Abstract
Background:
Although several studies have investigated the association between ovarian cancer risk and nonisoflavone flavonoids intake, these findings are inconsistent. This systematic review of published epidemiological studies was conducted to summarize and clarify the evidence on the association between ovarian cancer incidence and nonisoflavone flavonoids intake.
Materials and Methods:
PubMed, Scopus, Google Scholar, and EMBASE databases were searched based on MeSH term (ovarian neoplasm in combination with flavonoids) to identify related English and non-English papers published up to June 2016. We summarized the results of the relevant studies in this review.
Results:
In total, seven studies (four with cohort and three with case–control design) included in this review. The results of conducted cohort studies show no relation between ovarian cancer risk and total nonisoflavone flavonoids intake, and only one study reported a significant reduction between ovarian cancer incidence and kaempferol and luteolin intake. Similar to those in the cohort studies, also in case–control studies, no association was found between total nonisoflavone flavonoids intake and ovarian cancer risk, just an inverse association between flavonols intake and ovarian cancer was reported.
Conclusion:
Several studies investigated the relation of nonisoflavone flavonoids intake and ovarian cancer risk; none of them reported any association for total nonisoflavone flavonoids intake, but some reported an inverse association between certain subclasses or individual flavonoids. These findings are limited, and there is a need for further and more accurate researches to be confirmed.
Keywords: Flavanones, flavonols, nonisoflavone flavonoid, ovarian neoplasm
INTRODUCTION
Flavonoids are polyphenolic chemicals found naturally in fruits, vegetables, and plant-derived foods and beverages. There are more than 5000 individual flavonoid compounds and at least ten subgroups of flavonoids. The most common subgroups in human diet are flavones, flavonols, flavanols (catechins), flavanones, isoflavones, and anthocyanidins.[1,2,3,4] Flavonols (e.g., quercetin, kaempferol, and myricetin) are the most bountiful flavonoids in plant foods and are mainly present in leafy vegetables, apples, onions, broccoli, and berries. Flavones (e.g. apigenin and luteolin) and anthocyanidins are present in small quantities in grains, leafy vegetables, and herbs. Flavanols (e.g., catechin and epicatechin) are plentiful in tea, apples, grapes, chocolate, and red wine. Flavanones (e.g., naringenin and hesperetin) predominantly exist in citrus fruit and their juices. Isoflavones (e.g., daidzein and genistein) are mainly present in soybeans and soy-based products.[5,6]
Ovarian cancer, cancer that begins in an ovary, is the fifth leading cause of cancer death in women, and unfortunately, its symptoms (such as abdominal pain and swelling) usually occur late in the disease process.[7] In 2012, ovarian cancer occurred in 239,000 women and resulted in 152,000 deaths worldwide. This makes it the seventh most common cancer and the eighth most common cause of death from cancer among women. Ovarian cancer outcomes depend on the extent of cancer and its subtype. In comparison to North America and Europe, outcomes are worse in the developing world.[8]
Nowadays, there is a growing body of evidences about the effects of phytochemicals and their sources on various health condition[9,10,11] and cancers risk.[12,13,14] This scientific evidence suggests that flavonoids possess several biological effects that may play a valuable role in cancer prevention, including antiestrogenic, antimutagenic, antiproliferative, anti-inflammatory, and antioxidant properties.[1,15] These mentioned properties may involve in well-established association between fruits and vegetables high intake and lower cancers risk.[12,13,14]
There are several systematic reviews that analyze the association between ovarian cancer and consumption of tea,[16] wine,[17] and isoflavonoids,[18,19] but based on our knowledge, there is no systematic review on the relation of ovarian cancer risk and nonisoflavone flavonoids intake; moreover, published evidences in this field are inconsistent. Therefore, we scrutinize the issue in this paper to clarify unknown aspects. This systematic review of published epidemiological studies was conducted to summarize and clarify the evidence on the association between ovarian cancer incidence and nonisoflavone flavonoids intake.
