRCRESAHA.115.305139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jacot JG, Martin JC, Hunt DL. Mechanobiology of cardiomyocyte development. J. Biomech. 2010;43(1):93–98. doi: 10.1016/j.jbiomech.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aratyn-Schaus Y, Pasqualini FS, Yuan H, et al. Coupling primary and stem cell-derived cardiomyocytes in an in vitro model of cardiac cell therapy. J. Cell Biol. 2016;212(4):389–397. doi: 10.1083/jcb.201508026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alapan Y, Younesi M, Akkus O, Gurkan UA. Anisotropically stiff 3D micropillar niche induces extraordinary cell alignment and elongation. Adv. Healthc. Mater. 2016;5(15):1884–1892. doi: 10.1002/adhm.201600096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Young JL, Kretchmer K, Ondeck MG, Zambon AC, Engler AJ. Mechanosensitive kinases regulate stiffness-induced cardiomyocyte maturation. Sci. Rep. 2014;4:6425. doi: 10.1038/srep06425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heras-Bautista CO, Katsen-Globa A, Schloerer NE, et al. The influence of physiological matrix conditions on permanent culture of induced pluripotent stem cell-derived cardiomyocytes. Biomaterials. 2014;35(26):7374–7385. doi: 10.1016/j.biomaterials.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 113.Bhana B, Iyer RK, Chen WL, et al. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol. Bioeng. 2010;105(6):1148–1160. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- 114.Qiu Y, Bayomy AF, Gomez MV, et al. A role for matrix stiffness in the regulation of cardiac side population cell function. Am. J. Physiol. Heart Circ. Physiol. 2015;308(9):H990–H997. doi: 10.1152/ajpheart.00935.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am. J. Physiol. Cell Physiol. 2008;295(4):C1037–C1044. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- 116.Macri-Pellizzeri L, Pelacho B, Sancho A, et al. Substrate stiffness and composition specifically direct differentiation of induced pluripotent stem cells. Tissue Eng. Part A. 2015;21(9–10):1633–1641. doi: 10.1089/ten.TEA.2014.0251. [DOI] [PubMed] [Google Scholar]
- 117.Qi Y, Li Z, Kong CW, et al. Uniaxial cyclic stretch stimulates TRPV4 to induce realignment of human embryonic stem cell-derived cardiomyocytes. J. Mol. Cell. Cardiol. 2015;87:65–73. doi: 10.1016/j.yjmcc.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 118.Gwak SJ, Bhang SH, Kim IK, et al. The effect of cyclic strain on embryonic stem cell-derived cardiomyocytes. Biomaterials. 2008;29(7):844–856. doi: 10.1016/j.biomaterials.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 119.Pereira MB, Santos AM, Goncalves DC, et al. alphaB-crystallin interacts with and prevents stress-activated proteolysis of focal adhesion kinase by calpain in cardiomyocytes. Nat. Commun. 2014;5:5159. doi: 10.1038/ncomms6159. [DOI] [PubMed] [Google Scholar]
- 120.Foldes G, Mioulane M, Wright JS, et al. Modulation of human embryonic stem cell-derived cardiomyocyte growth: a testbed for studying human cardiac hypertrophy? J. Mol. Cell. Cardiol. 2011;50(2):367–376. doi: 10.1016/j.yjmcc.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lal H, Verma SK, Smith M, et al. Stretch-induced MAP kinase activation in cardiac myocytes: differential regulation through beta1-integrin and focal adhesion kinase. J. Mol. Cell. Cardiol. 2007;43(2):137–147. doi: 10.1016/j.yjmcc.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Suciu L, Nicolescu MI, Popescu LM. Cardiac telocytes: serial dynamic images in cell culture. J. Cell. Mol. Med. 2010;14(11):2687–2692. doi: 10.1111/j.1582-4934.2010.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vannucchi MG, Bani D, Faussone-Pellegrini MS. Telocytes contribute as cell progenitors and differentiation inductors in tissue regeneration. Curr. Stem Cell Res. Ther. 2016;11(5):383–389. doi: 10.2174/1574888x10666150528142741. [DOI] [PubMed] [Google Scholar]
