Abstract
Background:
The celiac axis, superior mesenteric artery (SMA), and hepatic artery are the most important branches of abdominal aorta due to their vascularization field. The aim of our study was to evaluate the prevalence of different anatomical variation of celiac axis, SMA, hepatic artery, and its branches with multidetector computed tomography (MDCT) angiography of upper abdomen arteries.
Materials and Methods:
MDCT of 607 kidney donor and traumatic patients that referred to MDCT unit at Al Zahra Hospital in Isfahan from 2012 to 2015 were retrospectively evaluated. We excluded patients with history of abdominal vascular surgery and hepatic or pancreatic surgery. Computed tomography images of the patient were obtained with 64-row MDCT scanner and anatomical variations were analyzed.
Results:
Three hundred and eighty-eight (63.9%) of the 607 patients had classic arterial anatomy and 219 (36.1%) patients had variant types. The most common type of variation was the origin of the right hepatic artery (RHA) from SMA (9.6%), and the next common variation was the origin of the left hepatic artery (LHA) from the left gastric artery (6.9%). Variations in the origin of the common hepatic artery (CHA) were seen in 16 (2.6%) patients. Buhler arc was identified in two patients. The RHA originated from the celiac axis in 11 (1.8%) patients and from the aorta in 8 (1.3%) patients. Trifurcation of CHA into gastroduodenal artery, RHA, and LHA was detected in 11 (1.8%) patients.
Conclusion:
The results of the present study showed that anatomical variation occurs in a high percentage of patients. Detection of these variations can guide surgical and radiological interventional planning.
Keywords: Major branches of abdominal aorta, multidetector computed tomography angiography, normal variation, prevalence
INTRODUCTION
The celiac axis and superior mesenteric artery (SMA) are the most important branches of the abdominal aorta. The importance of the celiac axis, SMA, and common hepatic artery (CHA) are regarded to their vascularization field. In the recent decades, with the advent of interventional and surgical options for patients with partial hepatectomy for liver transplantation, end-stage hepatic malignancy, determining tumor respectability in patient with pancreatic and hepatobiliary malignancy, surgeons, and interventional radiologist are dependent on accurate imaging before procedures.[1,2,3,4] In variant patterns, vessels do not arise from their usual source and present as accessory or replaced vessels. Accessory vessel is a branch addition to the normal artery supply and replaced is a vessel that representing the primary blood supply to the organs.[4,5] The first description of celiac axis variation refers to the research of Haller in 1756. In 1955, Michel published his classification scheme for describing the variation of hepatic arterial blood supply by dissecting 200 cadavers.[5] In the year 1969, Vandamme et al. search and describe patterns of the hepatic artery on 156 cadavers.[6] Then in 1971, Suzuki et al. evaluated angiography of 371 patients and highlighted the importance of anatomical variation of the hepatic artery in surgical procedures.[7]
The purpose of this study was to evaluate the prevalence of celiac axis, SMA, CHA, right hepatic artery (RHA), left hepatic artery (LHA), and gastroduodenal artery (GDA) in our country and compare the prevalence with previous studies.
MATERIALS AND METHODS
Patients
The study was performed on 607 patients who underwent multidetector computed tomography (MDCT) because of trauma or kidney transplantation from 2012 to 2015 at Al Zahra Hospital in Esfahan. In this retrospective study, population included 343 men and 262 women who were 13–90-year-old (mean, 51 ± 15 years). We included patients without a history of major abdominal surgery and patient without a history of abdominal pain. We supposed that patient with abdominal pain may have more prevalence of anatomical variations, and this results in bias in the sampling process.
Computed tomography examination protocol
All computed tomography (CT) examinations were performed using a 64-slice MSCT scanner (Medical Health Care GE Work Station RDW 4.3, GE, USA). Technical features of MSCT were as following: 64 mm × 1 mm collimation, minimum slice thickness of 0.625, gantry rotation time of 320 ms, kV of 120, and mAs of 320. A bolus of 80–100 ml of nonionic iodinated contrast agent (ultravist-300) followed by 50–60 ml of normal saline was injected by means of an 18-gauge intravenous catheter through an antecubital vein or a vein in the forearm at a flow rate of 4–6 ml/s.[8] A dose of 15 ml of contrast was used during the bolus timing scan calculated (by the apparatus software) at the level of the descending aorta.[9]
Image interpretation
MDCT images were processed using a various technique such as multiplanar reformation, maximum intensity projection, and volume rendering on a commercially available workstation.[8] The CT images were analyzed independently by two experienced interventional radiologists. Kappa statistics test was used to calculate interobserver reliability between two radiologists.
