Abstract
Background:
Echinococcosis is a parasitic disease with worldwide distribution which is caused by the tapeworms Echinococcus granulosus. Diagnosis of the disease relies on imaging techniques, but the techniques are not able to differentiate the cyst from benign or malignant tumors; hence, appropriate serologic methods are required for the differential diagnosis of the infection.
Materials and Methods:
In this investigation, different sheep hydatid cyst antigens probed with thirty sera of patients with hydatid cyst and also thirty human normal sera using Western immunoblotting technique. Considering results of surgery as gold standard, sensitivity and specificity of Western blotting was estimated.
Results:
Sera of 29, 26, and 16 patients with hydatid cyst reacted with specific bands of hydatid cyst fluid (HCF), protoscolex crude antigen, and cyst wall crude antigen, respectively. However, none of the normal human sera reacted with those specific bands.
Conclusion:
A 20 kDa band of sheep HCF is an appropriate antigen for serodiagnosis of hydatid cyst infection.
Keywords: Antigen, cyst wall, hydatid cyst fluid, protoscolex, Western immunoblotting
INTRODUCTION
Echinococcosis is a parasitic disease with worldwide distribution[1,2] affecting about 100 different countries.[3] Adult worm of Echinococcus granulosus lives in small intestine of carnivores, and infected eggs are excreted in their feces.[4] Following ingestion of eggs by the intermediate host (herbivores and humans), larval stage of the parasite, hydatid cyst, develops in internal viscera.[5] Clinical signs and symptoms of the disease are not specific and depend on the location of the cyst.[6] Diagnosis of the disease relies on paraclinical methods. Imaging techniques are able to identify the cyst in the body, but they are not able to differentiate the cyst from benign or malignant tumors.[7] Moreover, the infection may be associated with other infections such as fungal infection.[8] In these cases, also, differential diagnosis is important. Different immunological methods including Casoni test, complement fixation, indirect hemagglutination, latex agglutination, enzyme-linked immunosorbent assay (ELISA), and immunofluorescent methods have been used for this differential and specific diagnosis.[9] In previously published works, different sensitivity and specificity has been reported for the ELISA test. This variability may be due to the type of antigen and geographic region of the parasite.[10,11,12]
Western immunoblotting which provides fractionation of the parasite antigens may provide higher sensitivity and specificity. A few works have been performed using Western immunoblotting in diagnosis of human hydatid cyst. Sensitivity of 72%–97% and specificity of 96%–100% have been reported for this test in diagnosis of hydatidosis.[13,14,15] Considering this fact that certain genotype of the parasite exists in certain area of the world,[16] in this work, diagnosis of hydatid cyst with Western immunoblotting technique using antigens from local sheep hydatid cyst has been investigated.
MATERIALS AND METHODS
In this cross-sectional study, E. granulosus hydatid cysts were collected from sheep from a slaughter house in Isfahan, Iran. Hydatid fluid was aspirated from sheep hydatid cyst, and with observation of the protoscolices, the tubes containing fluids were centrifuged at 2000 ×g, for 2 min, and the supernatant was stored at −20°C as hydatid fluid antigen. The sediment which was compact of many protoscolices washed with isotonic saline, sonicated and then kept at −20°C as crude protoscolex antigen. Laminated and germinal layers of the cyst were removed, homogenized, sonicated, and then kept at −20°C as cyst wall antigen.[17]
Polyacrylamide gel electrophoresis with sodium dodecyl sulfate and Western immunoblotting were performed using BIO-RAD apparatus according to the methods of Laemmli[18] and Towbin et al.,[19] respectively, with some modification as we published before.[20,21] Briefly, antigens run on 15% acrylamide gel under reducing conditions and transferred to nitrocellulose papers. The papers were then probed with different sera at appropriate concentrations. Following washing with the buffer, the papers were probed with a secondary antibody against human IgG. Finally, appropriate substrate was added to develop reaction of sera with the bands of antigens. According to the previous investigation,[14,22] sample size of thirty was estimated for this work, so spare sera of thirty patients with hydatid cyst were collected when they were subjected to surgery for removing their cysts. For control group, sera of thirty blood donors whose fecal examinations were negative for parasitic infection were collected from blood bank. Sensitivity, specificity, positive predictive value, and negative predictive value of Western immunoblotting in diagnosis of human hydatid cyst were estimated considering results of surgery as gold standard. Kappa coefficient index was calculated for all three antigens.
