Abstract
Aims
The primary aim of the CANagliflozin cardioVascular Assessment Study‐Renal (CANVAS‐R) is to determine whether the favourable effects of inhibition of the sodium glucose co‐transporter 2 (SGLT2) on blood glucose, blood pressure and body weight are accompanied by protection against adverse renal outcomes.
Materials and methods
CANVAS‐R is a prospective, randomized, double‐blind, placebo‐controlled trial in patients with type 2 diabetes with a history or high risk of cardiovascular events. Patients were randomly assigned to once‐daily placebo or canagliflozin 100 mg (with optional uptitration to 300 mg) for a planned average of 2.5 years of follow‐up. The primary outcome is kidney disease progression, defined by class change in albuminuria. The two secondary outcomes are the composite of hospitalized heart failure or cardiovascular death, and cardiovascular death alone. Effects on end‐stage renal disease and a range of other outcomes will also be explored.
Results
A total of 5812 participants were recruited at 422 sites in 24 countries between January 2014 and May 2015. The mean baseline age was 64 years, mean duration of diabetes was 14 years, mean glycated haemoglobin level was 8.3% and mean body mass index was 32 kg/m2. Of these participants, 37% were women, 71% had a history of cardiovascular disease, 22.3% had microalbuminuria and 8.7% had macroalbuminuria. The mean baseline estimated glomerular filtration rate was 76 mL/min/1.73 m2. The study will have at least 90% power ( P = .05) to detect a 22% or greater reduction in the risk of progression of albuminuria.
Conclusions
The trial should define the potential renoprotective effect of canagliflozin and will provide additional important new data about its effects on vascular outcomes, death and kidney failure.
Keywords: cardiovascular disease, SGLT2 inhibitor, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is associated with a significant global health burden,1 and kidney disease is a frequent complication closely associated with an increased risk of adverse cardiovascular outcomes. Renal haemodynamic changes and increased leakage of albumin into the urine (microalbuminuria) are the first signs of kidney involvement and predict an increased risk of progression to end‐stage renal disease (ESRD), requiring dialysis, transplantation or both. The global epidemic of T2DM has made diabetes the leading reason for ESRD requiring dialysis in most countries.1 Interventions to prevent the development and progression of chronic kidney disease, in conjunction with the prevention of other cardiovascular events, are therefore a priority.
Effective control of blood glucose levels, blood pressure, blood lipids and albuminuria is the current mainstay of therapy for the prevention of cardiovascular and renal disease in patients with T2DM. Drugs that target these risk factors have proven effective in preventing serious renal and cardiovascular endpoints.2, 3, 4, 5, 6, 7 The first large trial of a sodium glucose co‐transporter 2 (SGLT2) inhibitor was completed last year and suggested protective effects of empagliflozin against a range of vascular and renal outcomes.8, 9 Summary data for all SGLT2 inhibitors suggest that similar effects can be anticipated from other compounds in the class.10
The CANagliflozin cardioVascular Assessment Study–Renal (CANVAS‐R) trial will test the effect of canagliflozin on the primary outcome of renal disease progression in T2DM by defining the effect on transitions between normoalbuminuria, microalbuminuria and macroalbuminuria. Alone, and in conjunction with the ongoing CANVAS trial,11 it will also provide important new data about the effects of the compound on vascular outcomes, death and chronic kidney disease.
The primary objective of CANVAS‐R is to determine the effects of canagliflozin compared with placebo on the progression of albuminuria against a background of standard of care. The primary null hypothesis to be tested is that there is no difference in the progression of albuminuria between patients treated with canagliflozin and those treated with placebo. The secondary objectives of the study are to determine the effects of canagliflozin compared with placebo on, first, the composite outcome of hospitalization for heart failure or cardiovascular death and, second, on cardiovascular death alone. Exploratory outcomes will include regression of albuminuria, albumin‐to‐creatinine ratio (ACR), estimated glomerular filtration rate (eGFR) and composite major renal outcomes. The cardiovascular outcome data from the CANVAS‐R trial will also be combined with the data from CANVAS (ClinicalTrials.gov Identifier: NCT01032629) in an integrated analysis to enable the better evaluation of the cardiovascular safety and efficacy of canagliflozin.
2. MATERIALS AND METHODS
CANVAS‐R is a randomized, double‐blind, placebo‐controlled, parallel‐group, multicentre trial (Figure 1). The study has received approval from national regulatory agencies in 24 countries and the local ethics committees of all participating sites. The study is being conducted in accordance with ethical principles that comply with the Declaration of Helsinki, Good Clinical Research Practices and applicable regulatory requirements. Recruitment commenced in January 2014 and was completed at 422 participating sites in May 2015. The trial is registered at ClinicalTrials.gov (NCT01989754).
