Abstract
Background
Delayed response to clinical deterioration of ward patients is common.
Methods
We performed a prospective before-and-after study in all patients admitted to two clinical ward areas in a district general hospital in the UK. We examined the effect on clinical outcomes of deploying an electronic automated advisory vital signs monitoring and notification system, which relayed abnormal vital signs to a rapid response team (RRT).
Results
We studied 2139 patients before (control) and 2263 after the intervention. During the intervention the number of RRT notifications increased from 405 to 524 (p = 0.001) with more notifications triggering fluid therapy, bronchodilators and antibiotics. Moreover, despite an increase in the number of patients with “do not attempt resuscitation” orders (from 99 to 135; p = 0.047), mortality decreased from 173 to 147 (p = 0.042) patients and cardiac arrests decreased from 14 to 2 events (p = 0.002). Finally, the severity of illness in patients admitted to the ICU was reduced (mean Acute Physiology and Chronic Health Evaluation II score: 26 (SD 9) vs. 18 (SD 8)), as was their mortality (from 45% to 24%; p = 0.04).
Conclusions
Deployment of an electronic automated advisory vital signs monitoring and notification system to signal clinical deterioration in ward patients was associated with significant improvements in key patient-centered clinical outcomes.
Trial registration
ClinicalTrials.gov, NCT01692847. Registered on 21 September 2012.
Electronic supplementary material
The online version of this article (doi:10.1186/s13054-017-1635-z) contains supplementary material, which is available to authorized users.
Background
Deterioration of patients on general hospital wards often goes unnoticed for prolonged periods of time [1]. This delay can result in otherwise preventable cardiopulmonary arrest and admission to the intensive care unit (ICU) [2, 3] despite the fact that, in most cases, measurable changes in vital signs [4] could identify patients at risk. Such delayed or absent response to deterioration has been labeled as “failure to rescue” [5], which may be due to a combination of factors [6]. In order to decrease the incidence and consequences of such failure to rescue, many hospitals have introduced rapid response systems (RRSs) [7]. Rapid response systems have been shown to reduce hospital mortality, the rate of cardiopulmonary arrests and preventable ICU admissions, in a number of settings [8]. Nevertheless, even in hospitals with an established RRS, failure-to-rescue events occur [9–11], mostly related to problems with the afferent (monitoring, identification and rapid response team (RRT) activation) component of the RRS. All these failings have in common the dependence on individual bedside staff to raise the alarm.
In contrast to human-based response, industrial high-reliability systems rely on redundancy to ensure that failure of a single part does not result in system failure [12, 13]. When this approach is applied to monitoring in health care, systems with automated notification can be deployed to notify remote and senior healthcare professionals or RRTs who are not at the bedside to respond to deterioration [14, 15]. This approach can be supplemented with continuous monitoring of selected vital signs such as heart rate, respiratory rate and oxygen saturation. Accordingly, we hypothesized that the application of monitoring technology with automated notification of the RRT would improve the reliability of escalation of care for clinically deteriorating patients on general wards, and result in improved patient outcomes.
Methods
Setting
We conducted a before-and-after study on two wards of a university-affiliated hospital serving a population of 220,000 inhabitants in the UK. The hospital covers all hospital specialties including a hematology and oncology unit and a dialysis unit but does not provide neuro-surgical, cardiothoracic or transplant surgery care. The ICU has eight beds and there are a further five high dependency and five coronary care beds.
The intervention was undertaken on two general medical wards with 30 and 24 beds, respectively, one admitting patients predominantly with respiratory disease and the other admitting patients with predominantly gastroenterological conditions. The two study wards were selected due to their large proportion of patients with abnormal and complex physiology as evidenced by high rates of critical care transfers and cardiopulmonary arrest prior to the commencement of the study.
Ethics
The hospital human research ethics committee (Reference 12/WA/0050, Protocol number SD-05163-BBN-IGS A.2) approved the study. Monitoring remained in line with hospital policy in the control and intervention phase. The ethics committee confirmed that no patient consent was required.
Study patients
We included all patients admitted to the study wards as acute emergencies if they had at least one overnight stay (more than 24 hours of admission). We excluded patients admitted for elective procedures (such as pulmonary biopsy, pleural procedures or endoscopy). Data from all patients were collected on age, gender, main diagnosis (ICD 10 code), date of admission, date of discharge, degree of physiological abnormality as described by the National Early Warning Score (NEWS) [16] on admission, survival status and limitation of medical treatment (LOMT).
Rapid response system
Hospital policy stipulates the recording of vital signs in acutely unwell patients at least twice per day and with increasing frequency in the presence of increasing severity, usually four times per day. Trained registered nurses and health care assistants obtained and recorded vital signs.