MATERIALS AND METHODS
We performed a systematic review of studies that evaluated the correlation between ovarian cancer risk and nonisoflavone flavonoids intake.
Search strategy
A systematic search for relevant publications was done using PubMed, Scopus, Google Scholar, and EMBASE databases. Two authors (Vida Mohammadi and Sirous Dehghani) independently searched English and non-English papers published up to June 2016 using the following keywords “Flavonoids,” “Flavone,” “Luteolin,” “Apigenin,” “Tangeritin,” “Flavonol,” “3-hydroxyflavone,” “Quercetin,” “Kaempferol,” “Myricetin,” “Fisetin,” “Galangin,” “Isorhamnetin,” “Pachypodol,” “Rhamnazin,” “Flavanone,” “Hesperetin,” “Naringenin,” “Eriodictyol,” “Homoeriodictyol,” “Flavanonol,” “3-Hydroxyflavanone,” “2,3-dihydroflavonol,” “Taxifolin,” “Dihydrokaempferol,” “Dihydroquercetin,” “Flavan,” “Anthocyanidin,” “Cyanidin,” “Delphinidin,” “Malvidin,” “Pelargonidin,” “Peonidin,” “Petunidin,” “Glycitein,” “Coumestan,” “Pterocarpan,” “Parsley,” “Blueberrie,” “Citrus,” “Dark chocolate” in combination with “ovarian cancer,” “ovarian neoplasm.”
We found 348 papers, but after reading the titles and abstracts, most of them excluded based on our exclusion criteria: investigating molecular and biochemical aspects or working on animal models. Eligible (cohort and case–control studies) studies scanned based on their title, abstract, and their major aims in first-step and related studies assessed based on their full texts, except one article[20] that we could not achieve its full text and our efforts to contact its authors were inconclusive. We also checked references of related studies to extract relevant studies.
RESULTS
Finally, we selected seven articles, which had our inclusion criteria (cohort and case–control studies) for systematic review. Figure 1 shows the pathway; we went through for selecting final articles.
Figure 1.
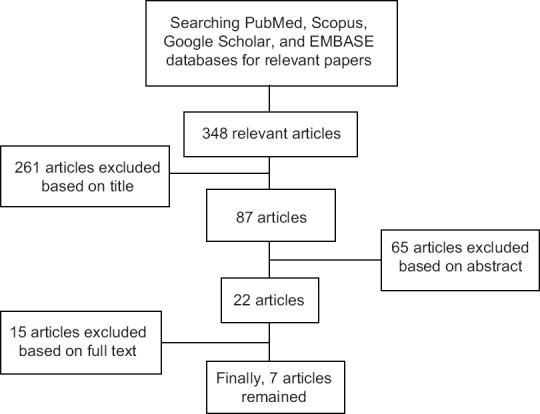
A summary of how selecting articles
In total, seven studies were included in the present review; four with cohort and three with case–control design. The articles, reviewed in this paper, were summarized in Table 1.