RESULTS
Hydatid cyst fluid (HCF) antigen on Western immunoblotting was probed with thirty individual sera of patients with hydatid cyst and also thirty normal sera. Of 30 sera, 29 (96.6%) reacted with antigen of HCF with molecular weight of about 20 kDa. However, none of the normal sera reacted with that band [Figure 1]. When protoscolex crud antigen was probed with sera of thirty patients with hydatid cyst or thirty normal sera, 26 (86.6%) of 30 hydatid cyst patients sera reacted with a band about 7 kDa in Western immunoblotting. Again, none of the normal sera reacted with this band [Figure 2]. Using cyst wall crud antigen, 16 (53.3) of 30 sera of patients with hydatid cyst reacted with a band about 7 kDa in Western immunoblotting. However, none of the normal sera reacted with this band [Figure 3].
Figure 1.
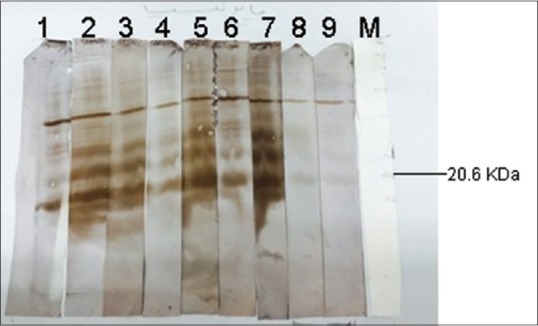
Western immunoblotting of hydatid cyst fluid probed with the sera of patients with hydatid cyst (1–7) or normal human sera (8 and 9). M stands for molecular weight marker
Figure 2.
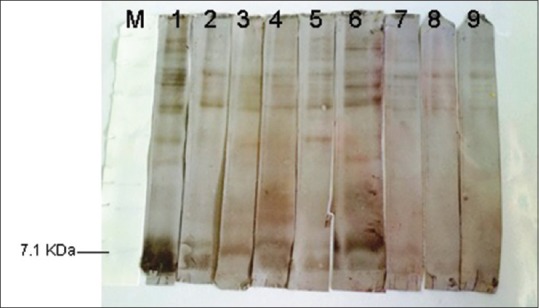
Western immunoblotting of protoscolex probed with the sera of patients with hydatid cyst (1–7) or normal human sera (8 and 9). M stands for molecular weight marker
Figure 3.
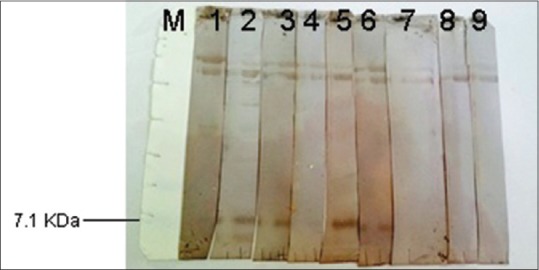
Western immunoblotting of cyst wall probed with the sera of patients with hydatid cyst (1–7) or normal human sera (8 and 9). M stands for molecular weight marker
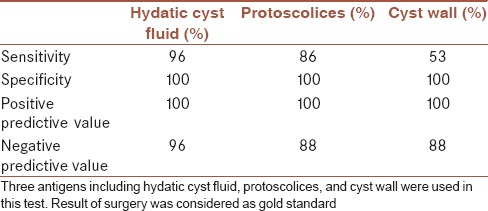
Sensitivity, specificity, positive predictive value, and negative predictive value of Western blotting for diagnosis of hydatid cyst infection were estimated considering the result of surgery as gold standard. With HCF as antigen, sensitivity of 96% and specificity of 100% was estimated. When protoscolex antigen was used, sensitivity of 86% and specificity of 100% was achieved. With cyst wall antigen, sensitivity of 53% and specificity of 100% was estimated for this test. Results of Western blotting test for thirty serum samples of hydatid cyst patients and thirty serum samples of healthy controls with different antigens are summarized in Table 1. In addition, sensitivity, specificity, positive predictive value, and negative predictive value of Western immunoblotting for diagnosis of hydatid cyst are summarized in Table 2. Kappa coefficient index for HCF, protoscolex, and cyst wall antigens was 96.6%, 86.4%, and 53.4%, respectively.
Table 1.