Figure 1.
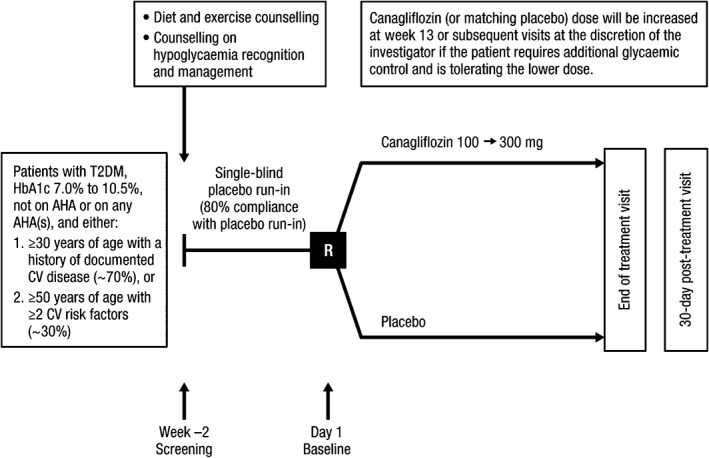
Trial design. AHA, antihyperglycaemic agent; CV, cardiovascular; R, randomization.
2.1. Participant inclusion and exclusion criteria
Participants in the CANVAS‐R trial are men and women with T2DM who had inadequate glycaemic control at baseline (glycated haemoglobin [HbA1c] ≥7.0% and ≤10.5%) and have a history or are at an elevated risk of cardiovascular disease. All participants were required to provide informed consent, to be willing and able to adhere to the study protocol and to successfully complete the trial's placebo run‐in period. To ensure the recruitment of a broad population group, there were minimal restrictions on the use of background therapies for glucose control or cardiovascular risk factor management. The inclusion criteria were designed to be identical to the twinned CANVAS study and were powered to achieve an event rate of ≥2.25% per year11 for the cardiovascular outcome (cardiovascular death, myocardial infarction or stroke) so that the data can be seamlessly integrated with data from CANVAS to address safety, as stipulated by the US Food and Drug Administration (FDA), and efficacy.12 Accordingly, participants were either aged ≥30 years with a history of symptomatic atherosclerotic vascular disease (coronary, cerebrovascular or peripheral) or aged ≥50 years with ≥2 of the following risk factors for vascular disease: duration of diabetes ≥10 years, systolic blood pressure >140 mmHg while on ≥1 antihypertensive agent, current smoker, microalbuminuria, or macroalbuminuria, or HDL cholesterol <1 mmol/L. The intent was to include 70% of individuals with a history of cardiovascular disease and 30% with ≥2 disease risk factors by the capping of recruitment. Other inclusion and exclusion criteria were consistent with those typically used for a trial of this stage in this patient group and are identical to those used in the separate CANVAS trial (Appendix S1). This study uses the same adjudication committee and Independent Data Monitoring Committee (IDMC) as CANVAS.
2.2. Screening and run‐in
All potential participants completed a 2‐week, single‐blind, placebo run‐in period if screening criteria were met. If eligibility was confirmed, the participant was counselled about diet and exercise, trained in the recognition of hypoglycaemia, provided with materials for making self‐monitored blood glucose measurements and then provided with single‐blind placebo therapy. The primary purpose of the run‐in period was to exclude individuals who were unlikely to adhere to the long‐term treatment and follow‐up regimen required by the trial.
2.3. Randomization
Randomization was carried out centrally through an interactive web response system. Participants were randomly assigned in a 1:1 ratio to canagliflozin or matching placebo on the basis of a computer‐generated randomization schedule that was prepared by the study sponsor using randomly permuted blocks.
2.4. Post‐randomization study treatment and control
Study treatment is provided in identical bottles for both canagliflozin and matching placebo. Canagliflozin was administered at an initial dose of 100 mg daily but, at week 13 (or any time thereafter), the dose of canagliflozin (or matching placebo) may be increased from 100 to 300 mg. Uptitration is encouraged if the participant requires additional glycaemic control (eg, if ≥50% of glucose measurements from fasting fingerstick readings exceeded 6 mmol/L [110 mg/dL] during the 2 weeks preceding) and there has been no hypoglycaemia, volume depletion or other adverse event that might preclude dose increase. Participants are instructed to take the study treatment once daily before the first meal of the day until completion of the study or premature treatment discontinuation. Participants and all study staff will be masked to individual treatment allocation until the completion of the study.
2.5. Background drug treatments
Use of SGLT2 inhibitors is precluded, but participants are otherwise free to use other background therapy for glycaemic management at the discretion of the responsible investigator, in line with applicable local guidelines. Likewise, the use of all other therapies, particularly for cardiovascular and renal risk factors, will be according to best practice throughout the course of the study, instituted according to local guidelines and policies.