RESULTS
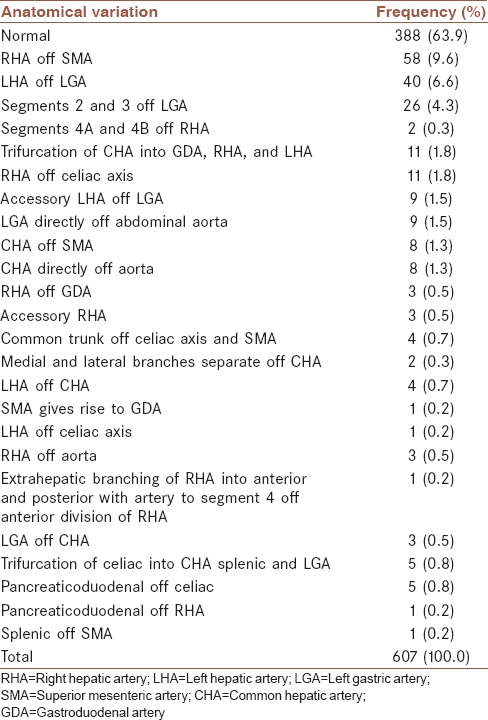
A total of 607 patients because of trauma or kidney transplantation underwent MDCT. Three hundred and forty-three of all patients were men, and 264 were women. The age range was 13–90-year-old (mean, 51 ± 15 years). Three hundred and eighty-eight (63.9%) of the 607 patients had classic arterial anatomy [Figure 1], and 219 (36.1%) patients had variant types. Thirty-four (5.6%) had two arterial variants, and five of patients had more than two arterial variations. Distribution of variation between genders was as following: 94 in women and 125 in men [Table 1]. The most frequent variation was a replaced RHA originating from the SMA which was seen in 58 (9.6%) of the patients [Figure 2]. The next variation according to frequency was a replaced LHA originating from the left gastric artery which was seen in forty (6.6%) patients. Variations in the origin of the CHA were seen in 16 patients (2.6%) (origin from SMA in eight and from the aorta in eight patients). The RHA originated from the celiac axis in 11 (1.8%) patients and from the aorta in 3 (0.5%) patients. Accessory RHA was detected in three (0.5%) patients, and accessory LHA were identified in nine (1.5%) patients. Trifurcation of CHA was seen in 11 (1.8%) of the patients. The common trunk of the celiac trunk and SMA is a rare variation and in this research, it was found in four (0.7) patients [Figure 3]. The arc of Buhler was detected in two patients [Figure 4]. We used Chi-square test to compared the prevalence of normal pattern and other patterns between men and women, and we find that gender has no influence on the pattern of variation distribution (P = 0.864). The agreement between two radiologists was good to excellent (kappa range: 0.75–0.85) the frequency of all different types of arterial variation are reported in Table 2.
Figure 1.