- 124.Gherghiceanu M, Popescu LM. Cardiac telocytes – their junctions and functional implications. Cell Tissue Res. 2012;348(2):265–279. doi: 10.1007/s00441-012-1333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Howard CM, Baudino TA. Dynamic cell-cell and cell-ECM interactions in the heart. J. Mol. Cell. Cardiol. 2014;70:19–26. doi: 10.1016/j.yjmcc.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 126.Tirziu D, Giordano FJ, Simons M. Cell communications in the heart. Circulation. 2010;122(9):928–937. doi: 10.1161/CIRCULATIONAHA.108.847731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Burridge PW, Metzler SA, Nakayama KH, et al. Multi-cellular interactions sustain long-term contractility of human pluripotent stem cell-derived cardiomyocytes. Am. J. Transl. Res. 2014;6(6):724–735. [PMC free article] [PubMed] [Google Scholar]
- 128.Hsieh PC, Davis ME, Lisowski LK, Lee RT. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu. Rev. Physiol. 2006;68:51–66. doi: 10.1146/annurev.physiol.68.040104.124629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen K, Bai H, Arzigian M, et al. Endothelial cells regulate cardiomyocyte development from embryonic stem cells. J. Cell. Biochem. 2010;111(1):29–39. doi: 10.1002/jcb.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou J, Chen J, Sun H, et al. Engineering the heart: evaluation of conductive nanomaterials for improving implant integration and cardiac function. Sci. Rep. 2014;4:3733. doi: 10.1038/srep03733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dvir T, Timko BP, Brigham MD, et al. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 2011;6(11):720–725. doi: 10.1038/nnano.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barash Y, Dvir T, Tandeitnik P, Ruvinov E, Guterman H, Cohen S. Electric field stimulation integrated into perfusion bioreactor for cardiac tissue engineering. Tissue Eng. Part C. 2010;16(6):1417–1426. doi: 10.1089/ten.TEC.2010.0068. [DOI] [PubMed] [Google Scholar]
- 133.Kim DH, Kshitiz, Smith RR, et al. Nanopatterned cardiac cell patches promote stem cell niche formation and myocardial regeneration. Integr. Biol. (Camb.) 2012;4(9):1019–1033. doi: 10.1039/c2ib20067h. [DOI] [PubMed] [Google Scholar]
- 134.Liau B, Christoforou N, Leong KW, Bursac N. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials. 2011;32(35):9180–9187. doi: 10.1016/j.biomaterials.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin YD, Ko MC, Wu ST, et al. A nanopatterned cell-seeded cardiac patch prevents electro-uncoupling and improves the therapeutic efficacy of cardiac repair. Biomater. Sci. 2014;2(4):567–580. doi: 10.1039/c3bm60289c. [DOI] [PubMed] [Google Scholar]
- 136.Lux M, Andree B, Horvath T, et al. In vitro maturation of large-scale cardiac patches based on a perfusable starter matrix by cyclic mechanical stimulation. Acta Biomater. 2016;30:177–187. doi: 10.1016/j.actbio.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 137.Wendel JS, Ye L, Zhang P, Tranquillo RT, Zhang JJ. Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng. Part A. 2014;20(7–8):1325–1335. doi: 10.1089/ten.tea.2013.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zimmermann W-H, Melnychenko I, Wasmeier G, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 2006;12(4):452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]; •• This seminal work demonstrated that implanted engineered myocardial tissue constructs could improve cardiac function.
- 139.Riegler J, Tiburcy M, Ebert A, et al. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ. Res. 2015;117(8):720–730. doi: 10.1161/CIRCRESAHA.115.306985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wendel JS, Ye L, Tao R, et al. Functional effects of a tissue-engineered cardiac patch from human induced pluripotent stem cell-derived cardiomyocytes in a rat infarct model. Stem Cells Transl. Med. 2015;4(11):1324–1332. doi: 10.5966/sctm.2015-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dvir T, Kedem A, Ruvinov E, et al. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc. Natl Acad. Sci. USA. 2009;106(35):14990–14995. doi: 10.1073/pnas.0812242106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ogle BM, Bursac N, Domian I, et al. Distilling complexity to advance cardiac tissue engineering. Sci. Transl. Med. 2016;8(342):342ps313. doi: 10.1126/scitranslmed.aad2304. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This opinion paper provides an overview from leading experts in the field of the current challenges in cardiac tissue engineering.
- 143.Feiner R, Engel L, Fleischer S, et al. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat. Mater. 2016;15(6):679–685. doi: 10.1038/nmat4590. [DOI] [PMC free article] [PubMed] [Google Scholar]