We deployed three different scoring algorithms to identify patients at risk: the NEWS was used as the default algorithm. The Chronic Respiratory Early Warning Score (CREWS) [17] was used in patients with chronic hypoxia due to chronic obstructive pulmonary disease or pulmonary fibrosis. The CREWS algorithm uses different weighting for abnormalities of oxygen saturation. A palliative algorithm scored all patients according to the NEWS algorithm but did not result in notifications. Patients were allocated to either NEWS, CREWS or the palliative algorithm by senior medical staff or the nurse in charge of the ward.
The local vital sign protocol recommends escalation to the nurse in charge of each ward in patients with a score of 3 or more. For a score of 6 or more, it recommends additional escalation to a resident doctor and an advanced nurse practitioner member of the RRT. For a score of 9 or more, it recommends escalation to the lead of the medical on-call team (a physician with a minimum of four years of clinical experience). We obtained data on several key interventions (i.e. fluid bolus, antibiotics, bronchodilators and time on ventilators). In order to avoid subjective judgment, all notifications were classified as independent events.
Study period
The control and intervention phases were separated by a washout period. Wards were allocated to the intervention phase of the study with a two-month difference. Patient data were collected from 15 October 2012 to 16 October 2013 for the control period in ward 1 and from 1 October 2012 to 2 October 2013 for the control period in ward 2.
Data were collected in the intervention phase from 17 February 2014 in ward 1 and 28 April 2014 in ward 2 until all the interventions ceased in both wards on 17 April 2015. Additional control data were collected in ward 2 from 17 February 2014 to 17 April 2014 to run parallel with the intervention start period in ward 1 for two months.
Intervention
During the intervention period an electronic automated advisory vital signs monitoring system (Intelivue Guardian Solution (IGS) with cableless sensors and MP5SC spot-check monitors, Philips Healthcare, Boeblingen, Germany) was deployed to each study ward. The monitoring system electronically transfers and displays respiratory rate, blood pressure, heart rate, pulse oximetry and temperature either obtained by the bedside nurses using spot-check monitors (Fig. 1a) or by cableless sensor devices. The spot-check monitors request the nurse to manually enter respiratory rate (RR) or to confirm an RR measurement from a wireless patient sensor. Information on conscious state was entered manually. Early warning scores (EWSs) were automatically calculated from these vital signs.
Fig. 1.
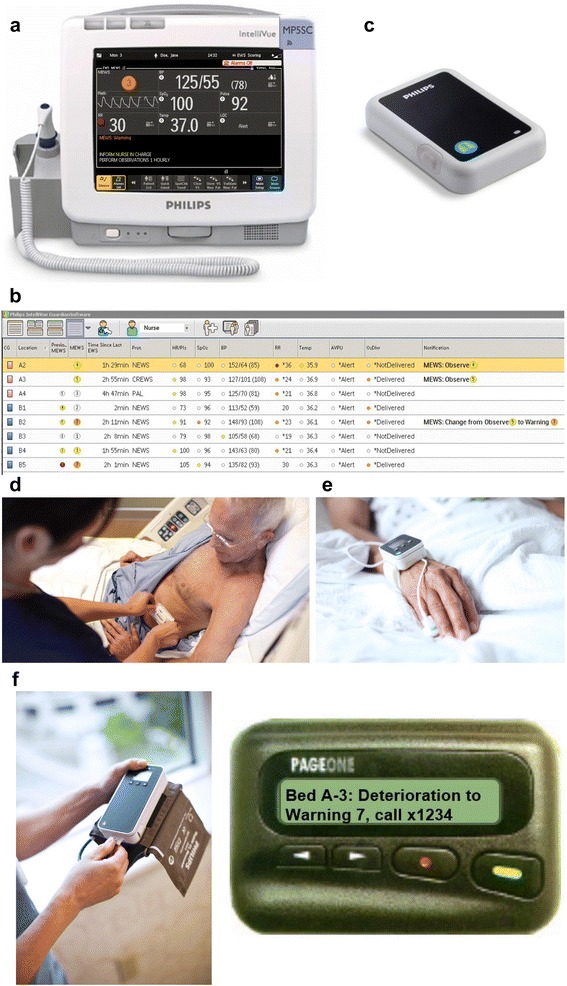
a-f Elements of the monitoring system with a spot-check monitor with connection to wireless area network (a), central screen (b), wireless respiratory sensor (c), application of wireless respiratory sensor (d), wireless oxygen saturation monitor (e), wireless blood-pressure monitor (f) and alphanumeric paging device (g)
The NEWS and CREWS values were displayed and color-coded at central screens (Fig. 1b) in the nursing station as: “safe range” in white; “observe range” in yellow; “warning range” in orange, and “urgent range” in red. The same color codes were used on screens in the offices of consultant physicians of the ward.