2.6. Follow‐up schedule
Post‐randomization, face‐to‐face follow‐up is scheduled for 13 and 26 weeks after randomization and at 6‐month intervals thereafter. Additionally, telephone follow‐up is scheduled at 6 weeks, 9 months and every 6 months thereafter, such that it alternates with the face‐to‐face visits. Every follow‐up contact includes an inquiry about adverse events and concomitant therapies. All face‐to‐face visits also include provision of study medication and recording of physical measurements. First morning urinary ACR and serum creatinine levels are measured every 6 months. After the year 1 visit, clinical laboratory assessments are performed every 6 or 12 months. Individuals who prematurely discontinue study treatment are encouraged to return for regular assessments to ensure full ascertainment of study outcomes and to support an intention‐to‐treat analysis for all outcomes. Patient retention is supported by flexibility in the follow‐up regimen such that investigators can schedule additional visits or reduce the intensity of follow‐up as required. Further, the consent process included agreement by the participant for the investigator to consult family members, the participant's physicians, medical records or public records in the event they are not reachable by conventional means.
2.7. Outcomes
The primary outcome is progression of albuminuria, which is defined as a change from normoalbuminuria to microalbuminuria or macroalbuminuria, or from microalbuminuria to macroalbuminuria, accompanied by an ACR value increase of ≥30% from baseline. The main analysis will be on incident events, with a supplementary analysis carried out that is restricted to events with duplicate measures of progressions in which a repeat ACR collection was carried out ~1 to 2 months later to identify sustained progression of albuminuria. In the circumstance where the last recorded ACR value meets the definition of progression, it will be deemed to be a sustained progression for the subsidiary analysis. ACR assessments will be based on values obtained from duplicate first morning void urines and analysed by a central laboratory. The secondary outcomes are (1) the composite of hospitalization for heart failure or cardiovascular death and (2) cardiovascular death.
Exploratory outcomes will be (1) regression of albuminuria, defined as change to normoalbuminuria in a patient with baseline microalbuminuria or macroalbuminuria or change to microalbuminuria in a patient with baseline macroalbuminuria, accompanied by a decrease in the urinary ACR value of ≥30% from baseline. A supplementary analysis of sustained regression will be performed that is restricted to events where regression is observed in duplicate assays; (2) change in eGFR from baseline to the last off‐treatment value, with eGFR calculated using the Modification of Diet in Renal Disease (MDRD) formula13; (3) urinary ACR; (4) the composite of a 40% reduction in eGFR, renal death or the requirement for renal replacement therapy; (5) the composite of a 40% reduction in eGFR, renal death, the requirement for renal replacement therapy or cardiovascular death; and (6) the composite of the development of macroalbuminuria, a 40% reduction in eGFR, renal death or the requirement for renal replacement therapy. The last three exploratory outcomes will also be assessed by substituting the 40% reduction in eGFR with doubling of serum creatinine (which equates to an approximate 57% reduction in eGFR). Other exploratory analyses include on‐treatment GFR slope, change in HbA1c and utilization of antihyperglycaemic therapy.
Given the planned concordance in study design and patient population enrolled between the CANVAS‐R and CANVAS studies, an integrated analysis will be performed to evaluate the cardiovascular safety and efficacy of canagliflozin using a closed testing procedure to control for type 1 error across the integrated analysis and CANVAS‐R study at 5%. Safety data on acidosis,14 malignancies, fatal pancreatitis, pancreatitis, severe hypersensitivity reactions, photosensitivity reactions, hepatic injury, acute kidney injury, venous thromboembolic events, fractures, male genital infections, amputation and pregnancy are also being systematically collected to provide better insight into the safety of the compound. The vascular safety events will all be reviewed by an Independent Endpoint Adjudication Committee, in addition to all other deaths, fractures, ESRD events and hospitalization for heart failure.
2.8. Statistical power
Based on the interim data from the canagliflozin development programme, it was projected that the annual albuminuria progression rate for the CANVAS‐R study will be ~7.4% in the placebo arm. Assuming an 18‐month accrual period, a minimum follow‐up period of 18 months and an annual discontinuation (from treatment) rate of 10%, this was projected to provide >90% power (2‐sided P = .05) to detect a 22% relative risk reduction for the primary outcome of albuminuria progression. This period of follow‐up will also allow the accrual of sufficient major adverse cardiovascular events between this trial and CANVAS, such that the FDA post‐marketing requirements can be met.