Results of Western immunoblotting of hydatid cyst antigens (hydatid cyst fluid, protoscolex, and cyst wall) probed with thirty sera of patients with hydatid cyst and thirty sera of healthy controls
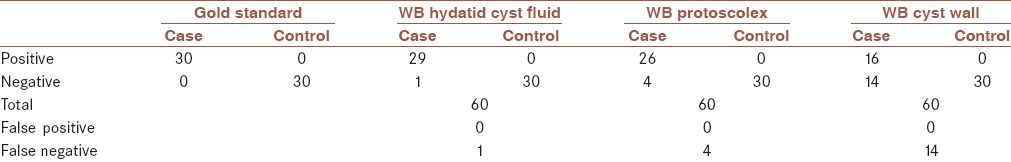
Table 2.
Sensitivity, specificity, positive predictive value, and negative predictive value of Western immunoblotting in diagnosis of human hydatidosis
DISCUSSION
In this investigation, 96.6% of sera of patients with hydatid cyst reacted with 20 kDa band of cyst fluid and 53.3% of sera also reacted with a 7 kDa band of cyst wall. Moreover, 86.6% sera of patients with hydatid cyst reacted with a 7 kDa of protoscolex crud antigen. However, none of the sera in control group reacted with those three bands.
Diagnosis of hydatid cyst disease is primarily based on imaging methods. However, those techniques are not able to differentiate hydatid cyst from other disorders such as benign or malignant tumors. Immunological methods would be very beneficial for this differential diagnosis.[7] Hence, different immunological techniques such as ELISA have been developed for this differential diagnosis. In ELISA test, HCF antigens including antigen 5 (Ag5) and AgB and also crude liquid antigen have been used for immunological diagnosis of hydatid cyst. Using crude antigen, sensitivity of 64%–100% and specificity of 41%–100% was achieved.[23,24,25] With AgB, sensitivity of 30%–92% and specificity of 77%–97% was detected.[25,26,27] With Arc5 as antigen, sensitivity of 70%–100% and specificity of 70%–100% was reported.[28]
In Western immunoblotting, different antigens including HCF,[24,29] AgB,[30] and E. granulosus 19 kDa[31] have been used for diagnosis of hydatid cyst. Sensitivity of 70%–100% and specificity 51%–100% was reported for this test.[24,29,30,31] Ortona et al. used Western blotting for immunodiagnosis of hydatid cyst. They reported a sensitivity of 72% and specificity of 100% for this test.[15] In another work, Jiang et al. reported a sensitivity of 91% and specificity of 92% for this test.[14] Finally, Doiz et al. used sheep hydatid fluid antigen for diagnosis of human hydatid cyst. They showed that Western blotting was able to detect 100% of cases of hydatidosis.[22]
In our investigation, we achieved sensitivity of 96% and specificity of 100% for HCF, 86% and 100% for protoscolex, and 53% and 100% for cyst wall antigen. Result of our study is in agreement with results of other works for Western blotting technique.
The main problem with the results of the different tests in diagnosis of hydatidosis is existence of a wide range of variation for reported sensitivity and specificity.[23,24,25,26,27,28] This variation may be related to the sample size of the study or batch of the antigen which has been used in the given tests.
In our work, when HCF used as antigen, sera of patients with hydatid cyst reacted with different bands. However, reaction against 20 kDa band was more consistent. In this regard, Doiz et al. reported that sera of patients with hydatid cyst reacted with bands with molecular weight of 12, 14, 16, 20, 24, 34, and 39 kDa of HCF.[22]
In our study, both sera of patients with hydatid cyst and normal sera reacted with different bands of cyst wall. In this regard, it has been shown that bands with molecular weight of about 55, 25, and 29 kDa of laminated layer reacted with anti-sheep IgG.[32]
CONCLUSION
Results of this investigation revealed that 96.6% of sera of patients with hydatid cyst reacted with a 20 kDa band of HCF. However, none of the thirty normal human sera reacted with this band. Considering results of surgery as gold standard, sensitivity of 96% and specificity of 100% was achieved for Western blotting. Hence, the 20 kDa band of HCF might be a good candidate for diagnosis of human hydatid cyst and more work in this regard is recommended.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION
MH: Performance of lab works
MN: Providing human samples and contribution in designing the research project
BS: Providing human samples and contribution in writing the manuscript
ZG: Contribution in designing the research project and writing the manuscript
SMS: Contribution in lab works and writing the manuscript
HY: Contribution in lab works and writing the manuscript
HYD: Designing research project, supervision of lab work, and writing the manuscript.