Cableless devices for respiratory rate (Philips IntelliVue CL Respiration Pod, Fig. 1c, d) and saturations (Fig. 1e) were set to record vital signs every 15 minutes. Cableless blood pressure devices (Fig. 1f) were set to start a series of 7 measurements every 15 minutes on demand only and at times of EWS. Deployment of cableless sensors was at the discretion of the treating clinicians. Overall, 278 patients had at least one cableless sensor attached during the intervention phase. Based on automated cableless measurements, “deterioration notifications” were paged and were displayed on a big central screen if a change in the cableless parameters resulted in a change of the EWS.
Nurses on the study wards and members of the RRT received notifications about abnormal vital signs via a dedicated alphanumeric paging device (Fig. 1g) that would display the value of the score, the location of the patient and information about the trend (for example “Bed A1, NEWS changed from 5 to 9, call (telephone number)”).
Training in how to use the EWS and the escalation protocol preceded the current study. Training in the use of the IGS was undertaken in two workshops and a dedicated trainer was on site at the hospital for the first week of the intervention period of each of the study wards for in-service training in day and night shifts until 80% of staff had been trained.
Outcome measures
We obtained information on the prevalence of predefined serious events (acute myocardial infarction, pulmonary embolism, acute pulmonary edema, respiratory failure, stroke, severe sepsis, acute renal failure, emergency admission to the ICU, cardiopulmonary arrest, death), activations of the RRT and patient outcomes, using the hospital administration system and clinical records. Trained research nurses extracted the data.
Data on cardiopulmonary arrests were extracted from clinical notes and cross-referenced with the clinical administration system of the hospital. The same process was followed for deaths in the study wards and admissions to the ICU.
The hospital also collected data as part of the Intensive Care National Audit and Research Centre (ICNARC) case-mix program including data on severity of illness on admission to the ICU as measured by the Acute Physiology and Chronic Health Evaluation II (APACHE II) score [18].
Statistical analysis
All statistical analysis was performed using Minitab (V16, Minitab Inc., PA, USA) and the Software Package for Social Sciences (SPSS V22). For continuous variables, descriptive statistics are presented as means with standard deviation or 95% confidence intervals (CIs). Where appropriate, medians and interquartile ranges (IQRs) are given. Categorical variables are presented as actual number and proportion of overall count. Continuous variables were compared using the Mann–Whitney test or the Student t test as appropriate, while categorical variables were compared using the chi-square, normal approximation or Fisher’s exact test. Binary logistic regression with a backward stepwise (Wald) procedure was used to confirm that mortality and cardiac arrests were independently associated. A p value <0.05 was considered statistically significant. The sample size of the study was based on limited data from local spot-check audits: 100 events per phase and ward were considered, according to pertinent nomograms and Gpower software calculations (SIG level 0.05, Power 0.8), to prove a relevant effect.
Results
Patient characteristics
During the control period, the 2139 patients with a mean age of 68.8 years (standard deviation (SD) 17 years) were admitted to the study wards compared to 2263 patients with a mean age of 68.6 years (SD 17.3) who were admitted during the intervention period. Patient characteristics are summarized in Table 1. Patients on the respiratory ward had greater severity of illness as measured by NEWS on admission to hospital (2.40, 95% CI 2.24–2.57; p < 0.001). The NEWS algorithm was used in 80% of patients, the CREWS algorithm was in used in 16% of patients; 4% of patients were palliative.
Table 1.
