Abstract
Lymphatic leakage can be seen as a detrimental phenomenon associated with fluid retention and deposition as well as gain of weight. Moreover, lymphatic dysfunction is associated with an inflammatory environment and can be a substrate for other health conditions. A number of treatments can ameliorate lymphatic vasculature: natural substances have been used as treatment options particularly suitable for their consolidated effectiveness and safety profile. Here we report the protective effect of AdipoDren®, an association of a series of plant-derived natural complexes, on lymphatic endothelium permeability promoted by interleukin-1 beta (IL-1β) and the associated molecular mechanisms. AdipoDren® demonstrated a protective effect on dermal lymphatic endothelial cell permeability increased by IL-1β. Reduced permeability was due to the maintenance of tight junctions and cell-cell localisation of occludin and zonula occludens-1 (ZO-1). Moreover, AdipoDren® reduced the expression of the inflammatory key element cyclooxygenase-2 (COX-2), while not altering the levels of endothelial and inducible nitric oxide synthases (eNOS and iNOS). The upregulation of antioxidant enzymatic systems (catalase and superoxide dismutase-1, SOD-1) and the downregulation of pro-oxidant markers (p22 phox subunit of NADPH oxidase) were also evident. In conclusion, AdipoDren® would be useful to ameliorate conditions of altered lymphatic vasculature and to support the physiological functionality of the lymphatic endothelium.
Keywords: Lymphatic endothelial cells, Permeability, Inflammation, Plant fractions, Rutin
Introduction
The lymphatic network is composed of blind-beginning thin-walled capillaries without pericyte coverage and with an incomplete basal lamina, collecting lymphatic vessels with a smooth muscle cell layer, a basement membrane, and valves that prevent lymph backflow. The lymphatic system is involved in the maintenance of the body fluid balance, protein homeostasis, lipid absorption, and immune function. A dysfunctional lymphatic vasculature can result in a wide range of clinical disorders, including oedema, altered lymphocyte circulation, depressed immune function, and impaired lipid metabolism. Indeed, dysregulation of lymphatic vessel integrity causes lymph leakage, which is associated with the development of obesity and cellulitis, atherosclerosis, and oedema, and it is thought to facilitate cancer cell entry into these vessels [1,2,3]. Impaired lymphatic vessel function is associated with increased adipocyte accumulation, whereas lymphatic vessel function is reduced in obese individuals as well as in obese mice [4,5].
Lymphoedema is a condition of localised fluid retention and tissue swelling caused by a compromised lymphatic system, which normally returns interstitial fluid to the thoracic duct and then the bloodstream [6]. Chronic inflammatory conditions, through the release of inflammatory mediators such as interleukins (IL-1, IL-6) and tumour necrosis factor-α (TNF-α), are responsible for lymphatic hyperpermeability and the perpetuation of detrimental inflammatory conditions [7,8,9].
The consumption of certain complex natural substances derived from plants is widely accepted for their protective role in microvascular and lymphatic physiological functionality. Among the natural substances demonstrated to maintain microvascular and lymphatic homeostasis, our attention was focused on specific complex natural substances derived from buckwheat, dandelion, butcher's broom, goldenrod, and java tea, comprising polyphenolic compounds, such as flavonoids and hydroxycinnamic acid derivatives, and steroidal saponins.
Polyphenols are secondary metabolites of plants and are mainly involved in plant defense mechanisms against pathogens and ultraviolet radiations. These compounds are classified into different groups depending on the number and position of phenol rings and the structural elements that bind these rings to one another. In this heterogeneous class, flavonoids, phenolic acids, stilbenes, and lignans can be found. Polyphenols can also be associated with various carbohydrates as well as organic acids [10].
Flavonoids are widely distributed in many fruits, vegetables, grains, bark, roots, stems, and flowers and share a common structure consisting of 2 aromatic rings bound together by 3 carbon atoms that form an oxygenated heterocycle. Flavonoids are classified into flavonols, flavones, flavanones, isoflavones, flavanols, proanthocyanidins, and anthocyanins [10,11]. Flavonoids are usually referred to as “venoactive” and “vascular protective and venous tonic agents” [12], and have high antioxidant properties [11].
Among flavonoids, there are the so-called flavonol glycosides that can be obtained from many plants, especially buckwheat (Fagopyrum esculentum Moench, from the Polygonaceae family). Flowering aerial parts of buckwheat are particularly rich in rutin, the glycoside of quercetin and the disaccharide rutinose (α-L-rhamnopyranosyl-[1→6])-β-D-glucopyranose), also called rutoside, quercetin-3-O-rutinoside, or sophorin [13]. Rutin inhibits platelet aggregation and decreases capillary permeability, improving circulation. Among the other protective activities, rutin has anti-inflammatory and antiadipogenic activity demonstrated in some animal and in vitro models [14,15]. All these properties account for the consolidated use of buckwheat as a venous and capillary tonic able to alleviate venous stasis and varicose veins [16].
Hyperoside, or quercetin-3-O-galactoside, is a flavonol glycoside that can be found in the co-extract between the flowering aerial parts of goldenrod (Solidago virgaurea L., from the Asteraceae family) and the leaves of java tea (Orthosiphon stamineus Benth, from the family of Lamiaceae). These plants have diuretic properties and are both traditionally used for the irrigation of the urinary tract in cases of inflammation [17,18].
Caffeic acid derivatives are polyphenolic compounds belonging to the class of hydroxycinnamic acids [10]. These non-flavonoid phenols can be obtained from the dandelion roots (Taraxacum officinale [L.] Weber ex. F.H. Wigg, from the family of Asteraceae). Dandelion has diuretic properties and it is usually indicated in those conditions where an enhancement of urinary secretion is useful [17,19].
Ruscogenins are steroidal saponins present in butcher's broom roots (Ruscus aculeatus L., from the Liliaceae family). Lymphatic and venotonic activities have been reported for this plant, which is indicated as supportive therapy for symptoms of chronic venous insufficiency, such as painful, tired and heavy legs, tingling, and swelling, and also as supportive therapy for haemorrhoid symptoms. There is evidence that this medicinal plant may also be beneficial in treating lymphoedema. Butcher's broom extracts have been shown to increase blood and lymph vessel contractions and peripheral lymph flow [17,20].
The aim of this paper was to evaluate the effect of selected complex natural substances extracted from the above-mentioned plants, both alone and combined to form a specific composition (AdipoDren®; patent pending, PCT/IB2016/050023), on lymphatic (and microvascular) endothelial cell permeability that is altered by IL-1β. The protective effect of the tested compositions was compared to rutin, which was used as a positive control of the functional response. The molecular mechanisms at the basis of the reduced hyperpermeability observed for AdipoDren® were analysed on lymphatic endothelium in terms of functional activity (endothelial nitric oxide synthase, eNOS), key markers of inflammatory pathways (inducible NOS, iNOS; cyclooxygenase-2, COX-2) and antioxidant/pro-oxidant enzymatic systems (superoxide dismutase-1, SOD-1; catalase, and p22 phox subunit of NADPH oxidase).