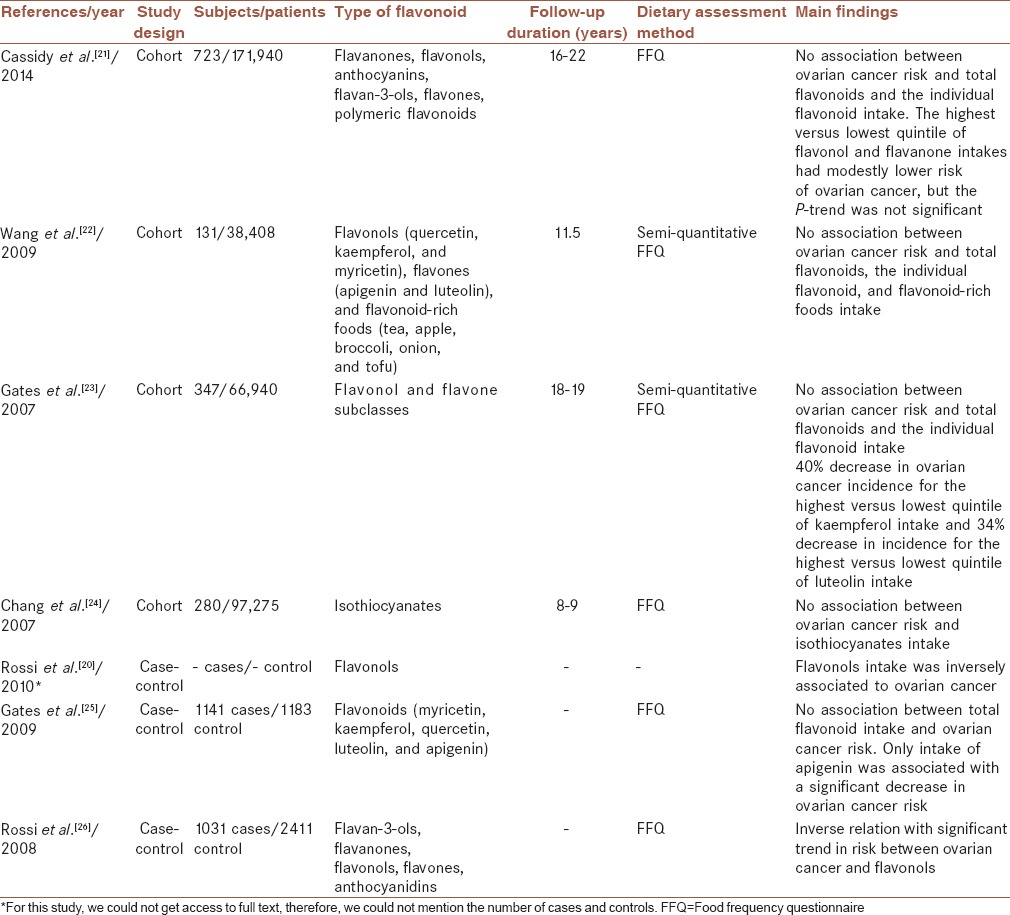
Table 1.
A summary of studies that reviewed in current article
Cohort studies
Cassidy et al.[21] followed 171,940 Nurses’ Health Study and Nurses’ Health Study II participants to examine associations between consumption of total flavonoids and their subgroups (flavanones, flavonols, anthocyanins, flavan-3-ols, flavones, and polymeric flavonoids) and risk of ovarian cancer. During 16–22 years of follow-up, 723 cases of ovarian cancer were confirmed through medical records. In this cohort study, food frequency questionnaire was used for collecting dietary data. They reported that in comparing top with the bottom quintile, total flavonoids intake was not significantly associated with ovarian cancer risk. However, participants in the highest quintiles of flavonol and flavanone consumption had modestly lower risk of ovarian cancer although the P-trend was not significant. Authors concluded that higher intakes of flavonols and flavanones might be associated with lower risk of ovarian cancer. Above authors conclusion, these findings were not statistically significant.
In another study, Wang et al.[22] prospectively investigated the association between the intake of selected flavonoids (flavonols [quercetin, kaempferol, and myricetin] and flavones [apigenin and luteolin]) and flavonoid-rich foods (tea, apple, broccoli, onion, and tofu) and risk of ovarian cancer in the Women's Health Study. A total of 131 incident ovarian cancer cases were identified during 11.5 years of follow-up among 38,408 women aged ≥45 years. Semi-quantitative food frequency questionnaire was used for collecting dietary data in this study. No significant association was found between total flavonoids consumption, the individual flavonoid, and flavonoid-rich foods and the incidence of ovarian cancer.