Patient characteristics
| All patients | Patients with RRT notification | |||||
|---|---|---|---|---|---|---|
| Parameter | Control period | Intervention period | p | Control period | Intervention period | p |
| Number of patients | 2139 | 2263 | 304/2139 (14.2) | 365/2263 (16.1) | 0.076 | |
| Ward 1 (gastroenterology) | 941/2139 (44.0) | 1062/2263 (46.9) | .051 | 131/304 (43.1) | 177/365 (48.5) | 0.163 |
| Ward 2 (pulmonology) | 1198/2139 (56.0) | 1201/2263 (53.1) | 0.051 | 173/304 (56.9) | 188/365 (51.5) | 0.163 |
| Age in years | 68.8 (68.1–69.5) | 68.6 (67.9–69.3) | 0.727 | 71.9 (70.2–73.6) | 70.3 (68.7–72.0) | 0.200 |
| Male gender | 1041/2139 (48.7) | 1069/2263 (47.2) | 0.343 | 152/304 (50.0) | 175/365 (47.9) | 0.596 |
| Ward LOS | 7 (4–11) | 6 (3–11) | 0.232 | 11 (7–18) | 10 (7–19) | 0.557 |
| NEWS value on hospital admission | 3.27 (3.14–3.41) | 3.26 (3.13–3.39) | 0.923 | 3.97 (3.63–4.31) | 4.13 (3.81–4.45) | 0.511 |
| NEWS value on hospital admission Ward 1 | 1.84 (1.70–1.98) | 2.01 (1.87–2.16) | 0.096 | 2.73 (2.31–3.15) | 2.62 (2.24–3.00) | 0.692 |
| NEWS value on hospital admission Ward 2 | 4.30 (4.12–4.48) | 4.37 (4.19–4.56) | 0.591 | 4.88 (4.42–5.34) | 5.46 (5.04–5.88) | 0.068 |
| COPD and CFA diagnosis | 526/2139 (24.6) | 551/2263 (24.3) | 0.851 | 104/304 (34.2)) | 122/365 (33.4) | 0.831 |
Data expressed as means with 95% CI, median (IQR) and number (%). LOS length of stay, NEWS National Early Warning Score, EWS early warning score, COPD chronic obstructive pulmonary disease, CFA cryptogenic fibrosing alveolitis
Notifications
During the control period, 755/2139 patients (35.3%) reached a score of 6 or more. Their mean age was 72.0 (SD 14.2) years and 387/755 patients (51%) were female. Notification of the RRT, however, occurred only 405 times in 304 patients (1.33 per patient, 189/1000 admissions (18.9%)). The mean age in this patient subgroup was 71.9 (SD 15.1) years. Of these, 320/2139 patients (15.0%) reached a score of 9 or more (Table 2).
Table 2.
Number of patients with at least one NEWS score above notification limits
| Parameter | Control period | Intervention period | p | Control period | Intervention period | p |
|---|---|---|---|---|---|---|
| NEWS score | 6 or more | 9 or more | ||||
| Number of patients | 755/2139 (35.3%) | 747/2263 (33.0%) | 0.262 | 320/2139 (15.0%) | 302/2263 (13.3%) | 0.182 |
| Ward 1 (gastroenterology) | 136/941 (14.5%) | 135/1062 (12.7%) | 0.321 | 49/941 (5.2%) | 49/1062 (4.6%) | 0.559 |
| Ward 2 (pulmonology) | 619/1198 (51.7%) | 612/1201 (51.0%) | 0.843 | 271/1198 (22.6%) | 253/1201 (21.1%) | 0.460 |
Vital signs for patients monitored with the Chronic Respiratory Early Warning Score were recalculated using the National Early Warning Score (NEWS) in order to allow for comparison. Data expressed as number (%).
During the intervention period, 747/2263 patients (33.0%) reached a score of 6 or more. Their mean age was 72.3 (SD 14.4) years and 396/747 (53%) patients were female. Notification of the RRT occurred 524 times in 365 patients in the intervention phase (1.43 per patient, 231/1000 admissions (23.1%), p = 0.001 for comparison with control period). The mean age of this patient subgroup was 70.3 (SD 15.7). Of these patients, 302/2263 (13.3%) reached a score of 9 or more.
Response to notifications
During the 929 notifications (405 notifications in 304 patients in the control phase and 524 in 365 patients in the intervention phase), the RRT undertook a range of medical interventions (Table 3) with mortality in affected patients changing from 67/304 (22.0%) in the control phase to 63/365 (17.3%) in the intervention phase (p = 0.122).
Table 3.