Materials and Methods
Preparation of Test Samples
The dried and ground plant materials of each species were extracted by 1 or 2 steps. Depending on the characteristics of the fractions, the extraction processes were performed with different percentages of EtOH/H2O. After 6-12 h, the EtOH/H2O herb mixtures were dropped for 1 h and filtered to remove the exhausted material and the corresponding extracted fractions were concentrated under vacuum to evaporate ethanol. The obtained concentrates underwent freeze-drying for 72 h and the resulting freeze-dried extracted fractions were stored until analysis.
AdipoDren® (patent pending No. 102015902320174) is a mixture of natural freeze-dried fractions enriched in flavonoids from buckwheat, non-flavonoid phenols from dandelion, steroidal saponins from butcher's broom, flavonoids from goldenrod and java tea contained in Lynfase, a marketed food supplement in the form of oral liquid concentrate. Lynfase is indicated in those situations where a support to vascular and lymphatic function, together with an improvement of body fluids drainage, is required. The above-mentioned freeze-dried fractions and AdipoDren® were supplied by the manufacturer Aboca S.p.A.
Single freeze-dried extracted fractions obtained from the medicinal plants and AdipoDren® were freshly solubilised before testing on cell cultures. The powders were dissolved in DMSO at doses of 5-10 mg/mL and the experimental control was set up with the final concentration of DMSO present in the culture medium.
UPLC-QToF Method
MeOH 50% v/v (100 mL) was added to a sample of AdipoDren® (0.25 g). The resulting mixture was extracted in an ultrasonic bath at 35°C for 10 min and then centrifuged for 10 min at 4,000 rpm. The pellet was suspended again in MeOH 50% v/v (100 mL) and extracted in the same conditions. After centrifugation, the second extract was combined and the solution so obtained was transferred into a 200-mL flask. The sample was filtered on a 0.22-μm cellulose acetate syringe filter and then employed for the acquisition of the ESI-MS chromatographic profile (negative and positive ion mode).
The measurements were recorded by a MS/Q-ToF (Xevo G2XS, Waters) system equipped with an ESI source (LockSpray, Waters). The system was coupled to an UPLC (H Class Aquity, Waters), equipped with a quaternary pump, a thermostated autosampler at 10°C and a column compartment thermostated at 50°C.
An MSE method was performed using 2 scanning functions simultaneously: the first at low energy providing precursor ion spectra, and the second characterised by a collision energy ramp from 20 to 45 eV, providing fragment ions. The parameters used were the following:
(ESI[-]): polarity ES-, analyser resolution mode, capillary 2.5 kV, sampling cone 40, source temperature 150°C, source offset 80, desolvation temperature 500°C, cone gas flow 50 L/h, desolvation gas flow 1,000 L/h.
(ESI[+]): polarity ES+, analyser resolution mode, capillary 2.5 kV, sampling cone 40, source temperature 150°C, source offset 80, desolvation temperature 500°C, cone gas flow 50 L/h, desolvation gas flow 1,000 L/h.
The column used was an RP-C18 (Cortecs C18 Column, 2.1 × 100 mm × 1.6 μm coupled to a VanGuard Pre-Column 2.1 × 5 mm, Waters). The elution was performed with H2O/HCOOH 125 mM (solvent A), MeOH (solvent B), H2O (solvent C), MeOH/HCOOH 125 mM (solvent D). The gradient program used was: 0 min 20% A, 5% B, 75% C, 0% D; 1 min 20% A, 5% B, 75% C, 0% D; 4 min 20% A, 45% B, 35% C, 0% D; 13 min 20% A, 80% B, 0% C, 0% D; 14 min 20% A, 80% B, 0% C, 0% D; 14.10 min 5% A, 80% B, 0% C, 15% D; 16 min 5% A, 80% B, 0% C, 15% D; 16.10 min 20% A, 5% B, 80% C, 0% D; 18 min 20% A, 5% B, 75% C, 0% D. The flow rate was 0.5 mL/min. The injection volume was 3 µL.
The quantitative determination of sinensetin was carried out using the same conditions reported above except for the column temperature and the flow rate, which were 45°C and 0.3 mL/min, respectively.
The acquired data were processed using MassLynx v.4.1. The quantitative analysis was performed building calibration curves of each identified standard reference compound. Sulfadimethoxine-d6 was used as an internal standard to correct the analyte response and to monitor the matrix effect. Each standard reference compound was quantified on the first MS function. All calibration curves showed an R2 ≥0.98. All the samples were acquired in triplicate and the quantitative results represent the average of 3 acquisitions with a CV% ≤15%, except for isorhamnetin and quercetin, which are characterised by a CV% of 18 and 16%, respectively.
Endothelial Cells
Human dermal lymphatic endothelial cells (HDLEC) and human dermal blood endothelial cells (HDBEC) were purchased from Promocell (Heidelberg, Germany). Both cell lines were grown in complete endothelial cell basal medium (MV2; Promocell, Heidelberg, Germany), supplemented with 10% foetal bovine serum. The cells were cultured at 37°C in 5% CO2, were split 1:3 twice a week, and used until passage 10.
Cell Survival
Cell viability was determined by MTT test [21]. Subconfluent cells (5 × 103 cells/well in 96 multiwell plates) were incubated in the presence of increasing concentrations of test sample in medium with the addition of 2% serum. After 24 h of incubation, cultures were exposed for 4 h to 1.2 mM MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; Sigma-Aldrich, St. Louis, MO, USA) in fresh medium (without phenol red). After the solubilisation of formazan crystal in DMSO, absorbance was measured with a microplate absorbance reader (Infinite 200 Pro SpectraFluor; Tecan, Männedorf, Switzerland) at 540 nm. Data are reported as the fold increase of absorbance units (at 540 nm), taking as reference the condition of medium with the addition of 2% serum.
Measurement of Permeability
Cells (8 × 104/insert) were seeded on collagen-coated insert membranes (Corning, Sigma Aldrich) containing a high density of 0.4-µm-diameter pores, and the inserts were placed into 12 multiwell plates for 72 h. Confluent monolayers were pretreated with test samples (18 h) and then IL-1β (10 ng/mL, 1 h) was added where indicated. Where specified, the eNOS inhibitor L-NMMA (100 μM) was added together with AdipoDren®. FITC-Dextran (3 kDa, 10 μM) was used as a fluorescent marker of permeability, which was evaluated after 15 min by measuring the fluorescence in a plate reader (Infinite 200 Pro, SpectraFluor) at 485 and 535 nm excitation and emission, respectively [22]. Data are reported as fluorescence units, taking as reference the condition of medium with the addition of 2% serum (control condition).
ROS Measurement
ROS were evaluated as previously reported [23]. A total of 1.5 × 103 cells (HDLEC) were seeded in a 96-multiwell plate and, after adherence, were pretreated with AdipoDren® (10 µg/mL, 18 h) and then with IL-1β (10 ng/mL, 1 h) in a medium without phenol red. DCFH2-DA (2,-7-dichlorodihydrofluorescein diacetate; Invitrogen, Milan, Italy) was added (10 μM, 30 min) and intracellular levels of ROS were evaluated with a microplate reader (excitation/emission 495/527; Infinite 200 Pro, SpectraFluor). The results are reported as relative fluorescence units (RFU) corrected for the cell number counted.