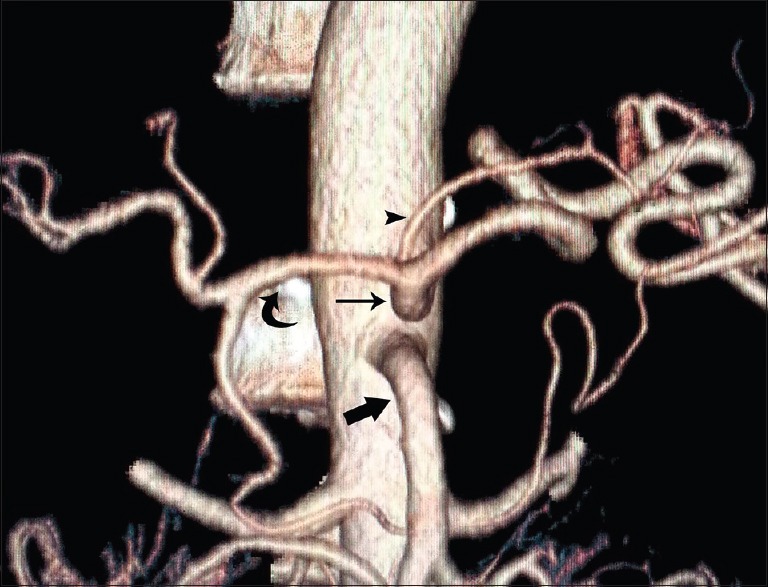
Volume-rendered image showing normal anatomy of celiac trunk ([thin arrow], superior mesenteric artery [thick arrow], left gastric artery [arrowhead], common hepatic artery [curved arrow])
Table 1.
Frequency of different type of variation in men and women (percentage within gender)
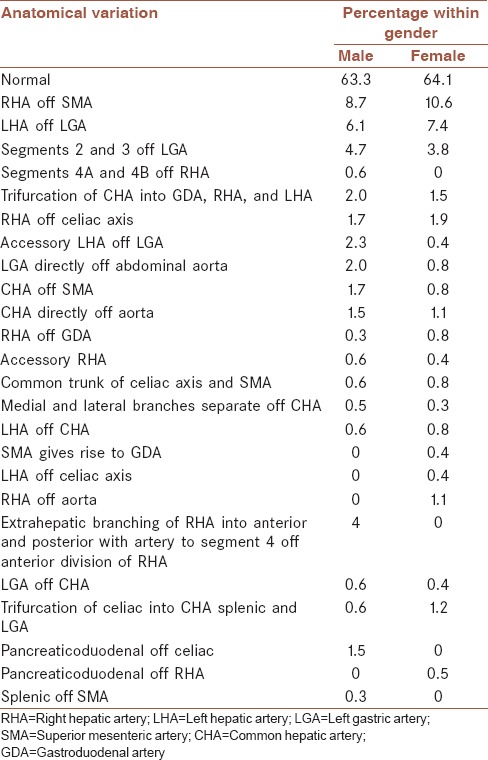
Figure 2.
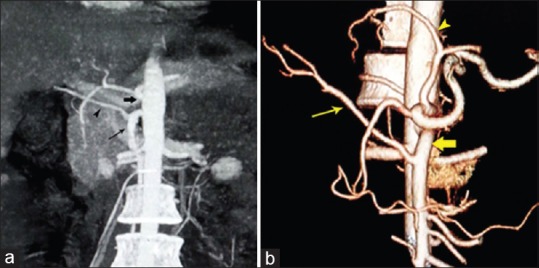
(a) Coronal maximum intensity projection image showing replaced right hepatic artery (arrowhead) from a superior mesenteric artery (thin arrow). Thick arrow points to celiac trunk. (b) Volume-rendered image showing the right hepatic artery (thin arrow) is originated from superior mesenteric artery (fat arrow), also notice to the left hepatic artery off left gastric artery (arrowhead)
Figure 3.
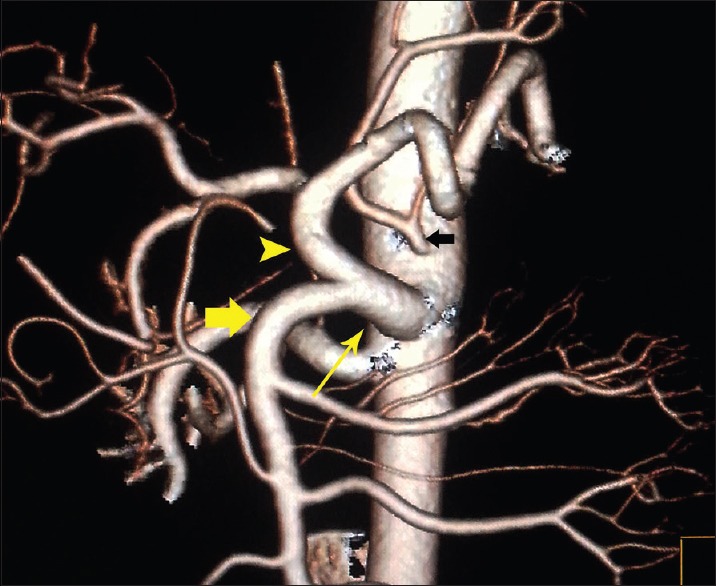
Volume-rendered image showing common trunk (thin yellow arrow) of the celiac artery (arrowhead) and the superior mesenteric artery (fat yellow arrow), also notice to the left gastric artery directly off aorta (black arrow)
Figure 4.