Gates et al.[23] analyzed the association between intake of five common dietary nonisoflavone flavonoids from the flavonol and flavones subclasses (myricetin, kaempferol, quercetin, luteolin, and apigenin) and incidence of epithelial ovarian cancer among 66,940 women in the Nurses’ Health Study. In this cohort study, dietary information was collected using semi-quantitative food frequency questionnaire, and data were available from multiple time points during 18 years of follow-up. They observed no clear association between total intake of the five examined flavonoids and ovarian cancer risk. However, there was a significant 40% and 34% reduction in ovarian cancer incidence for the highest versus lowest quintile of kaempferol and luteolin intake, respectively. These data suggest dietary intake of certain flavonoids may reduce ovarian cancer risk.
In a study by Chang et al.,[24] the association between consumption of some phytochemical compounds including isothiocyanates and ovarian cancer risk has been investigated. Among 97,275 women participated in the California Teachers Study, 280 women developed invasive or borderline ovarian cancer. Food frequency questionnaire was used for gathering dietary data, but in this study, usual dietary intake was assessed only at one point. Intake of isothiocyanates or foods high in isothiocyanates was not associated with ovarian cancer risk.
In general, the results of conducted cohort studies in this field are diverse. Remarkable point is that no study shows a significant relation between ovarian cancer risk and total flavonoids intake,[21,22,23,24] and only one study reported a significant 40% and 34% reduction in ovarian cancer incidence for the highest versus lowest quintile of kaempferol and luteolin intake (flavonoids subgroups), respectively.[23]
Case–control studies
In a network of case–control studies from Italy, Rossi et al.[20] assessed the relation of total flavonoids, flavanones, flavonols consumption, and ovarian cancer risk. They expressed that just flavonols intake (odds ratio = 0.63) was inversely associated with ovarian cancer.
In another study, Gates et al.[25] evaluated the association between ovarian cancer risk and intake of five common dietary flavonoids (myricetin, kaempferol, quercetin, luteolin, and apigenin), as well as total intake of these flavonoids, for 1141 cases and 1183 frequency-matched controls. In this study, food frequency questionnaire was used for gathering flavonoid intake. No association was observed between total flavonoid intake and ovarian cancer risk. In analyses of each individual flavonoid, only intake of apigenin was associated with a significant decrease in ovarian cancer risk.
Another study by Rossi et al.[26] investigated the relation of six classes of flavonoids (flavan-3-ols, flavanones, flavonols, flavones, anthocyanidins, and isoflavones) with ovarian cancer risk. The study included 1031 cases with confirmed epithelial ovarian cancer and 2411 controls. Food frequency questionnaire was used for collecting data. In this study, an inverse relation was found between ovarian cancer and flavonols intake, which had a trend toward significance.
Similar to those in the cohort studies, also in case–control studies, no association was found between total flavonoids consumption and ovarian cancer; just Rossi et al. reported an inverse association between flavonols intake and ovarian cancer,[20] and in another study, an inverse relation with significant trend was found between ovarian cancer risk and flavonols intake.[26]
DISCUSSION
In this systematic review of epidemiological studies, we investigated the relationship between ovarian cancer risk and nonisoflavone flavonoids and their subgroups intake. For this purpose, we systematically reviewed the related cohort and case–control studies. Based on the present evidences, it seems that there is no association between ovarian cancer risk and total nonisoflavone flavonoids intake, but if the members of flavonoids subgroups considered individually, there is a significant adverse association between some of them and ovarian cancer risk.
Based on scientific evidence, there is a well-established association between high fruits and vegetables consumption and reduced cancers risk. Their phytochemicals content has been suggested for these effects.[12,13,14] As you know, like other phytochemicals, flavonoids and its subgroups have several biological effects, including antiestrogenic, antimutagenic, antiproliferative, anti-inflammatory, and antioxidant properties, that may play a valuable role in cancer prevention.[1,5,27] Certain flavonoids, including quercetin, luteolin, and apigenin, appear to decrease inflammation through inhibiting cyclooxygenase-2 and inducible nitric oxide synthase which are important mediators of inflammatory reaction.[1,28,29]
Despite the considerable strengths of cohort studies, including their large size, prospective exposure assessment, and effective complete case ascertainment, there are several limitations to take into account. For example, Chang et al.[24] evaluated dietary intake only at one point, and their study has a wide age range, so the effects of dietary intake during some other age or period or accumulative effects or change in dietary habits over time are not considered.