2.9. Analysis
Analyses will be performed using the intention‐to‐treat population, and a P value of 0.05 will be taken to indicate a statistically significant effect. The primary efficacy analysis will seek to demonstrate the superiority of canagliflozin compared with placebo for the prevention of albuminuria progression. The geometric mean of the duplicate ACR measurements collected at each visit will be computed, and patients will be classified as having normoalbuminuria (urinary ACR of <3.5 mg/mmol [<30 mg/g]), microalbuminuria (ACR ≥3.5 mg/mmol [≥30 mg/g] and ≤35 mg/mmol [≤300 mg/g]) or macroalbuminuria (ACR of >35 mg/mmol [>300 mg/g]) at each time point. The time from randomization to the first visit date at which progression of albuminuria is recorded will be analysed using a Cox proportional hazards regression model. Appropriate correction will be made for regression to the mean. The model will include treatment and baseline albuminuria status as covariates. The hazard ratio comparing canagliflozin and placebo will be estimated with its 95% confidence interval. For participants who do not experience progression of albuminuria, censoring will be the visit date of the last albuminuria measurement. The cumulative progression rate derived from the Kaplan‐Meier estimate will be displayed graphically to illustrate the timing of progression and to explore the consistency of the treatment effect over time. The secondary efficacy analyses will use Cox proportional hazards models. A closed testing procedure will be implemented to control for the overall type 1 error at 5% for the primary and secondary endpoints across CANVAS‐R and CANVAS with the hypotheses for the primary and secondary endpoints in CANVAS‐R tested sequentially as part of a testing family. There are no interim analyses planned.
2.10. Trial management
Scientific responsibility for the design, analysis and reporting of the trial lies with the Steering Committee, which comprises 6 independent academic researchers and a representative of the trial sponsor. After trial completion, the Steering Committee will have full access to the trial database and will conduct analyses of the main trial outcomes independent of the sponsor. Day‐to‐day operation of the trial is managed jointly by the sponsor and an academic research organization; broadly, the academic research organization is responsible for the management of the various trial committees and operations in the Asia‐Pacific region, with the sponsor having primary responsibility for other geographic regions and functions. An independent data monitoring committee has been established to provide interim monitoring of unblinded safety data throughout the course of the trial.
3. RESULTS/CURRENT STATUS
During a recruitment period of 16 months, 7800 individuals were screened and 5812 were randomized (Figure 2). A total of 1988 individuals were not randomized; more than two‐thirds of these individuals were excluded because HbA1c was out of range, with smaller proportions excluded on the basis of other laboratory results, cardiovascular risk criteria, a low eGFR or diverse other reasons. One individual was randomized at 2 sites and has been removed from follow‐up and analysis. Findings are therefore reported for 5811 individuals, amongst whom accrued follow‐up was 8737 patient‐years by August 2016. The corresponding mean duration of follow‐up was 78.5 weeks, the median duration was 78 weeks and the maximum duration was 122 weeks.
Figure 2.
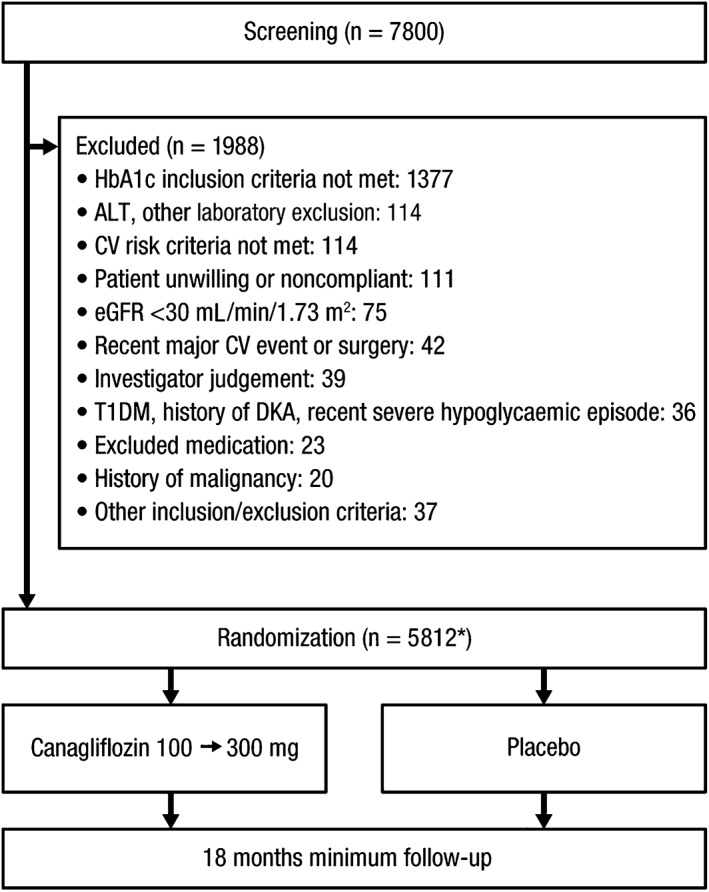
CONSORT diagram. ALT, alanine aminotransferase; CV, cardiovascular; T1DM, type 1 diabetes mellitus; DKA, diabetic ketoacidosis. *One subject who was randomized twice in error has been excluded from follow‐up and will be excluded from analysis.