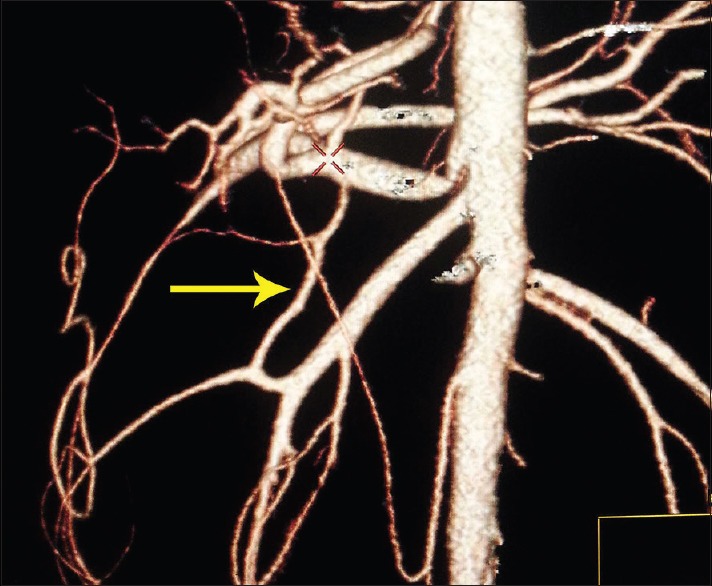
Volume-rendered image showing the Buhler arc (arrow) is an anastomosis between the celiac trunk and the superior mesenteric artery
Table 2.
Frequency of different types of arterial variation
DISCUSSION
During human embryogenesis, four roots of omphalomesenteric artery arise from abdominal aorta and are interconnected by a ventral longitudinal anastomosis. Two central roots of these four aortic branches disappear during embryogenesis and the first and fourth root connects to each other with longitudinal anastomosis. The splenic, left gastric, and CHA will come from this longitudinal anastomosis and the SMA from the fourth roots of omphalomesenteric artery. Remain or regression of any of these arteries leads to the development of vascular variations of the celiac trunk or SMA. In the CTA, the relationship between arteries and adjacent organs is displayed so that the course of arterial variation anatomy is clearly determined.[7] Preoperative knowledge of variant arterial anatomy may reduce extensive exploration during surgery and consequently decrease the risk of vascular damage.[8] In the classic anatomy, celiac trunk divided into three branches; left gastric artery and then common hepatic and splenic artery. The CHA bifurcate into GDA and the proper hepatic artery.[3,4,6] PHA divided into RHA and LHA. In this study, classical arterial anatomy was seen in 63.6% of the patients who underwent CTA, while previous studies showed different results, i.e., 89% by Michel,[5] 51% by Winston et al.,[8] 66% by De Cecco et al.,[9] 89% by Song et al.,[10] and 91% by Sureka et al.[11] The most common frequent variant in this study was a replaced RHA originating from SMA, seen in 9.6% of patients. It was found in 15% and 9.2% of patients in a study of Winston and Cecco, respectively. In patients with pancreatic head or pancreatic uncinated process cancer, the involvement of replaced RHA may cancel the plan of surgery. Hence, identification of a replaced RHA is important in these patients. If replaced RHA is not involved, special care is need to reduce the risk of damage to this artery during dissecting. The second most frequent variant was a replaced LHA originate off the left gastric artery, identified in 6.9% of the patients. The prevalence of this variation in Cecco et al. study was 5.2%, and it was seen in 8% of patients in the study of both Winston and Michel. Before the left hepatectomy, this variant should be identified and ligated.[3,12,13] An unusual course of RHA that originates from the celiac axis or aorta may result in iatrogenic injury to this vessel if the location of the vessel is unknown for the surgeon. Accessory left and RHA were seen in 3.3% of patients. Accessory arteries provide an additional source of blood supply to the hepatic lobes. These accessory arteries needed to be occluded separately when surgeons want to control inflow to hepatic lobes. On the other hands, variant arterial anatomy has an important role in chemotherapy as an adjuvant to resection in controlling hepatic disease.[13,14] The catheter is surgically placed in the GDA. The hepatic variant artery can result in a nonuniform perfusion of the chemotherapeutic agent through the liver so a replaced or accessory artery should be ligated during chemotherapy.