Characteristics of patients with notifications and escalation events
| Parameter | Control period | Intervention period | p |
|---|---|---|---|
| Number of patients | 304/2139 | 365/2263 | 0.077 |
| Number of notifications | 405/2139 | 524/2263 | 0.001 |
| Age in years | 71.9 (70.2–73.6) | 70.3 (68.7–72.0) | 0.200 |
| Male gender | 152/304 (50.0) | 175/365 (47.9) | 0.597 |
| EWS value at time of first notification of rapid response team | 5.68 (5.23–6.12) | 5.53 (5.14–5.93) | 0.641 |
| Emergency ICU admissions | 26/304 (8.6) | 21/365 (5.8) | 0.158 |
| Patients with a DNAR order in place | 99/304 (32.6) | 135/365 (37.0) | 0.233 |
| Patients with DNAR order before first notification | 35/99 (35.4) | 70/135 (51.9) | 0.012 |
| Patients with a DNAR order after first notification | 63/99 (63.6) | 60/135 (44.4) | 0.004 |
| Patients with a DNAR order and unknown time | 3/99 (3.0) | 5/135 (3.7) | 0.779 |
| Fluid bolus | 193/405 (47.7) | 279/524 (53.2) | 0.091 |
| Antibiotics | 192/405 (47.4) | 317/524 (60.5) | <0.001 |
| Bronchodilators | 93/405 (23.0) | 174/524 (33.2) | 0.001 |
| Ventilation in the ICU after RRT review (total hours) | 1554.5 | 752 | 0.256 |
Data expressed as means with 95% CI and number (%) unless not indicated otherwise. DNAR do not attempt resuscitation, RRT rapid response team, EWS early warning score
Outcomes
There were 14 cardiac arrests (6.5/1000 discharges, 3.5% of RRT notifications) in the control period compared with two (0.8/1000 discharges, 0.4% of RRT notifications) in the intervention period (p = 0.002). We observed 320 deaths in 4402 patients (mortality 7.3%). Hospital mortality was 173/2139 patients (8.1%) during the control and 147/2263 (6.5%) during the intervention period (difference 1.59%, 95% CI 0.05–3.13%; p = 0.042). After exclusion of readmissions, the mortality benefit remained significant in the higher acuity (as measured by higher NEWS) respiratory ward (decrease from 110/983 (11.2%) to 79/945 (8.4%); difference 2.83%, 95% CI 0.19–5.48%; p = 0.036), but not in the gastroenterology ward.
Data from the 47 patients admitted to the ICU were extracted from the ICNARC database (1% of 4402 patients) these comprised 26 patients (12/1000 discharges) admitted during the control phase and 21 patients (9/1000 discharges) during the intervention phase (p = 0.158). Mean APACHE II scores on admission to the ICU were significantly lower during the intervention phase (18 ± 8 vs. 26 ± 9, p < 0.007). This was associated with lower predicted mortality (24% vs. 45%, p < 0.006) and a decrease in actual mortality (3/21 (14%) vs. 11/26 (42%), p < 0.04).
In total, there were 268 serious events (268 in 2139 patients, 125 per 1000 patients) in the control and 185 serious events (185 in 2263 patients, 82 per 1000 patients) in the intervention period (p < 0.001) (Table 4).
Table 4.
Serious events in the control and intervention period
| Parameter | Control period (n = 2139) | Intervention period (n = 2263) | p |
|---|---|---|---|
| Number of patients with serious events | 208 (9.72) | 166 (7.34) | 0.005 |
| Total number of serious events | 268 (12.53) | 185 (8.17) | <0.001 |
| Acute myocardial infarction | 4 (0.19) | 0 | 0.056 |
| Pulmonary embolism | 3 (0.14) | 2 (0.09) | 0.679 |
| Acute pulmonary edema | 5 (0.23) | 1 (0.04) | 0.115 |
| Respiratory failure | 19 (0.89) | 10 (0.44) | 0.070 |
| Stroke | 0 | 0 | 1.000 |
| Severe sepsis | 21 (0.98) | 1 (0.04) | <0.001 |
| Acute renal failure | 3 (0.14) | 1 (0.04) | 0.361 |
| Emergency admission to the ICU | 26 (1.22) | 21 (0.93) | 0.355 |
| Cardiopulmonary arrest | 14 (0.66) | 2 (0.09) | 0.002 |
| Death | 173 (8.09) | 147 (6.50) | 0.042 |
Serious events occurred after admission to the study ward and were not the reason for admission. Data expressed as number (%). Denominator: all patients admitted to the study wards (2139 patients in control period, 2263 patients in intervention period). Fisher’s exact test was used instead of the normal approximation test if the number of events in either sample was fewer than five
Binary logistic regression
Reduced mortality was maintained in stepwise binary logistic regression analysis including age, gender and acuity (measured by type of ward) at step 1: there was reduced mortality for patients admitted during the intervention period (OR = 0.79, 95% CI 0.63–0.99; p = 0.043). The same was true for the rate of patients with cardiopulmonary arrest (OR = 0.15, 95% CI 0.03–0.64; p = 0.011) (Additional file 1: Table S1).
Discussion
Key findings
We conducted a before-and-after study of an electronic automated advisory vital signs monitoring and notification system, which relayed abnormal vital signs to an RRT. We found that implementation of such a system led to a significant increase in RRT notifications and more notifications, which triggered interventions such fluid boluses, bronchodilators, and antibiotics. Moreover, implementation was associated with a significant decrease in cardiac arrests, overall mortality in the sicker of the two clinical areas, the severity of illness in those patients admitted to ICU, and decreased ventilation time and mortality in such patients, and more patients received “do not attempt resuscitation” orders.