The mean age of the participants at baseline was 64 years and 37% were women (Table 1). A total of 4765 (82.0%) participants were white, with most of the remainder being of Asian descent. The average time from diagnosis of diabetes was 13.7 years, with 4652 participants (80.1%) using metformin, 2325 (40.0%) a sulphonylurea, 310 (5.3%) a glucagon‐like peptide‐1 receptor agonist and 2905 (50.0%) insulin. As would be anticipated for a group with long‐standing diabetes, many patients were on multiple glucose‐lowering therapies. The majority were also using a statin (76.6%) and large numbers of participants were prescribed antithrombotic therapy (75.1%). A total of 1263 (21.7%) and 1114 (19.2%) of those randomized had diagnoses of retinopathy and nephropathy, respectively, while 1762 participants (30.3%) had neuropathy. A total of 70.5% reported a history of macrovascular atherosclerotic vascular disease at entry into the study and 15.9% reported a history of heart failure. The mean baseline body mass index was 32 kg/m2 and the mean HbA1c was 8.3%. Baseline blood pressure was 137/78 mmHg, total cholesterol was 4.4 mmol/L, HDL cholesterol was 1.2 mmol/L, LDL cholesterol was 2.3 mmol/L and triglycerides were 2.1 mmol/L. Microalbuminuria was present in 22.3% (median urinary ACR 8.0 mmol/mg) of participants and 8.7% had macroalbuminuria (median urinary ACR 83.5 mmol/mg). The mean eGFR was 76 mL/min/1.73 m2 and the median was 75 mL/min/1.73 m2.
Table 1.
Baseline characteristics of randomized participants
| Total randomized intention‐to‐treat N = 5811* | |
|---|---|
| Mean (s.d.) age, years | 64.0 (8.4) |
| Female, n (%) | 2162 (37.2) |
| Race, n (%) | |
| White | 4765 (82.0) |
| Asian | 489 (8.4) |
| Black or African American | 229 (3.9) |
| Other | 328 (5.6) |
| Current smoker, n (%) | 1030 (17.7) |
| History of hypertension, n (%) | 5109 (87.9) |
| History of heart failure, n (%) | 924 (15.9) |
| Mean (s.d.) duration of diabetes, years | 13.7 (7.9) |
| Drug therapy, n (%) | |
| Insulin | 2905 (50.0) |
| Sulphonylurea | 2325 (40.0) |
| Metformin | 4652 (80.1) |
| GLP‐1 receptor agonist | 310 (5.3) |
| Statin | 4454 (76.6) |
| Antithrombotic | 4363 (75.1) |
| RAAS inhibitor | 4593 (79.0) |
| Microvascular disease history, n (%) | |
| Retinopathy | 1263 (21.7) |
| Nephropathy | 1114 (19.2) |
| Neuropathy | 1762 (30.3) |
| Atherosclerotic vascular disease history, n (%)† | |
| Coronary | 2922 (50.3) |
| Cerebrovascular | 828 (14.2) |
| Peripheral | 1126 (19.4) |
| Any | 4098 (70.5) |
| Mean (s.d.) body mass index, kg/m2 | 31.9 (5.7) |
| Mean (s.d.) systolic BP, mmHg | 136.9 (15.8) |
| Mean (s.d.) diastolic BP, mmHg | 77.6 (9.6) |
| Mean (s.d.) HbA1c, % | 8.3 (1.0) |
| Mean (s.d.) total cholesterol, mmol/L | 4.4 (1.2) |
| Mean (s.d.) triglycerides, mmol/L | 2.1 (1.5) |
| Mean (s.d.) HDL cholesterol, mmol/L | 1.2 (0.3) |
| Mean (s.d.) LDL cholesterol, mmol/L | 2.3 (0.9) |
| Mean (s.d.) LDL cholesterol:HDL cholesterol ratio | 2.1 (0.9) |
| Mean (s.d.) eGFR, mL/min/1.73 m2 | 75.9 (21.7) |
| eGFR ≥90 mL/min/1.73 m2, n (%) | 1439 (24.8) |
| eGFR ≥60 to <90 mL/min/1.73 m2, n (%) | 3044 (52.4) |
| eGFR ≥45 to <60 mL/min/1.73 m2, n (%) | 941 (16.2) |
| eGFR ≥30 to <45 mL/min/1.73 m2, n (%) | 363 (6.2) |
| eGFR ≥15 to <30 mL/min/1.73 m2, n (%) | 23 (0.4) |
| eGFR <15 mL/min/1.73 m2, n (%) | 1 (<0.1) |
| Mean (s.d.) ACR, mg/mmol‡ ‡ | 15.2 (56.3) |
| Normoalbuminuria, n (%) | 3944 (68.9) |
| Microalbuminuria, n (%) | 1279 (22.3) |
| Nephrotic range macroalbuminuria, n (%) | 41 (0.7) |
| Non‐nephrotic range macroalbuminuria, n (%) | 459 (8.0) |
Abbreviations: BP, blood pressure; GLP‐1, glucagon‐like peptide‐1; RAAS, renin angiotensin aldosterone system; s.d., standard deviation.