Reviewed cohort studies are also subject to the limitations of all observational studies that assessed dietary intake. For instance, food frequency questionnaires were used for collecting data in all studies that have a limited capacity to gather detailed information about food and nutrient consumption.[30] The semi-quantitative food frequency questionnaire used in some studies was not specifically designed to estimate flavonoid intake; as a result, not all dietary sources of flavonoids were included in the questionnaire.
In addition, we assessed case–control studies, and these data need to be explicated watchfully because of the retrospective collection of dietary data and the long common period of cancer development.
In the present paper, we reviewed cohort and case–control studies, which evaluated the association of nonisoflavone flavonoids and its subgroup components consumption and ovarian cancer risk. According to the results of reviewed studies, there is no association between ovarian cancer risk and total nonisoflavone flavonoids intake, unanimously. However, some studies reported an inverse association between certain nonisoflavone flavonoids subclasses (flavonols)[20,26] or individual flavonoids (kaempferol and luteolin[23] and apigenin[25]) and ovarian cancer risk. These results provide limited support for an association between nonisoflavone flavonoids intake and ovarian cancer risk, and we should be aware that there is a need for further and more accurate researches to be confirmed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
VM and SD contributed in the conception of the work, conducting the study, searched all target databases and merged the results, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. BL and LA contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. All steps were under supervision of LA.
Acknowledgments
We thank all personnel of Food Security Research Center.
REFERENCES
- 1.Le Marchand L. Cancer preventive effects of flavonoids – A review. Biomed Pharmacother. 2002;56:296–301. doi: 10.1016/s0753-3322(02)00186-5. [DOI] [PubMed] [Google Scholar]
- 2.Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65:337–53. doi: 10.1016/s0024-3205(99)00120-4. [DOI] [PubMed] [Google Scholar]
- 3.Ross JA, Kasum CM. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 4.Aherne SA, O’Brien NM. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition. 2002;18:75–81. doi: 10.1016/s0899-9007(01)00695-5. [DOI] [PubMed] [Google Scholar]
- 5.Neuhouser ML. Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr Cancer. 2004;50:1–7. doi: 10.1207/s15327914nc5001_1. [DOI] [PubMed] [Google Scholar]
- 6.Moon YJ, Wang X, Morris ME. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 7.Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and body size: Individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med. 2012;9:e1001200. doi: 10.1371/journal.pmed.1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Cancer Report 2014. Ch. 5.12. World Health Organization. 2014 [Google Scholar]
- 9.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr. 2006;84:1489–97. doi: 10.1093/ajcn/84.6.1489. [DOI] [PubMed] [Google Scholar]
- 10.Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Padyab M, Hu FB, et al. Soy inclusion in the diet improves features of the metabolic syndrome: A randomized crossover study in postmenopausal women. Am J Clin Nutr. 2007;85:735–41. doi: 10.1093/ajcn/85.3.735. [DOI] [PubMed] [Google Scholar]
- 11.Azadbakht L, Atabak S, Esmaillzadeh A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: A longitudinal randomized clinical trial. Diabetes Care. 2008;31:648–54. doi: 10.2337/dc07-2065. [DOI] [PubMed] [Google Scholar]