Immunofluorescence Analysis
The tight junction proteins occludin and zonula occludens-1 (ZO-1), expressed at the cell surface, were visualised by confocal analysis. A total of 5 × 104 cells were seeded on 1-cm circular glass coverslips. After 24 h, the cells were washed and treated with the indicated stimuli. Immunofluorescence analysis was performed as previously reported [22,24] and fluorescence was quantified in digitalised images by using ImageJ software.
Western Blot
Cells (3 × 105/6-cm plate) at 90% of confluence were treated or not with AdipoDren® (10 µg/mL, 18 h) in the absence and presence of IL-1β (10 ng/mL). The expression of markers of inflammatory pathways (iNOS, eNOS, COX-2) and antioxidant/pro-oxidant enzymatic systems (SOD-1, catalase, p22 phox) was evaluated by Western blot as previously described [22,24]. Data are reported as arbitrary densitometric units (ADU) of the target protein with respect to β-actin used as the loading control.
Materials and Reagents
Neochlorogenic acid, caffeic acid, p-coumaric acid, chicoric acid, homoorientin, orientin, m-coumaric acid, hyperoside, rutin, kaempferol-7-O-glucoside, rosmarinic acid, isorhamnetin 3-O-rutinoside, quercetin, kaempferol, isorhamnetin, sinensetin, and eupatorin were purchased from Extrasynthese (Lyon, France); chlorogenic acid, sulfadimethoxine-d6 and formic acid were purchased from Sigma Aldrich; crypto chlorogenic acid was purchased from Phytolab (Vestenbergsgreuth, Germany); desglucoruscin was purchased from LGC (Teddington, UK). Methanol was purchased from Chebios (Rome, Italy). Ultrahigh purified water used in this study was prepared in a PurelabUltra water purification system (ELGA, Lane End, UK).
Cell culture reagents, rutin, and L-NMMA were from Sigma Aldrich. Foetal bovine serum was from Hyclone (Euroclone, Milan, Italy). 3 kDa FITC-Dextran was from Life Technologies (Carlsbad, CA, USA). Anti-ZO-1 and anti-eNOS were from BD Transduction Laboratories (Milan, Italy). Anti-occludin was from Life Technologies. Anti-iNOS and anti-p22 phox were from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Anti-COX-2 was from Cayman Chemical Company (Ann Arbor, MI, USA). Anti-SOD-1 was from Millipore (Temecula, CA, USA). Anti-catalase and anti-β-actin were from Sigma-Aldrich.
Data Analysis and Statistical Procedures
Results are either representative or the average of at least 3 independent experiments done in triplicate. Statistical analysis was performed using ANOVA test followed by the Bonferroni test and the Student t test when appropriate (GraphPad). p < 0.05 was considered statistically significant.
Results
Characterisation of AdipoDren®
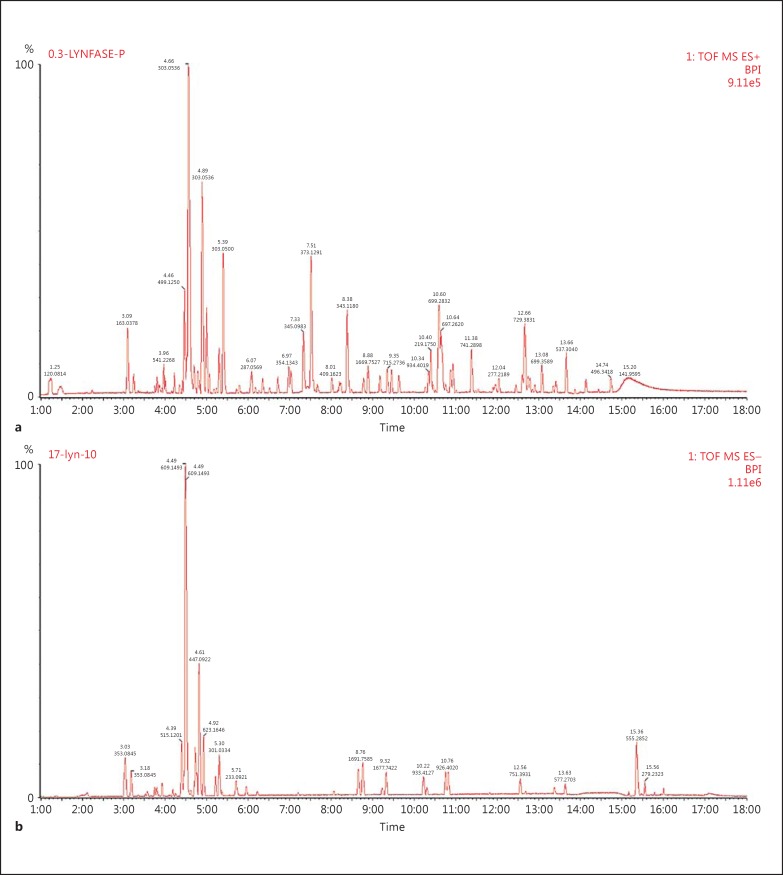
AdipoDren® is a mixture of selected complex natural substances extracted from dandelion roots, the flowering aerial parts of buckwheat and goldenrod, java tea leaves, and butcher's broom roots. The components of each fraction are reported in Table 1. A complex fraction containing hydroxycinnamic acids, particularly caftaric acid, chlorogenic acid, cichoric acid, and caffeic acid (minimum 0.6%), were obtained from dandelion roots; the complex fraction containing flavonoids particularly with a high rutin concentration (12%) was obtained from the flowering aerial parts of buckwheat; another complex fraction containing different flavonoids (calculated as hyperoside 4.6%) was obtained by co-extracting the flowering aerial parts of goldenrod and java tea leaves (ratio 3:1). The last complex fraction containing steroidal saponins (calculated as ruscogenins 10%) was obtained from butcher's broom roots. AdipoDren® was prepared by combining the above-mentioned compounds according to the pending patent (PCT/IB2016/050023). AdipoDren® was characterised by means of a metabolomic fingerprint, and the compounds reported in Table 2 were identified and quantified. The chromatographic profiles of AdipoDren® are reported in Figure 1.
Table 1.
Characteristics of AdipoDren® components
| Main class of compounds | Specific marker compounds | Herb | Latin name | Part of plant |
|---|---|---|---|---|
| Hydroxycinnamic acid derivatives | Caftaric acid Chlorogenic acid Cichoric acid Caffeic acid |
Dandelion | T. officinale (L.) Weber ex. F.H. Wigg | Roots |
| Flavonoids | Rutin | Buckwheat | F. esculentum Moench | Flowering aerial parts |
| Flavonoids | Hyperoside | Goldenrod | S. virgaurea L. | Flowering aerial parts |
| Java tea | O. stamineus Benth | Leaves | ||
| Steroidal saponins | Ruscogenins | Butcher's broom | R. aculeatus L. | Roots |
Table 2.