Relationship to previous studies
Automated assessment of physiological abnormalities has been shown to reduce mortality among patients seen by the RRT [19] but the effect on cardiac arrests has not been reported. However, the intervention reduced the number of abnormal sets of vital signs from four sets to three before a call to the RRT was undertaken, implying earlier activation. Introduction of electronic documentation of vital signs was also associated with a reduction in standardized hospital mortality in two large UK university hospitals [20], but it was unclear in which patient group this reduction occurred and whether other concurrent changes might have accounted for the improvement in outcomes.
There is a lack of studies measuring the impact of new monitoring technology on clinical outcomes, with much of the published work focused around technical feasibility [21] and user acceptance [22]. In contrast, we used a combination of wireless sensors to support care in the more unstable patients, in effect facilitating more frequent recording of vital signs with limited need for extra staffing.
Strengths and limitations
Our study has several strengths. It is the first detailed investigation of the effect of automated notifications on team behavior and clinical outcomes in clinically deteriorating patients. To our best knowledge, this is also the first study of cableless sensors deployed in a clinical trial. The large number of patients, large number of activations, changes in clinical interventions and changes in clinical outcomes documents for the first time that the impact of an automated system across the whole pathway of deterioration in patients was coherent.
Concerns have been raised about the feasibility of implementation of the EWS in patients with respiratory illness [23, 24]. We have shown that by adapting the scoring algorithm, clinically meaningful results can be achieved, even in this group of patients. To our knowledge, this study is also the first that has used a range of scoring algorithms in order to individualize monitoring plans, albeit in a single center.
Our study has some limitations: We did not collect data on admission diagnosis and comorbidity beyond the presence of significant lung disease. It also remains untested whether findings would be different, for example, in a surgical population in whom the number of notifications might be fewer [25, 26] and the patterns of expected physiological derangement likely to be dominated by hypotension.
The fact that RRT responders received automated notification during the intervention period meant that they might have initiated communication more frequently than in the observation period. Resulting phone calls and conversations within parent teams are likely not to have been fully documented in the clinical notes. In the absence of an electronic patient record, we were also unable to find reliable documentation to confirm the timing of interventions. Our study was conducted in a very specific environment and in a UK hospital. Its external validity to other institutions and patient cohorts, therefore, needs to be assessed. Nursing teams were appreciative of the technology used. We are aware that especially with regards to continuous monitoring these finding might not be generalizable [27, 28].
We do not have detailed data to explain the increase in DNAR orders, not only overall, but also before RRT notification. It is possible that the increase is notification might have increased overall awareness of the overall status of all patients and of the limitations of what medical intervention could do for such patients, thereby increasing pro-active decision making on end-of-life care.
Mortality was reduced in the study ward with the sicker patient population. The fact that overall mortality was not affected could be due to the fact that many of the included patients would have suffered from advanced chronic diseases with limited reversibility after a study period of more than two years. Further research is needed in order to define the characteristics of patients who most benefit from the type of intervention described. Finally, the low rates of adverse events limit any clinical inference made from statistical tests related to their incidence during the two study periods.
Implications of study findings
This study implies that deployment of an electronic automated advisory vital signs monitoring and notification system, which relays abnormal vital signs to an RRT, has the ability to significantly increase the number of activations of the RRT. Moreover, it implies that such an increase in activations can lead to decreased overall mortality, cardiac arrests and illness severity in those patients admitted to ICU, resulting in a specific decrease in mortality among such patients. Finally, our study implies that the more frequent activation affects decision-making on end-of-life care toward greater use of DNAR orders.
Future research
The findings of our study will need to be confirmed in other patient groups (i.e. surgical, obstetric and pediatric patients) and in other health care settings to tease out how much our findings are context dependent [29]. The mechanisms that result in improved outcomes also require further research: Human-factor-led design with reduction of cognitive load due to a graphic interface or system design with modular redundancy and increase in the number of staff members to are aware of emerging deteriorations might be candidate mechanisms.
Conclusions
Implementation of an electronic automated advisory vital signs monitoring and notification system appears to increase RRT notifications and RRT interventions associated with decreases in cardiac arrests, overall mortality, illness severity and mortality in those patients admitted to ICU and an increase in pro-active decision-making on end-of-life care. Wider testing of this system in different populations and health care settings now appears justified.