[13,14,15,16,17] The common trunk of the celiac artery and the SMA is a rare variation and according to the earlier studies, it has been found in <2% of patients.[18,19] In our research, it was found in five patients. The arc of Buhler that is a persistence of embryonal ventral anastomotic shunt joining SMA and celiac trunk[20] was seen in two patients. Buhler's arc is present in <4% in Dubel et al. study (2007),[21] and 1.7% in Ferrari et al. study.(2007).[22] According to our finding, the prevalence of variation was significant, so we suggest to apply reconstruction method for evaluation of variation at least in patients who are candidate for mentioned surgical or interventional procedures.
CONCLUSION
The results of the present study showed that anatomical variation occurs in a high percentage of patients. Knowledge of variations in the hepatic arteries and the SMA prepares more information for revascularization in surgical- and imaging-guided interventional planning, selection of appropriate treatment options, and help in reducing the chance of surgical iatrogenic injuries.
Financial support and sponsorship
The authors would like to appreciate the financial support of Isfahan University of Medical Sciences, Isfahan (Research Project Number: 395004).
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION
MF contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work, MM contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work AH contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work FM contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work MMBM contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
REFERENCES
- 1.Catalano OA, Singh AH, Uppot RN, Hahn PF, Ferrone CR, Sahani DV. Vascular and biliary variants in the liver: Implications for liver surgery. Radiographics. 2008;28:359–78. doi: 10.1148/rg.282075099. [DOI] [PubMed] [Google Scholar]
- 2.Ognjanović N, Jeremić D, Živanović-Mačužić I, Sazdanović M, Sazdanović P, Tanasković I, et al. MDCT angiography of anatomical variations of the celiac trunk and superior mesenteric artery. Arch Biol Sci. 2014;66:233–40. [Google Scholar]
- 3.Blumgart L, Fong Y. Surgery of the Liver and Biliary Tract. New York: Saunders; 2000. pp. 10–4. [Google Scholar]
- 4.Buljoud MS, Kim DY, Yoshida A, Arenas J, Jerius J, Malinzak L, Raoufi M, Brown KA, Moonka DK. Impact of aberrant arterial anatomy and location of anastomosis on technical outcomes after liver transplantation. J Gastrointest Surg. 2005;9:672–678. doi: 10.1016/j.gassur.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Michel NA. Blood supply and anatomy of the upper abdominal organs, with a descriptive atlas. Philadelphia, Pa: Lippincott; 1955. Observatons on the blood supply of the liver and gallbladder (200 dissections) pp. 64–9. [Google Scholar]
- 6.Vandamme JP, Bonte J, Van der Scheueren G. A reevaluation of hepatic and cystic arteries: The importance of aberrant hepatic branches. ActaAnat. 1969;73:192–209. doi: 10.1159/000143296. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T, Nakayasu A, Kawabe K, Takeda H, Honjo I. Surgical significance of anatomic variations of the hepatic artery. Am J Surg. 1971;122:505–12. doi: 10.1016/0002-9610(71)90476-4. [DOI] [PubMed] [Google Scholar]
- 8.Winston CB, Lee NA, Jarnagin WR, Teitcher J, DeMatteo RP, Fong Y, et al. CT angiography for delineation of celiac and superior mesenteric artery variants in patients undergoing hepatobiliary and pancreatic surgery. AJR Am J Roentgenol. 2007;189:W13–9. doi: 10.2214/AJR.04.1374. [DOI] [PubMed] [Google Scholar]