- 12.Liu RH. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J Nutr. 2004;134(12 Suppl):3479S–85S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 13.Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78(3 Suppl):517S–20S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 14.Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131(11 Suppl):3027S–33S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 15.López-Lázaro M. Flavonoids as anticancer agents: Structure-activity relationship study. Curr Med Chem Anticancer Agents. 2002;2:691–714. doi: 10.2174/1568011023353714. [DOI] [PubMed] [Google Scholar]
- 16.Boehm K, Borrelli F, Ernst E, Habacher G, Hung SK, Milazzo S, et al. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst Rev. 2009;8(3) doi: 10.1002/14651858.CD005004.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HS, Kim JW, Shouten LJ, Larsson SC, Chung HH, Kim YB, et al. Wine drinking and epithelial ovarian cancer risk: A meta-analysis. J Gynecol Oncol. 2010;21:112–8. doi: 10.3802/jgo.2010.21.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crane TE, Khulpateea BR, Alberts DS, Basen-Engquist K, Thomson CA. Dietary intake and ovarian cancer risk: A systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23:255–73. doi: 10.1158/1055-9965.EPI-13-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myung SK, Ju W, Choi HJ, Kim SC Korean Meta-Analysis (KORMA) Study Group. Soy intake and risk of endocrine-related gynaecological cancer: A meta-analysis. BJOG. 2009;116:1697–705. doi: 10.1111/j.1471-0528.2009.02322.x. [DOI] [PubMed] [Google Scholar]
- 20.Rossi M, Bosetti C, Negri E, Lagiou P, La Vecchia C. Flavonoids, proanthocyanidins, and cancer risk: A network of case-control studies from Italy. Nutr Cancer. 2010;62:871–7. doi: 10.1080/01635581.2010.509534. [DOI] [PubMed] [Google Scholar]
- 21.Cassidy A, Huang T, Rice MS, Rimm EB, Tworoger SS. Intake of dietary flavonoids and risk of epithelial ovarian cancer. Am J Clin Nutr. 2014;100:1344–51. doi: 10.3945/ajcn.114.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Lee IM, Zhang SM, Blumberg JB, Buring JE, Sesso HD. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutr. 2009;89:905–12. doi: 10.3945/ajcn.2008.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gates MA, Tworoger SS, Hecht JL, De Vivo I, Rosner B, Hankinson SE. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer. 2007;121:2225–32. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]
- 24.Chang ET, Lee VS, Canchola AJ, Clarke CA, Purdie DM, Reynolds P, et al. Diet and risk of ovarian cancer in the California Teachers Study cohort. Am J Epidemiol. 2007;165:802–13. doi: 10.1093/aje/kwk065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gates MA, Vitonis AF, Tworoger SS, Rosner B, Titus-Ernstoff L, Hankinson SE, et al. Flavonoid intake and ovarian cancer risk in a population-based case-control study. Int J Cancer. 2009;124:1918–25. doi: 10.1002/ijc.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossi M, Negri E, Lagiou P, Talamini R, Dal Maso L, Montella M, et al. Flavonoids and ovarian cancer risk: A case-control study in Italy. Int J Cancer. 2008;123:895–8. doi: 10.1002/ijc.23549. [DOI] [PubMed] [Google Scholar]
- 27.Dastjerdi MN, Kavoosi F, Valiani A, Esfandiari E, Sanaei M, Sobhanian S, et al. Inhibitory effect of genistein on PLC/PRF5 hepatocellular carcinoma cell line. Int J Prev Med. 2015;6:54. doi: 10.4103/2008-7802.158914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raso GM, Meli R, Di Carlo G, Pacilio M, Di Carlo R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001;68:921–31. doi: 10.1016/s0024-3205(00)00999-1. [DOI] [PubMed] [Google Scholar]
- 29.Guerra JA, Molina M, Abad MJ, Villar AM, Paulina B. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids isolated from Tanacetum microphyllum. Int Immunopharmacol. 2006;6:1723–8. doi: 10.1016/j.intimp.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Kristal AR, Peters U, Potter JD. Is it time to abandon the food frequency questionnaire? Cancer Epidemiol Biomarkers Prev. 2005;14:2826–8. doi: 10.1158/1055-9965.EPI-12-ED1. [DOI] [PubMed] [Google Scholar]