Acknowledgements
The authors would like to thank our tireless nursing and medical teams from Moelwyn and Tryfan ward and our excellent Rapid Response Team for participating in the set-up and conduct of the study. We are indebted to the research nurses Alison Owen, Tracy Savjin, Melissa Van Der Bijl, Karen Jones, Pam Martin-Forbes, Sarah Evans from the NISCHR CRC Research Network for collecting the data and to Lisa Roberts and Kate Hughes for secretarial support.
Funding
Funding of the monitoring equipment and the research nurses was through a grant from Philips Healthcare.
Availability of data and materials
Data and material can be made available on request.
Authors’ contributions
CPS, BD and RB planned the study design, CPS was the principal investigator, BD and CPS undertook the statistical analysis; CPS and RB wrote the manuscript. All three authors made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data, have been involved in drafting the manuscript and revising it critically for important intellectual content and have given final approval of the version to be published. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
Dr CP Subbe and Professor R Bellomo have received consultancy payments from Philips Healthcare for undertaking and evaluating the VITAL II study. Dr B Duller is a consultant for Philips Healthcare. The authors have no other competing interest in relation to this study.
Consent for publication
The photos used in the figures are from actors and form part of Philips Healthcare promotional material.
Ethics approval and consent to participate
Ethics approval was granted by the North Wales Research Ethics Committee (REC reference: 12/WAL/0050). The ethics committee confirmed that no patient consent was required.
Additional file
a Results of the multivariate stepwise binary logistic regression for mortality (backward Wald). b Results of the multivariate stepwise binary logistic regression for cardiac arrest patients (backward Wald). (DOCX 22 kb)
Contributor Information
Christian P. Subbe, Email: csubbe@hotmail.com
Bernd Duller, Email: bernd@perpet.de.
Rinaldo Bellomo, Email: rinaldo.bellomo@austin.org.au.
References
- 1.Kause J, Smith G, Prytherch D, Parr M, Intensive Care Society (UK); Australian and New Zealand Intensive Care Society Clinical Trials Group A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom — the ACADEMIA study. Resuscitation. 2004;62:275–82. doi: 10.1016/j.resuscitation.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Sax FL, Charlson M. Medical patients at high risk for catastrophic deterioration. Crit Care Med. 1987;15:510–5. doi: 10.1097/00003246-198705000-00012. [DOI] [PubMed] [Google Scholar]
- 3.McQuillan P, Pilkington S, Allan A, Taylor B, Short A, Morgan G, et al. Confidential inquiry into quality of care before admission to intensive care. BMJ. 1998;316(7148):1853–8. doi: 10.1136/bmj.316.7148.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schein RMH, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388–92. doi: 10.1378/chest.98.6.1388. [DOI] [PubMed] [Google Scholar]
- 5.Silber JH, Williams SV, Krakauer H, Schwartz JS. Hospital and patient characteristics associated with death after surgery: a study of adverse occurrence and failure to rescue. Med Care. 1992;30(7):615–29. doi: 10.1097/00005650-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Subbe CP, Welch JR. Failure to rescue: using rapid response systems to improve care of the deteriorating patient in hospital. AVMA Med Leg J. 2013;19(1):6–11. [Google Scholar]
- 7.Devita MA, Bellomo R, Hillman K, Kellum J, Rotondi A, Teres D, et al. Findings of the First Consensus Conference on Medical Emergency Teams. Crit Care Med. 2006;34(9):2463–78. doi: 10.1097/01.CCM.0000235743.38172.6E. [DOI] [PubMed] [Google Scholar]
- 8.Winters BD, Weaver SJ, Pfoh ER, Yang T, Pham JC, Dy SM. Rapid-response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:417–25. doi: 10.7326/0003-4819-158-5-201303051-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammer JA, Jones TL, Brown SA. Rapid response teams and failure to rescue. J Nurs Care Qual. 2012;27(4):352–8. doi: 10.1097/NCQ.0b013e31825a8e2f. [DOI] [PubMed] [Google Scholar]
- 10.Hillman K, for the MERIT study investigators Introduction of the medical emergency team (MET) system: A cluster-randomised controlled trial. Lancet. 2005;365(9477):2091–7. doi: 10.1016/S0140-6736(05)66733-5. [DOI] [PubMed] [Google Scholar]