Compounds identified in AdipoDren®
| Compound | RT, min | % (w/w)1 | Ion polarity | Adduction | Experimental mass (scan base peak mass), m/z | Theoretical neutral mass, m/z | Delta, ppm |
|---|---|---|---|---|---|---|---|
| Neochlorogenic acid | 2.12 | 0.201 | negative | [M-H]− | 353,0884 | 354,0950 | 3.9 |
| Chlorogenic acid | 3.03 | 0.833 | negative | [M-H]− | 353,0845 | 354,0950 | 3.9 |
| Crypto chlorogenic acid | 3.18 | 0.068 | negative | [M-H]− | 353,0845 | 354,0950 | 3.9 |
| Coumaric acid (para) | 3.67 | 0.082 | negative | [M-H]− | 163,0398 | 164,0473 | 8.4 |
| Orientin | 4.12 | 0.007 | negative | [M-H]− | 447,0966 | 448,1006 | 1.1 |
| Homoorientin | 4.18 | 0.018 | negative | [M-H]− | 447,0966 | 448,1006 | 2.9 |
| Coumaric acid (meta) | 4.22 | 0.011 | negative | [M-H]− | 163,0398 | 164,0473 | 9.0 |
| Rutin | 4.49 | 4.330 | negative | [M-H]− | 609,1493 | 610,1534 | 1.0 |
| Hyperoside | 4.5 | 0.047 | negative | [M-H]− | 463,0892 | 464,0954 | 1.7 |
| Rosmarinic acid | 4.75 | 0.173 | negative | [M-H]− | 359,0762 | 360,0845 | 3.8 |
| Kaempferol-7-O-glucoside | 4.82 | 1.300 | negative | [M-H]− | 447,0922 | 448,1005 | 2.2 |
| Isorhamnetin 3-O-rutinoside | 4.92 | 0.077 | negative | [M-H]− | 623,1646 | 624,1690 | 0.2 |
| Quercetin | 5.3 | 0.087 | negative | [M-H]− | 301,0334 | 302,0426 | 6.2 |
| Kaempferol | 5.95 | 0.038 | negative | [M-H]− | 285,0402 | 286,0477 | 5.2 |
| Isorhamnetin | 6.21 | 0.008 | negative | [M-H]− | 315,0493 | 316,0582 | 6.6 |
| Eupatorin | 7.20 | 0.013 | negative | [M-H]− | 343,0815 | 344,0896 | 5.8 |
| Sinensetin | 7.51 | 0.007 | positive | [M+H]+ | 373,1291 | 372,1209 | 1.7 |
| Ruscin | 8.06 | 0.004 | negative | [M+FA-H]− | 913,4493 | 868,4456 | 3.8 |
All of the compounds have a CV% <15%, except isorhamnetin and quercetin, which are characterized by a CV% of 18 and 16%, respectively.
Fig. 1.
Chromatographic profile of AdipoDren®. a Negative ion. b Positive ion.
Effect of Phytocomplexes on Endothelial Survival
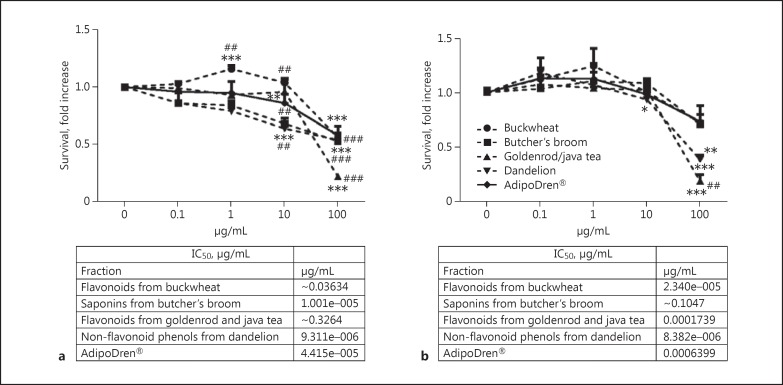
In order to test the efficacy of selected complex natural substances on endothelial cell functional responses, a first set of experiments was run to evaluate the effect of increasing concentrations (0.1-100 μg/mL) of test samples on cell survival, measured through the MTT test after 24 h of incubation in medium containing 2% serum. The results, reported in Fig. 2a and b for HDLEC and HDBEC, respectively, demonstrated that all the tested compositions induced a significant toxicity at 100 μg/mL on both cell lines. When tested at 10 μg/mL on HDLEC, only a few of them continued to show cytotoxicity, while at 1 and 0.1 μg/mL no toxicity was found. Interestingly, at 1 μg/mL, flavonoids extracted from buckwheat increased HDLEC number to a significant extent.
Fig. 2.
Endothelial cell survival in the presence of phytocomplexes or their combination (AdipoDren®). Results of MTT survival tests in HDLEC (a) and HDBEC (b). Data are reported as the fold increase of relative absorbance measured per well in the cells after 24 h of incubation in medium containing 2% serum and increasing concentrations of plant extracts. n = 3. * p < 0.05, ** p < 0.01, and *** p < 0.001 versus basal; ##p < 0.01 and ###p < 0.001 versus AdipoDren® preparation. The IC50 values (μg/mL) are reported under each graph.
Comparing the 2 sets of experiments (Fig. 2a, b), these results document a higher sensitivity of HDLEC than HDBEC in the tested compositions. The dose-response curve produced by AdipoDren® had an average tendency with respect to the single components. Different concentration ranges were chosen for the extracts and AdipoDren® to be evaluated in the next experiments, carefully avoiding cytotoxic concentrations that could interfere with the permeability measurements.