One subject who was randomized twice will be excluded from analysis.
Some participants had ≥1 type of atherosclerotic disease.
Values for albuminuria categories calculated based on N = 5723.
4. DISCUSSION
The CANVAS‐R trial has successfully randomized 5811 individuals with T2DM. The characteristics of the participants are typical of those of a high‐risk population with diabetes, both in terms of the nature of the complications reported and the types of treatments that they are receiving. The proportion of participants with a history of atherosclerotic vascular disease is higher than is typically observed in this patient group but this reflects the inclusion criteria that targeted the enrolment of individuals with ischaemic macrovascular complications. The CANVAS‐R population characteristics are directly comparable with those of the ongoing CANVAS trial,11 which will ensure that a robust assessment of effects on cardiovascular and other outcomes can be made across the integrated data from the twin studies. Individuals with these characteristics are at a substantially elevated risk of both cardiovascular and renal complications, and CANVAS‐R will provide substantial new insight into the effects of canagliflozin on renal function and vascular disease.
The exploration of canagliflozin as a novel strategy for the prevention of the renal complications of diabetes is based on strong preliminary data. SGLT2 inhibitors have been shown to lower glucose, blood pressure and body weight.12, 15 Initial data from a subset of participants in the canagliflozin development programme that were available at the time of study design suggested protection against albuminuria, with mean changes in the ACR of +28 mg/g for placebo, −22 mg/g for canagliflozin 100 mg and −41 mg/g for canagliflozin 300 mg.16 The mechanism of the glucose lowering based on tubular wasting of glucose is well understood and a plausible explanation of the antihypertensive effect is sodium excretion resulting from the inhibition of the joint sodium glucose co‐transporter.13 The mechanism of the albuminuria reduction remains to be determined. Possibilities include reduction in intraglomerular pressure induced by glomerular tubular feedback or atrial natriuretic peptide, modification of tubular albumin reabsorption, changes in the glycocalyx or reduced filtration as a result of reduction in eGFR.17, 18
The change in albuminuria class selected as the primary outcome for CANVAS‐R has been used in multiple previous renal outcome trials,2, 4, 19, 20, 21, 22 and positive effects on albuminuria class change resulted in the European Medicines Agency (EMA) providing a labelled indication for irbesartan as a treatment for patients with microalbuminuria and diabetes. Consistent with the move towards earlier intervention in diabetic nephropathy, there are ongoing discussions regarding the potential for albuminuria class transition to serve as an outcome for which an indication might be provided in other clinical settings. In the meantime, however, it will be necessary to demonstrate that any benefits of canagliflozin on albuminuria will translate into protection against ESRD. This line of investigation has received support in recent months after post hoc analyses of the EMPA‐REG OUTCOME trial that have identified potential large protective effects of the compound against both albuminuria and ESRD.9 Accordingly, exploratory analyses of the effects of canagliflozin on a range of clinical renal outcomes have been added to the CANVAS‐R protocol. Analysis of these outcomes within CANVAS‐R, along with analyses of the same exploratory outcomes across the integrated data from the CANVAS‐R and CANVAS trials, will provide additional near‐term information about the effects of canagliflozin and the broader SGLT2 class on hard renal outcomes. Ultimately, the effects of SGLT2 inhibition on the development of ESRD will need to be defined in a dedicated renal trial, and the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study (NCT02065791) has been initiated for that purpose.
In addition to the renal outcomes, the EMPA‐REG OUTCOME trial also showed positive findings for cardiovascular outcomes, with particularly large benefits observed for vascular death and heart failure.10 Confirmation of these findings as a class effect for SGLT2 inhibitors is urgently required and these outcomes have been defined as secondary outcomes for the CANVAS‐R trial in a protocol update. As for the other CANVAS‐R outcomes, there will be a parallel evaluation of the efficacy effects across the integrated data from the twin CANVAS‐R and CANVAS studies in order to maximize statistical power.