- 11.Morris A, Owen HM, Jones K, Hartin J, Welch J, Subbe CP. Objective patient-related outcomes of rapid-response systems - a pilot study to demonstrate feasibility in two hospitals. Crit Care Resusc. 2013;15(1):33–8. [PubMed] [Google Scholar]
- 12.Pham H, Galyean WJ. Reliability analysis of nuclear fail-safe redundancy. Reliab Eng Syst Saf. 1992;37(2):109–12. doi: 10.1016/0951-8320(92)90003-4. [DOI] [Google Scholar]
- 13.Moehlenbrink C, Wies M, Jipp M. Conference Proceedings - IEEE International Conference on Systems, Man and Cybernetics. 2011. Monitoring principles in aviation and the importance of operator redundancy; pp. 2828–35. [Google Scholar]
- 14.Groves RH, Holcomb BW, Smith ML. Intensive care telemedicine: evaluating a model for proactive remote monitoring and intervention in the critical care setting. Stud Health Technol Inform. 2008;131:131–46. [PubMed] [Google Scholar]
- 15.Thomas EJ, Lucke JF, Wueste LR, Weavind L, Patel B. Association of telemedicine for remote monitoring of intensive care patients with mortality, complications, and length of stay. JAMA. 2009;302(24):2671–8. doi: 10.1001/jama.2009.1902. [DOI] [PubMed] [Google Scholar]
- 16.Jones M. NEWSDIG: The national early warning score development and implementation group. Clin Med. 2012;12(6):501–3. doi: 10.7861/clinmedicine.12-6-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eccles SR, Subbe C, Hancock D, Thomson N. CREWS: Improving specificity whilst maintaining sensitivity of the National Early Warning Score in patients with chronic hypoxaemia. Resuscitation. 2014;85(1):109–11. doi: 10.1016/j.resuscitation.2013.08.277. [DOI] [PubMed] [Google Scholar]
- 18.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Bellomo R, Ackerman M, Bailey M, Beale R, Clancy G, Danesh V, et al. A controlled trial of electronic automated advisory vital signs monitoring in general hospital wards. Crit Care Med. 2012;40(8):2349–61. doi: 10.1097/CCM.0b013e318255d9a0. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt PE, Meredith P, Prytherch DR, Watson D, Watson V, Killen RM, et al. Impact of introducing an electronic physiological surveillance system on hospital mortality. Qual Saf Health Care. 2015;24(1):10–20. doi: 10.1136/bmjqs-2014-003073. [DOI] [PubMed] [Google Scholar]
- 21.Aminian M. A hospital healthcare monitoring system using wireless sensor networks. J Heal Med Informatics. 2013;04(02):4–9. [Google Scholar]
- 22.Kummer TF, Schäfer K, Todorova N. Acceptance of hospital nurses toward sensor-based medication systems: a questionnaire survey. Int J Nurs Stud. 2013;50(4):508–17. doi: 10.1016/j.ijnurstu.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Kane B, Decalmer S, Murphy P, Turkington P, O’Driscoll BR. The proposed national early warning system (NEWS) could be hazardous for patients who are at risk of hypercapnic respiratory failure. Thorax. 2012;67:A16–7. doi: 10.1136/thoraxjnl-2012-202678.035. [DOI] [Google Scholar]
- 24.O’Driscoll BR, Murphy PTP. Acute monitoring of patients with chronic respiratory disease during hospital admission. Clin Med. 2012;12(1):79–81. doi: 10.7861/clinmedicine.12-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmes FM, Schoonhoven L, Mintjes J, Fikkers BG, van der Hoeven JG. Incidence of cardiac arrests and unexpected deaths in surgical patients before and after implementation of a rapid response system. Ann Intensive Care. 2012;2(1):20. doi: 10.1186/2110-5820-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellomo R, Goldsmith D, Uchino S, Buckmaster J, Hart G, Opdam H, et al. Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Care Med. 2004;32(4):916–21. doi: 10.1097/01.CCM.0000119428.02968.9E. [DOI] [PubMed] [Google Scholar]
- 27.Prgomet M, Cardona-Morrell M, Nicholson M, Lake R, Long J, Westbrook J, Braithwaite J, Hillman K. Vital signs monitoring on general wards: clinical staff perceptions of current practices and the planned introduction of continuous monitoring technology. Int J Qual Health Care. 2016;28(4):515–21. doi: 10.1093/intqhc/mzw062. [DOI] [PubMed] [Google Scholar]
- 28.Cardona-Morrell M, Prgomet M, Turner RM, Nicholson M, Hillman K. Effectiveness of continuous or intermittent vital signs monitoring in preventing adverse events on general wards: a systematic review and meta-analysis. Int J Clin Pract. 2016;70(10):806–24. doi: 10.1111/ijcp.12846. [DOI] [PubMed] [Google Scholar]
- 29.Dixon-Woods M, Leslie M, Tarrant C, Bion J. Explaining matching Michigan: an ethnographic study of a patient safety program. Implement Sci. 2013;8(1):70. doi: 10.1186/1748-5908-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and material can be made available on request.