Effect of Phytocomplexes on Endothelial Permeability and Monolayer Integrity
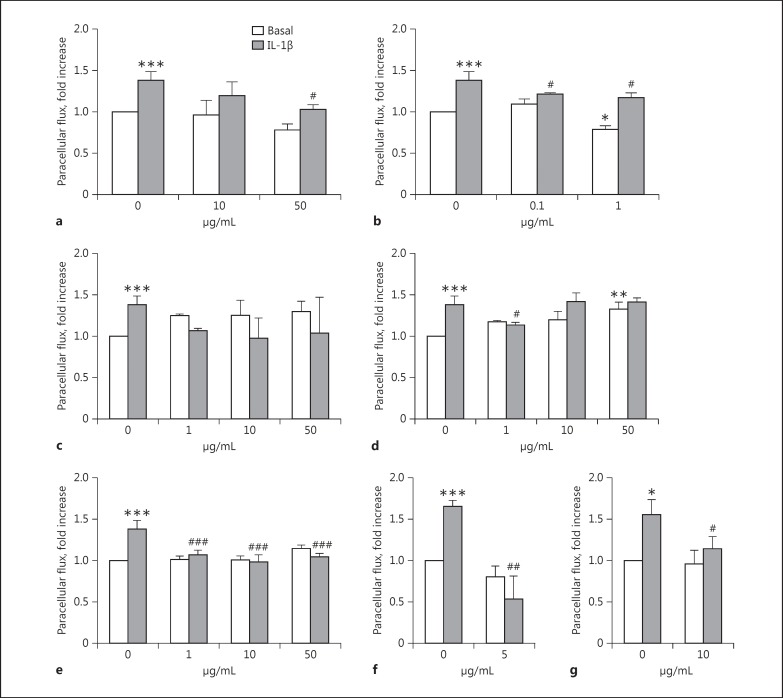
The basal and IL-1β (10 ng/mL, 1 h)-induced paracellular permeability to fluorescent dextran was evaluated in confluent endothelial monolayers exposed to test samples for 18 h. The following concentrations were tested on HDLEC: 10-50 μg/mL for buckwheat flavonoids, 0.1-1 μg/mL for dandelion non-flavonoid phenols, and 1-10-50 μg/mL for butcher's broom saponins, goldenrod/java tea flavonoids, and AdipoDren®. IL-1β (10 ng/mL) induced a significant increase in paracellular flux of FITCH-dextran after 1 h of incubation (Fig. 3). Test samples per se were devoid of any activity except non-flavonoid phenols from dandelion which, at 1 μg/mL, reduced basal permeability, and flavonoids from goldenrod/java tea which, at 50 μg/mL, increased spontaneous permeability, the latter effect presumably due to a partial cytotoxic effect (Fig. 3d, black columns). The pre-incubation with test samples prevented IL-1β-induced hyperpemeability with a significant and complete abolishment of the cytokine-induced effect brought about by AdipoDren® at all of the concentrations tested (Fig. 3e, grey columns). As can be seen, differently from the single plant-derived compositions, AdipoDren® was able to maintain the physiologic permeability level of HDLEC at all the concentrations tested, demonstrating that this specific association of non-flavonoid phenols, flavonoids, and saponins from different plant sources, rather than each single plant-derived phytocomplex, exerts an optimal effect respective of HDLEC physiologic permeability.
Fig. 3.
Effect of phytocomplexes on lymphatic endothelial cell permeability induced by IL-1β. HDLEC were treated with plant extracts: flavonoids from buckwheat (a); non-flavonoid phenols from dandelion (b); saponins from butcher's broom(c); flavonoids from goldenrod and java tea (d), or their association (AdipoDren®) (e) for 18 h; rutin (5 µg/mL) was used as a positive control (f). g HDBEC were treated with AdipoDren® (10 μg/mL, 18 h). FITC-dextran transcellular transport was measured after 1 h of stimulation with IL-1β (10 ng/mL). n = 3. * p < 0.05, ** p < 0.01, and *** p < 0.001 versus basal; #p < 0.05 and ###p < 0.001 versus IL-1β alone.
As a reference, the incubation of HDLEC with rutin (5 μg/mL, 18 h) significantly reduced basal and IL-1β-induced permeability, reinforcing the protective effect of polyphenols on endothelial cell monolayers (Fig. 3f). It is interesting to note that the concentration tested for isolated rutin (5 µg/mL) was consistently higher than the corresponding rutin concentration from the buckwheat sample tested at 10 µg/mL (which corresponds to 1.2 µg/mL of rutin). However, it is noteworthy that, at this concentration, the extract showed a good effect in preventing IL-1β-induced hyperpermeability on HDLEC, indicating that probably many other components of buckwheat flavonol glycoside-enriched extract contribute to the overall observed effect.
As seen on HDLEC, the hyperpermeability of HDBEC induced by IL-1β (10 ng/mL, 1 h) was reduced by cell exposure to AdipoDren® (10 μg/mL; Fig. 3g). Note that, as seen above on cell survival, HDBEC were less responsive to AdipoDren® compared to HDLEC, the permeability response of which was completely abolished by AdipoDren® pre-incubation (Fig. 3e vs. g).
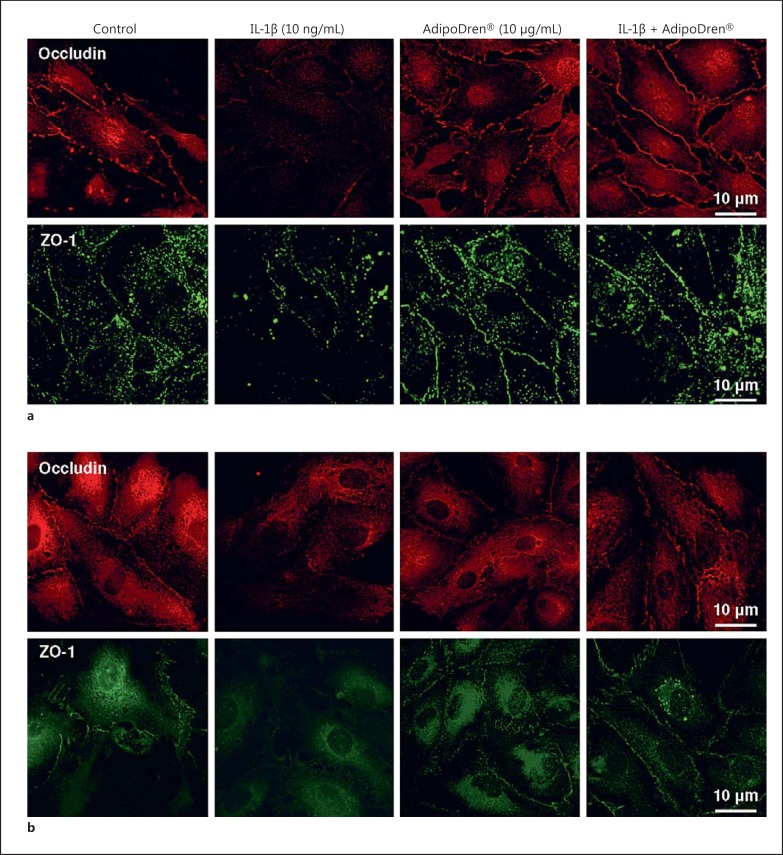
To reinforce the permeability data, immunofluorescence analysis of cell-cell contacts in endothelial cell cultures was performed in cells pre-incubated with AdipoDren® (10 μg/mL, 18 h) and then exposed to IL-1β (10 ng/mL, 1 h). Occludin and ZO-1 were evaluated as representative markers of endothelial tight junctions. While confluent cells (both lymphatic and blood EC) expressed these 2 proteins at cell-contacts, IL-1β induced their fainting. The overnight pre-incubation with AdipoDren® maintained cell-cell contact markers and did not allow their fading after IL-1β treatment (Fig. 4a, b; Table 3). Notably, an increase in cytoplasmic staining, presumably a sign of active protein synthesis, was visible in both cell types following AdipoDren® incubation.
Fig. 4.