Several recent concerns have been raised regarding potential adverse effects of SGLT2 inhibitors, and better safety data are required. Most recently, an elevated risk of amputation was reported by the data monitoring committee of CANVAS,11 and the EMA is now seeking data about this outcome for all registered drugs in the SGLT2 class.23 Diabetic ketoacidosis has also been noted as an uncommon, but serious, side effect of SGLT2 inhibitors,14 and a possible increased risk of fracture has also been identified for canagliflozin24 and in patients with moderate renal impairment treated with dapagliflozin.25 While the absolute risk of serious side effects appears to be small, and the IDMC for the trials has recommended continuation, these observations provide a further imperative to quantify fully the overall impact of SGLT2 inhibition on all important outcomes. Only with data from large‐scale trials, such as CANVAS‐R, will a robust estimate of the overall balance of risks and benefits be obtained. Future studies that include, for example, serial imaging of the heart might provide helpful additional insight into the mechanism of the macrovascular protection afforded. Likewise, retinal imaging studies would reveal whether protective effects on the renal microvasculature are mirrored elsewhere.
The ongoing large renal/cardiovascular outcome trials for canagliflozin will deliver substantial new data and will provide patients and clinicians with the information they need to make fully informed decisions about the optimum use of canagliflozin for the management of diabetes in general and, in particular, progression of the identified comorbidities.
Supporting information
Appendix S1. Detailed inclusion and exclusion criteria.
Appendix S2. Collaborating investigators.
ACKNOWLEDGMENTS
Steering Committee: D. R. Matthews (Co‐chair), B. Neal (Co‐chair), G. Fulcher, K. Mahaffey, V. Perkovic, G. Meininger/M. Desai, D. de Zeeuw. Independent Data Monitoring Committee: P. Home (Chair), J. Anderson, I. Campbell, J. Lachin, D. Scharfstein, S. Solomon and R. Uzzo. Endpoint Adjudication Committee: G. Fulcher, J. Amerena, C. Chow, G. Figtree, J. French, G. Hillis, M. Hlatky, B. Jenkins, N. Leeper, R. Lindley, B. McGrath, A. Street, J. Watson, S. Shahinfar, T. Chang, A. Sinha and P. August. Sponsor: Janssen Research & Development, LLC. Collaborating sites: see Appendix S2 for full listing.
The Steering Committee designed the study in conjunction with the Sponsor. B. N. wrote the first draft of the paper. The Sponsor provided the data. All authors provided input into subsequent drafts. Technical editorial assistance was provided by Kimberly Dittmar, PhD, of MedErgy, and was funded by Janssen Global Services, LLC.
Conflict of interest
B. N. is supported by an Australian National Health and Medical Research Council Principal Research Fellowship; holds a research grant for this study from Janssen; has held research grants for other large‐scale cardiovascular outcome trials from Philips Respironics, Roche, Servier and Merck Schering Plough; and his institution has received consultancy, honoraria or travel support for contributions he has made to advisory boards and/or the continuing medical education programmes of Abbott, Janssen, Novartis, Pfizer, Roche and Servier. V. P. is supported by a Senior Research Fellowship from the Australian National Health and Medical Research Council; has served on advisory boards and/or spoken at scientific meetings sponsored by Janssen, Baxter, AbbVie, Astellas, Boehringer Ingelheim, AstraZeneca, Eli Lilly, Merck and GlaxoSmithKline; and has a policy of honoraria going to his employer. D. R. M. has served on advisory boards or as a consultant for Novo Nordisk, GlaxoSmithKline, Novartis, Eli Lilly, Sanofi‐Aventis, Janssen and Servier; receives current research support from Janssen and the NIHR; and has given lectures for Novo Nordisk, Servier, Sanofi‐Aventis, Eli Lilly, Novartis, Janssen and Aché Laboratories. K. W. M.’s financial disclosures prior to August 1, 2013 can be viewed at https://www.dcri.org/about‐us/conflict‐of‐interest/Mahaffey‐COI_2011‐2013.pdf; disclosures after August 1, 2013 can be viewed at http://med.stanford.edu/profiles/kenneth‐mahaffey. G. F. has served on advisory boards for Johnson & Johnson and as a consultant to Janssen. G. M., N. E., M. D., W. S., J. Y. and H. D. are full‐time employees of Janssen Research & Development, LLC. F. V. is a full‐time employee of Janssen Research & Development. F. V. and G. M. are shareholders of Johnson & Johnson. D. d. Z. serves as a consultant for AbbVie, Astellas, Chemocentryx, Eli Lilly, Fresenius, Janssen and Merck Darmstadt; all consultancy honoraria are paid to his institution.
Author contributions
B. N. contributed to the design and conduct of the study and wrote the first draft of the paper. V. P., D. R. M., K. W. M., G. F. and D.d. Z. contributed to the design and conduct of the study. G. M., N. E., M. D., W. S., F. V. and J. Y. contributed to the design and conduct of the study. H. D. contributed to the data analysis. All authors reviewed and approved the manuscript.