- 9.De Cecco CN, Ferrari R, Rengo M, Paolantonio P, Vecchietti F, Laghi A. Anatomic variations of the hepatic arteries in 250 patients studied with 64-row CT angiography. Eur Radiol. 2009;19:2765–70. doi: 10.1007/s00330-009-1458-7. [DOI] [PubMed] [Google Scholar]
- 10.Song SY, Chung JW, Yin YH, Jae HJ, Kim HC, Jeon UB, et al. Celiac axis and common hepatic artery variations in 5002 patients: Systematic analysis with spiral CT and DSA. Radiology. 2010;255:278–88. doi: 10.1148/radiol.09090389. [DOI] [PubMed] [Google Scholar]
- 11.Sureka B, Mittal MK, Mittal A, Sinha M, Bhambri NK, Thukral BB. Variations of celiac axis, common hepatic artery and its branches in 600 patients. Indian J Radiol Imaging. 2013;23:223–33. doi: 10.4103/0971-3026.120273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas PG, Baer HU, Matthews JB, Gertsch P, Blumgart LH. Post-operative hepatic necrosis due to reduction in hepatic arterial blood flow during surgery for chronic biliary obstruction. Dig Surg. 1990;7:31–5. [Google Scholar]
- 13.Kapoor V, Brancatelli G, Federle MP, Katyal S, Marsh JW, Geller DA. Multidetector CT arteriography with volumetric three-dimensional rendering to evaluate patients with metastatic colorectal disease for placement of a floxuridine infusion pump. AJR Am J Roentgenol. 2003;181:455–63. doi: 10.2214/ajr.181.2.1810455. [DOI] [PubMed] [Google Scholar]
- 14.Allen PJ, Stojadinovic A, Ben-Porat L, Gonen M, Kooby D, Blumgart L, et al. The management of variant arterial anatomy during hepatic arterial infusion pump placement. Ann Surg Oncol. 2002;9:875–80. doi: 10.1007/BF02557524. [DOI] [PubMed] [Google Scholar]
- 15.Hazirolan T, Metin Y, Karaosmanoglu AD, Canyigit M, Turkbey B, Oguz BS, et al. Mesenteric arterial variations detected at MDCT angiography of abdominal aorta. AJR Am J Roentgenol. 2009;192:1097–102. doi: 10.2214/AJR.08.1532. [DOI] [PubMed] [Google Scholar]
- 16.Yi SQ, Terayama H, Naito M, Hayashi S, Moriyama H, Tsuchida A, et al. A common celiacomesenteric trunk, and a brief review of the literature. Ann Anat. 2007;189:482–8. doi: 10.1016/j.aanat.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz MT, Tezer M, Cicecibasi AE, Aydin AD, Salbacak A. A case report of coeliacomesenteric trunk. Biomed Res. 2013;24:150–2. [Google Scholar]
- 18.Tandler J. About the varieties of the celiac artery and Their Development. Anat Hefte. 1904;25:473–500. [Google Scholar]
- 19.Nebesar RA, Kornblith PL, Pollard JJ, Michels NA. Celiac and Superior Mesenteric Arteries: A Correlation of Angiograms with Dissections. Boston: Little, Brown; 1969. [Google Scholar]
- 20.Gordon DH, Martin EC, Kim YH, Kutcher R. Accessory blood supply to the liver from the dorsal pancreatic artery: An unusual anatomic variant. Cardiovasc Radiol. 1978;1:199–201. doi: 10.1007/BF02552033. [DOI] [PubMed] [Google Scholar]
- 21.Dubel GJ, Ahn SH, Saeed MA. Interventional management of arc of buhler aneurysm. Semin Intervent Radiol. 2007;24:76–81. doi: 10.1055/s-2007-971193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrari R, De Cecco CN, Iafrate F, Paolantonio P, Rengo M, Laghi A. Anatomical variations of the coeliac trunk and the mesenteric arteries evaluated with 64-row CT angiography. Radiol Med. 2007;112:988–98. doi: 10.1007/s11547-007-0200-2. [DOI] [PubMed] [Google Scholar]