Maintenance of cell-cell contact by AdipoDren®. Immunofluorescence analysis of occludin (upper panels) and ZO-1 protein (lower panels) in confluent HDLEC (a) and HDBEC (b) treated with the following stimuli: none, IL-1β (10 ng/mL, 1 h), AdipoDren® (10 μg/mL, 18 h), AdipoDren® (18 h) + IL-1β (1 h). Confocal images were taken at ×63 magnification.
Table 3.
Occludin and ZO-1 fluorescence quantification
| None | IL-1β | AdipoDren® | AdipoDren® + IL-1β | |
|---|---|---|---|---|
| HDLEC | ||||
| Occludin | 538,204 ± 189,997 | 61,057 ± 59,245* | 1,020,593 ± 134,129 | 655,570 ± 103,590## |
| ZO-1 | 1,986,154 ± 185,007 | 268,772 ± 77,869*** | 1,455,483 ± 66,005 | 1,186,348 ± 271,571## |
| HDBEC | ||||
| Occludin | 401,247 ± 21,304 | 108,283 ± 38,457*** | 262,024 ± 21,983* | 230,980 ± 39,389## |
| ZO-1 | 194,926 ± 44,295 | 73,387 ± 27,183* | 144,625 ± 4,250 | 162,937 ± 38,487# |
Cell fluorescence were measured using ImageJ software. Data are expressed as the corrected total cell fluorescence ± SD, calculated as: integrated density – (area of selected cell × mean fluorescence of background readings).
p < 0.05
p < 0.01 versus None
p < 0.05
p < 0.01 versus IL-1β.
Collectively, these data indicated that the selected non-flavonoid phenols, flavonoids, and saponins show interesting features on lymphatic endothelium and a protective effect is particularly evident when they are associated in AdipoDren® on both lymphatic and blood vessel EC stimulated with the inflammatory cytokine.
Molecular Mechanisms Responsible for Endothelial Protection by AdipoDren®
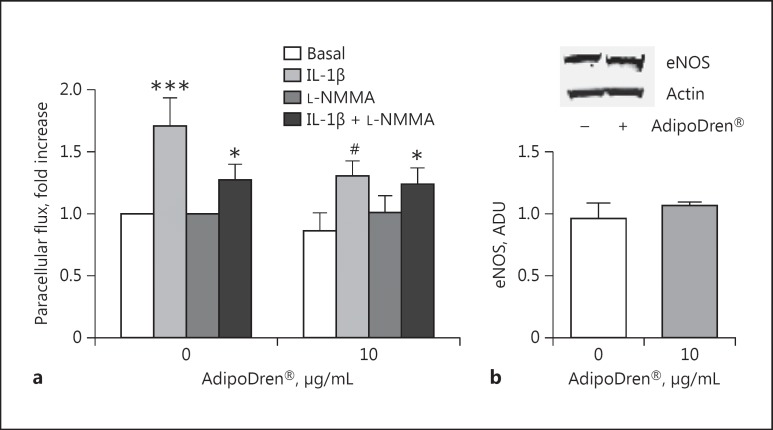
The biochemical and molecular mechanisms associated with the protective features expressed by AdipoDren® on lymphatic endothelium were then evaluated. Preservation of vascular function and homeostasis can be proposed as a preventive strategy to limit inflammatory damage. The simultaneous incubation of cells with AdipoDren® and the NOS inhibitor L-NMMA did not affect the protective effect elicited by AdipoDren® on IL-1β permeability (Fig. 5a). Furthermore, the treatment of cells with AdipoDren® (10 μg/mL, 18 h) in the basal condition maintained an unchanged eNOS expression (Fig. 5b). These data show that eNOS activity is not necessary for the protective effect of AdipoDren® in an inflammatory environment, while AdipoDren® maintained the endothelial functions unaltered.
Fig. 5.
eNOS-independent effect of AdipoDren® on IL-1β-induced permeability. a Cells were treated with L-NMMA (100 μM) and AdipoDren® (10 μg/mL, 18 h). FITC-dextran transcellular transport was measured after 1 h of stimulation with IL-1β (10 ng/mL). n = 3. * p < 0.05; *** p < 0.001 versus basal; #p < 0.05 versus IL-1β alone. b eNOS expression in HDLEC following exposure to AdipoDren® (10 μg/mL, 18 h). A representative gel of 3 tests is reported. Data are reported as ADU. n = 3.
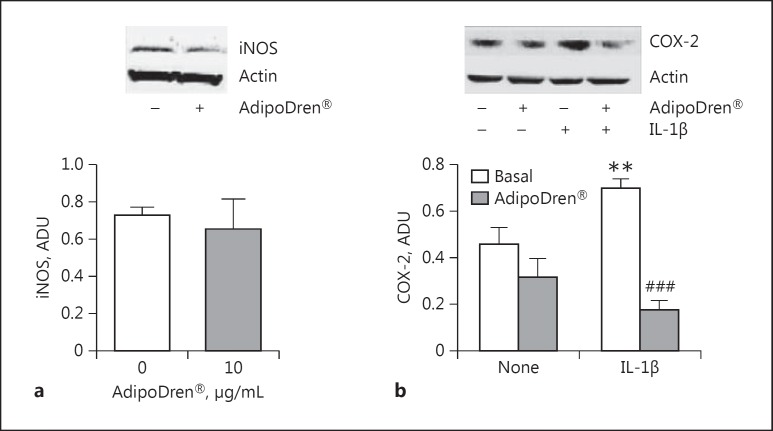
Key enzymes involved in the production/processing of inflammatory mediators were additionally evaluated in response to AdipoDren®. iNOS and COX-2 are inducible enzymes responsible for the production of a high quantity of nitrogen species and prostaglandins, mediators with vasodilating/propermeability activities and inflammatory cell infiltration. AdipoDren® (10 μg/mL) induced a slight non-significant downregulation of iNOS, while it reduced COX-2 levels. IL-1β-induced COX-2 protein contents were significantly reduced following AdipoDren® exposure (Fig. 6a, b). These data reinforce the finding of a protective function of AdipoDren® on physiological endothelial activities.
Fig. 6.
Effect of AdipoDren® on the expression of enzymes involved in inflammation. iNOS (a) and COX-2 (b) expression was carried out in HDLEC following exposure to AdipoDren® (10 μg/mL, 18 h) in the absence and presence of IL-1β (10 ng/mL). A representative gel of 3 tests is reported. Data are reported as ADU. n = at least 3. ** p < 0.01 versus basal; ###p < 0.001 versus IL-1β alone.