Acknowledgments
This work was supported by the grant from the Isfahan University of Medical Sciences (Research project number: 394253).
REFERENCES
- 1.Sarmast AH, Showkat HI, Shah NF, Mujtaba B, Malik AA, Malik NK, et al. Hydatids everywhere: A 15-year experience of unusual locations of the disease in an endemic area. J Res Med Sci. 2016;21:25. doi: 10.4103/1735-1995.179894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev. 2003;16:18–36. doi: 10.1128/CMR.16.1.18-36.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckert J, Gemmell MA, Meslin FX, Pawłowski ZS. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. 2001:100–118. [Google Scholar]
- 4.Markell EK, Voge M. Vol. 40. Saunders Publisher; 1965. Medical Parasitology. Academic Medicine; p. 719. [Google Scholar]
- 5.Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–35. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmona C, Perdomo R, Carbo A, Alvarez C, Monti J, Grauert R, et al. Risk factors associated with human cystic echinococcosis in Florida, Uruguay: Results of a mass screening study using ultrasound and serology. Am J Trop Med Hyg. 1998;58:599–605. doi: 10.4269/ajtmh.1998.58.599. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, McManus DP. Recent advances in the immunology and diagnosis of echinococcosis. FEMS Immunol Med Microbiol. 2006;47:24–41. doi: 10.1111/j.1574-695X.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- 8.Yazgan S, Gursoy S, Usluer O, Ucvet A. Hydatid cyst with intracavitary fungal ball: Does it require lung resection? J Res Med Sci. 2015;20:204–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Nasrieh MA, Abdel-Hafez SK. Echinococcus granulosus in Jordan: Assessment of various antigenic preparations for use in the serodiagnosis of surgically confirmed cases using enzyme immuno assays and the indirect haemagglutination test. Diagn Microbiol Infect Dis. 2004;48:117–23. doi: 10.1016/j.diagmicrobio.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Iacona A, Pini C, Vicari G. Enzyme-linked immunosorbent assay (ELISA) in the serodiagnosis of hydatid disease. Am J Trop Med Hyg. 1980;29:95–102. doi: 10.4269/ajtmh.1980.29.95. [DOI] [PubMed] [Google Scholar]
- 11.Craig PS, Rogan MT, Allan JC, Gillespie S, Hawkey P. New York, USA: Oxford University Press Inc; 1995. Hydatidosis and cysticercosis-larval cestodes. Medical Parasitology: A Practical Approach; pp. 209–37. [Google Scholar]
- 12.Maddison SE, Slemenda SB, Schantz PM, Fried JA, Wilson M, Tsang VC. A specific diagnostic antigen of Echinococcus granulosus with an apparent molecular weight of 8 kDA. Am J Trop Med Hyg. 1989;40:377–83. doi: 10.4269/ajtmh.1989.40.377. [DOI] [PubMed] [Google Scholar]
- 13.Ito A, Ma L, Schantz PM, Gottstein B, Liu YH, Chai JJ, et al. Differential serodiagnosis for cystic and alveolar echinococcosis using fractions of Echinococcus granulosus cyst fluid (antigen B) and E. multilocularis protoscolex (EM18) Am J Trop Med Hyg. 1999;60:188–92. doi: 10.4269/ajtmh.1999.60.188. [DOI] [PubMed] [Google Scholar]
- 14.Jiang L, Wen H, Ito A. Immunodiagnostic differentiation of alveolar and cystic echinococcosis using ELISA test with 18-kDa antigen extracted from Echinococcus protoscoleces. Trans R Soc Trop Med Hyg. 2001;95:285–8. doi: 10.1016/s0035-9203(01)90235-4. [DOI] [PubMed] [Google Scholar]
- 15.Ortona E, Riganò R, Margutti P, Notargiacomo S, Ioppolo S, Vaccari S, et al. Native and recombinant antigens in the immunodiagnosis of human cystic echinococcosis. Parasite Immunol. 2000;22:553–9. doi: 10.1046/j.1365-3024.2000.00336.x. [DOI] [PubMed] [Google Scholar]