Neal B, Perkovic V, Matthews DR, Mahaffey KW, Fulcher G, Meininger G, Erondu N, Desai M, Shaw W, Vercruysse F, Yee J, Deng H, de Zeeuw D ; on behalf of the CANVAS‐R Trial Collaborative Group . Rationale, design and baseline characteristics of the CANagliflozin cardioVascular Assessment Study–Renal (CANVAS‐R): A randomized, placebo‐controlled trial, Diabetes Obes Metab, 2017;19(3):387–393.
Funding information This study is funded by Janssen Research & Development, LLC. Technical editorial assistance was funded by Janssen Global Services, LLC.
REFERENCES
- 1. United States Renal Data Systems . USRDS annual data report: epidemiology of kidney disease in the United States. 2015. http://www.usrds.org/2015/view/Default.aspx. Accessed October 6, 2016.
- 2. Parving HH, Lehnert H, Brochner‐Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870‐878. [DOI] [PubMed] [Google Scholar]
- 3. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861‐869. [DOI] [PubMed] [Google Scholar]
- 4. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851‐860. [DOI] [PubMed] [Google Scholar]
- 5. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo‐controlled trial. Lancet. 2011;377(9784):2181‐2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560‐2572. [DOI] [PubMed] [Google Scholar]
- 7. Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829‐840. [DOI] [PubMed] [Google Scholar]
- 8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 9. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323‐334. [DOI] [PubMed] [Google Scholar]
- 10. Wu JH, Foote C, Blomster J, et al. Effects of sodium‐glucose cotransporter‐2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2016;4(5):411‐419. [DOI] [PubMed] [Google Scholar]
- 11. Neal B, Perkovic V, de Zeeuw D, et al. Rationale, design, and baseline characteristics of the canagliflozin cardiovascular assessment study (CANVAS)–a randomized placebo‐controlled trial. Am Heart J. 2013;166(2):217‐223. [DOI] [PubMed] [Google Scholar]
- 12. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium‐glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta‐analysis. Ann Intern Med. 2013;159(4):262‐274. [DOI] [PubMed] [Google Scholar]
- 13. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015;38(9):1680‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independent of glycemic effects. J Am Soc Nephrol. 2017;28(1):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration . FDA Briefing Document. NDA 204042. Invokana (canagliflozin) Tablets. Applicant: Janssen Pharmaceuticals, Inc. Endocrinologic and Metabolic Drugs Advisory Committee Meeting. January 10, 2013. 2013.
- 17. Thomson SC, Rieg T, Miracle C, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302(1):R75‐R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lambers Heerspink HJ, Gansevoort RT. Albuminuria is an appropriate therapeutic target in patients with CKD: the pro view. Clin J Am Soc Nephrol. 2015;10(6):1079‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Makino H, Haneda M, Babazono T, et al. Microalbuminuria reduction with telmisartan in normotensive and hypertensive Japanese patients with type 2 diabetes: a post‐hoc analysis of The Incipient to Overt: angiotensin II blocker, telmisartan, investigation on type 2 diabetic nephropathy (INNOVATION) study. Hypertens Res. 2008;31(4):657‐664. [DOI] [PubMed] [Google Scholar]
- 20. Ruggenenti P, Fassi A, Ilieva AP, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351(19):1941‐1951. [DOI] [PubMed] [Google Scholar]
- 21. Haller H, Ito S, Izzo JL Jr, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364(10):907‐917. [DOI] [PubMed] [Google Scholar]
- 22. Chaturvedi N, Porta M, Klein R, et al. Effect of candesartan on prevention (DIRECT‐Prevent 1) and progression (DIRECT‐Protect 1) of retinopathy in type 1 diabetes: randomised, placebo‐controlled trials. Lancet. 2008;372(9647):1394‐1402. [DOI] [PubMed] [Google Scholar]
- 23. European Medicines Agency (EMA) . SGLT2 inhibitors (previously Canagliflozin) Article‐20 procedure – Amended review started. 2016. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/SGLT2_inhibitors_(previously_Canagliflozin)/human_referral_prac_000059.jsp&mid=WC0b01ac05805c516f. Accessed October 6, 2016.
- 24. US Food and Drug Administration . Invokana and Invokamet (canagliflozin): drug safety communication—new information on bone fracture risk and decreased bone mineral density. 2015. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm461876.htm. Accessed November 11, 2015.
- 25.FARXIGA® (dapagliflozin) tablets, for oral use [package insert]. Princeton, NJ: Bristol‐Myers Squibb Company; 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Detailed inclusion and exclusion criteria.
Appendix S2. Collaborating investigators.