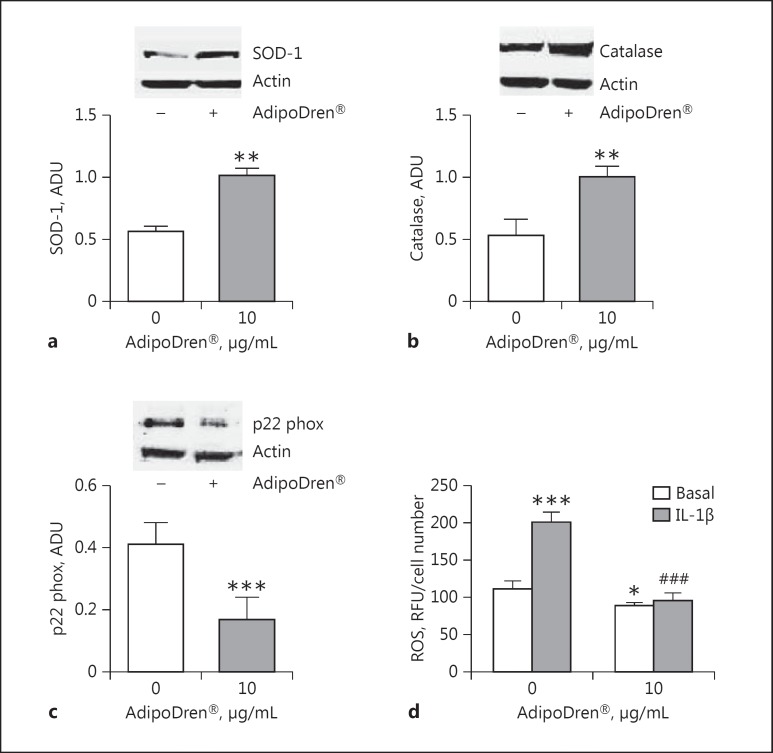
Due to the antioxidant properties of rutin and the other polyphenolic compounds characterising the tested compositions, cells were monitored for a panel of key oxidative stress enzymes. The expression of the antioxidant enzymes SOD-1 and catalase in HDLEC were increased by AdipoDren® stimulation, while the levels of the subunit of NADPH oxidase p22 phox was reduced (Fig. 7). Moreover, AdipoDren® further increased the levels of SOD-1 and catalase in the presence of IL-1β (respectively, +28.5 and +25.5% increase over the IL-1β effect, p < 0.01, n = 3). The antioxidant effect of AdipoDren® was confirmed by the measurement of ROS levels, which were reduced after the treatment with AdipoDren® both in the absence and in the presence of IL-1β (Fig. 7d).
Fig. 7.
AdipoDren® activity on the expression of enzymes involved in oxidative stress. SOD-1 (a), catalase (b), and p22 phox (c) expression in HDLEC following exposure to AdipoDren® (10 μg/mL, 18 h). Blots are representative of 3 experiments with overlapping results. Data are reported as ADU (n = 3). ** p < 0.01 versus basal. d ROS production in cells stimulated with IL-1β (1 h) with or without AdipoDren® (10 μg/mL, 18 h). n = 3. * p < 0.05 and *** p < 0.001 versus basal; ###p < 0.001 versus IL-1β.
Thus, the treatment of lymphatic endothelium with AdipoDren® upregulated all of the protective features of endothelium, while the key enzymes involved in cell damage and activation toward an inflammatory phenotype were downregulated, predisposing endothelium to react to the inflammatory insult.
Discussion
In this paper we report for the first time the protective effect of AdipoDren®, an association of a series of complex natural substances on lymphatic and blood endothelial cell permeability, promoted by the inflammatory cytokine IL-1β, and the molecular mechanisms associated with the protective effects. In particular, we could demonstrate that non-flavonoid phenols, flavonoids, and steroidal saponins extracted from dandelion, buckwheat, goldenrod/java tea, and butcher's broom, respectively, exert a protective effect on endothelial cell permeability induced by IL-1β. This is particularly true when the selected complex natural substances are combined together to form a specific composition (AdipoDren®). AdipoDren® is able to reduce IL-1β-induced cell hyperpermeability at all the concentrations tested, and is more effective with respect to its single components (especially ruscogenins from butcher's broom and rutin-rich extract from buckwheat), indicating that the association of several substances amplifies their protective effect on lymphatic endothelial cells in an inflammatory environment. Moreover, as permeability is correlated with cell retraction and the re-organisation of cell-cell junctional proteins, AdipoDren® resumes the cell-cell junctions compromised by IL-1β. In particular, proteins demonstrated to be in tight and adherens junctions of lymphatic endothelium (HDLEC) are occludin and ZO-1, and VE cadherin, respectively [25,26]. Inflammatory mediators such as TNF-α have been demonstrated to increase discontinuous junctions and to reduce VE-cadherin peripheral localisation [9,26]. Here we demonstrate that IL-1β produces the fainting and disappearance of occludin and ZO-1 at the periphery of both HDLEC and HDBEC. At the cellular level, the reduced permeability by AdipoDren® is due to the maintenance of tight junctions and cell-cell localisation of occludin and ZO-1. Lymphatic and blood vessel endothelial cells show overlapping results. Indeed, given that lymphatic and vascular endothelial cells have a common developmental origin [27], there are likely to also be similarities with the mechanisms underlying permeability regulation in both the lymphatic and blood endothelium. In addition, since CCR7- and IRF4-dependent dendritic cells around lymphatic collectors have been reported to regulate lymphatic permeability [28], an effect of AdipoDren® on these cells cannot be excluded.
Considering the molecular mechanisms underlying the protective effect observed, AdipoDren® reduces the expression of key inflammatory markers, such as iNOS and COX-2, while eNOS is not altered or involved in the functional responses. Since most of the protective effect elicited by polyphenols or other bioactive components is due to ROS scavenging activities, the expression pattern of antioxidant/pro-oxidant enzymes has been evaluated. Treatment of lymphatic endothelial cells with AdipoDren® induces the upregulation of antioxidant enzymatic systems (catalase and SOD-1) and the downregulation of pro-oxidant markers (p22 phox subunit of NADPH oxidase), moving the balance toward detoxification pathways, as also demonstrated by the measurement of ROS levels. All these features are established both in healthy lymphatic cells and following their exposure to IL-1β, indicating that AdipoDren® per se predisposes endothelial cells to counteract further inflammatory stimuli.
In addition to the protective effect on lymphatic endothelial cells here demonstrated for AdipoDren®, it could also be speculated that AdipoDren® exerts another beneficial effect, useful in lymphoedema conditions, thanks to its rutin content. Mouse models of lymphatic vascular dysfunction show adipose tissue accumulation and there is also evidence that lymph promotes the differentiation of pre-adipocytes in adipocytes in vitro [29], suggesting that the leakage of lymph, in lymphoedema conditions, could stimulate adipose tissue expansion. At the same time, adipose tissue expansion and accumulation, observed in mice fed a high-fat diet, has been shown to impair the functionality of the lymphatic vasculature, supporting the hypothesis that there could be a direct link between lymphatic alteration and obesity [4]. Adipose tissue acts as a secretory organ that generates signals that have a negative impact on lymphatic vasculature tone and permeability. In this scenario, lymphatic dysfunction could lead to further adiposity, thus worsening the condition in a vicious cycle [30,31]. Plant fractions enriched in rutin (i.e. buckwheat fraction and AdipoDren®), a flavonol glycoside endowed with antiadipogenic activity as shown in both in vitro and in vivo models [14], could be useful in preventing lymphatic impairment in the presence of lymphoedema conditions and/or obesity.