- 16.Sharafi SM, Rostami-Nejad M, Moazeni M, Yousefi M, Saneie B, Hosseini-Safa A, et al. Echinococcus granulosus genotypes in Iran. Gastroenterol Hepatol Bed Bench. 2014;7:82–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Aref N, Shirzad H, Yousefi M, Darani H. Effect of different hydatid cyst molecules on hela and vero cell lines growth in vitro. J Immunodefic Disord. 2012;2:1. [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darani HY, Doenhoff MJ. An association between Schistosoma mansoni worms and an enzymatically-active protease/peptidase in mouse blood. Parasitology. 2008;135:467–72. doi: 10.1017/S0031182007003988. [DOI] [PubMed] [Google Scholar]
- 21.Darani HY, Curtis RH, McNeice C, Price HP, Sayers JR, Doenhoff MJ. Schistosoma mansoni: Anomalous immunogenic properties of a 27 kDa larval serine protease associated with protective immunity. Parasitology. 1997;115(Pt 3):237–47. doi: 10.1017/s0031182097001303. [DOI] [PubMed] [Google Scholar]
- 22.Doiz O, Benito R, Sbihi Y, Osuna A, Clavel A, Gómez-Lus R. Western blot applied to the diagnosis and post-treatment monitoring of human hydatidosis. Diagn Microbiol Infect Dis. 2001;41:139–42. doi: 10.1016/s0732-8893(01)00293-0. [DOI] [PubMed] [Google Scholar]
- 23.Virginio VG, Hernández A, Rott MB, Monteiro KM, Zandonai AF, Nieto A, et al. A set of recombinant antigens from Echinococcus granulosus with potential for use in the immunodiagnosis of human cystic hydatid disease. Clin Exp Immunol. 2003;132:309–15. doi: 10.1046/j.1365-2249.2003.02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Sherbiny MM, Farrag AA, Fayad MH, Makled MK, Tawfeek GM, Ali NM. Application and assessment of a dipstick assay in the diagnosis of hydatidosis and trichinosis. Parasitol Res. 2004;93:87–95. doi: 10.1007/s00436-004-1076-x. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo C, Ferreira HB, Monteiro KM, Rosenzvit M, Kamenetzky L, García HH, et al. Comparative analysis of the diagnostic performance of six major Echinococcus granulosus antigens assessed in a double-blind, randomized multicenter study. J Clin Microbiol. 2005;43:2764–70. doi: 10.1128/JCM.43.6.2764-2770.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadjjadi SM, Abidi H, Sarkari B, Izadpanah A, Kazemian S. Evaluation of enzyme linked immunosorbent assay, utilizing native antigen B for serodiagnosis of human hydatidosis. Iran J Immunol. 2007;4:167–72. [PubMed] [Google Scholar]
- 27.Kalantari E, Bandehpour M, Pazoki R, Taghipoor-Lailabadi N, Khazan H, Mosaffa N, et al. Application of recombinant Echinococcus granulosus antigen B to ELISA kits for diagnosing hydatidosis. Parasitol Res. 2010;106:847–51. doi: 10.1007/s00436-010-1726-0. [DOI] [PubMed] [Google Scholar]
- 28.Khabiri AR, Bagheri F, Assmar M, Siavashi MR. Analysis of specific IgE and IgG subclass antibodies for diagnosis of Echinococcus granulosus. Parasite Immunol. 2006;28:357–62. doi: 10.1111/j.1365-3024.2006.00837.x. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Zhang WB, Wilson M, Ito A, McManus DP. A novel recombinant antigen for immunodiagnosis of human cystic echinococcosis. J Infect Dis. 2003;188:1951–60. doi: 10.1086/379976. [DOI] [PubMed] [Google Scholar]
- 30.de la Rue ML, Yamano K, Almeida CE, Iesbich MP, Fernandes CD, Goto A, et al. Serological reactivity of patients with Echinococcus infections (E. granulosus, E. vogeli, and E. multilocularis) against three antigen B subunits. Parasitol Res. 2010;106:741–5. doi: 10.1007/s00436-009-1707-3. [DOI] [PubMed] [Google Scholar]
- 31.Delunardo F, Ortona E, Margutti P, Perdicchio M, Vacirca D, Teggi A, et al. Identification of a novel 19 kDa Echinococcus granulosus antigen. Acta Trop. 2010;113:42–7. doi: 10.1016/j.actatropica.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Taherkhani H, Zeyhle E, Rogan MT. Antibody responses in human cystic hydatid disease to the laminated layer of Echinococcus granulosus. Parasitol Res. 2007;101:647–52. doi: 10.1007/s00436-007-0534-7. [DOI] [PubMed] [Google Scholar]