In conclusion, this work provides a cellular and biochemical characterisation of the lymphatic and venous protective effect of a series of complex natural substances against an inflammatory stimulus. These results can be exploited for the clinical use of a particular composition (AdipoDren®) comprising hydroxycinnamic acid derivatives, flavonol glycosides, and steroidal saponins to preserve and promote the physiological endothelial functionality of lymphatic vessels.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;7:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 2.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 3.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 4.Blum KS, Karaman S, Proulx ST, Ochsenbein AM, Luciani P, Leroux JC, Wolfrum C, Detmar M. Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PLoS One. 2014;4:e94713. doi: 10.1371/journal.pone.0094713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karaman S, Hollmén M, Robciuc MR, Alitalo A, Nurmi H, Morf B, Buschle D, Alkan HF, Ochsenbein AM, Alitalo K, Wolfrum C, Detmar M. Blockade of VEGF-C and VEGF-D modulates adipose tissue inflammation and improves metabolic parameters under high-fat diet. Mol Metab. 2014;4:93–105. doi: 10.1016/j.molmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rockson SG. The unique biology of lymphatic edema. Lymphat Res Biol. 2009;7:97–100. doi: 10.1089/lrb.2009.7202. [DOI] [PubMed] [Google Scholar]
- 7.Zgraggen S, Ochsenbein AM, Detmar M. An important role of blood and lymphatic vessels in inflammation and allergy. J Allergy (Cairo) 2013;2013:672381. doi: 10.1155/2013/672381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai Y, Kaidoh M, Yokoyama Y, Ohhashi T. Pivotal roles of lymphatic endothelial cell layers in the permeability to hydrophilic substances through collecting lymph vessel walls: effects of inflammatory cytokines. Lymphat Res Biol. 2014;12:124–135. doi: 10.1089/lrb.2014.0002. [DOI] [PubMed] [Google Scholar]
- 9.Cromer WE, Zawieja SD, Tharakan B, Childs EW, Newell MK, Zawieja DC. The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis. 2014;17:395–406. doi: 10.1007/s10456-013-9393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 11.Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 12.Bruneton J. Pharmacognosy, Phytochemistry, Medicinal Plants. ed 2. Cachan: Lavoisier; 1999. [Google Scholar]
- 13.Sedej I, Sakač M, Mandić A, Mišan A, Tumbas V, Čanadanović-Brunet J. Buckwheat (Fagopyrum esculentum Moench) grain and fractions: antioxidant compounds and activities. J Food Sci. 2012;77:C954–C959. doi: 10.1111/j.1750-3841.2012.02867.x. [DOI] [PubMed] [Google Scholar]
- 14.Choi I, Park Y, Choi H, Lee EH. Anti-adipogenic activity of rutin in 3T3-L1 cells and mice fed with high-fat diet. Biofactors. 2006;26:273–281. doi: 10.1002/biof.5520260405. [DOI] [PubMed] [Google Scholar]
- 15.Chua LS. A review on plant-based rutin extraction methods and its pharmacological activities. J Ethnopharmacol. 2013;150:805–817. doi: 10.1016/j.jep.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 16.Gruenwald J, Brendler T, Jaenicke C, editors. PDR for Herbal Medicine. ed 4. New York: Thomson Healthcare; 2007. [Google Scholar]
- 17.ESCOP (European Scientific Cooperative on Phytotherapy) Monographs. ed 2. New York: Thieme Medical Publishers; 2003. [Google Scholar]
- 18.Ameer OZ, Salman IM, Asmawi MZ, Ibraheem ZO, Yam MF. Orthosiphon stamineus: traditional uses, phytochemistry, pharmacology, and toxicology. J Med Food. 2012;15:678–690. doi: 10.1089/jmf.2011.1973. [DOI] [PubMed] [Google Scholar]
- 19.Blumenthal M, Goldberg A, Brinckmann J. Herbal Medicine: Expanded Commission E Monographs. New York: Thieme Medical Publishers; 2000. [Google Scholar]
- 20.Pouget G, Ducros L, Marcelon G. Effect of Ruscus extract on pheripheral lymphatic vessel pressure and flow. In: Vanhoutte PM, editor. Return Circulation and Norepinephrine: An Update. Paris: John Lybbey Eurotext; 1991. pp. 89–95. [Google Scholar]
- 21.Pezzatini S, Morbidelli L, Solito R, Paccagnini E, Boanini E, Bigi A, Ziche M. Nanostructured HA crystals up-regulate FGF-2 expression and activity in microvascular endothelium promoting angiogenesis. Bone. 2007;41:523–534. doi: 10.1016/j.bone.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Terzuoli E, Meini S, Cucchi P, Catalani C, Cialdai C, Maggi CA, Giachetti A, Ziche M, Donnini S. Antagonism of bradykinin B2 receptor prevents inflammatory responses in human endothelial cells by quenching the NF-kB pathway activation. PLoS One. 2014;9:e84358. doi: 10.1371/journal.pone.0084358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monti M, Terzuoli E, Ziche M, Morbidelli L. The sulphydryl containing ACE inhibitor zofenoprilat protects coronary endothelium from doxorubicin-induced apoptosis. Pharmacol Res. 2013;76:171–181. doi: 10.1016/j.phrs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Monti M, Solito R, Puccetti L, Pasotti L, Roggeri R, Monzani E, Casella L, Morbidelli L. Protective effects of novel metal-nonoates on the cellular components of the vascular system. J Pharmacol Exp Ther. 2014;351:500–509. doi: 10.1124/jpet.114.218404. [DOI] [PubMed] [Google Scholar]
- 25.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kakei Y, Akashi M, Shigeta T, Hasegawa T, Komori T. Alteration of cell-cell junctions in cultured human lymphatic endothelial cells with inflammatory cytokine stimulation. Lymphat Res Biol. 2014;12:136–143. doi: 10.1089/lrb.2013.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanov S, Scallan JP, Kim KW, Werth K, Johnson MW, Saunders BT, Wang PL, Kuan EL, Straub AC, Ouhachi M, Weinstein EG, Williams JW, Briseño C, Colonna M, Isakson BE, Gautier EL, Förster R, Davis MJ, Zinselmeyer BH, Randolph GJ. CCR7 and IRF4-dependent dendritic cells regulate lymphatic collecting vessel permeability. J Clin Invest. 2016;126:1581–1591. doi: 10.1172/JCI84518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harvey NL. The link between lymphatic function and adipose biology. Ann NY Acad Sci. 2008;1131:82–88. doi: 10.1196/annals.1413.007. [DOI] [PubMed] [Google Scholar]
- 30.Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro and microcirculation of adipose tissue. FEBS J. 2009;276:5738–5746. doi: 10.1111/j.1742-4658.2009.07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von der Weid PY, Rainey KJ. Review article: lymphatic system and associated adipose tissue in the development of inflammatory bowel disease. Aliment Pharmacol Ther. 2010;32:697–711. doi: 10.1111/j.1365-2036.2010.04407.x. [DOI] [PubMed] [Google Scholar]