Abstract
This review study aimed to compare the electronic prescription systems in five selected countries (Denmark, Finland, Sweden, England, and the United States). Compared developed countries were selected by the identified selection process from the countries that have electronic prescription systems. Required data were collected by searching the valid databases, most widely used search engines, and visiting websites related to the national electronic prescription system of each country and also sending E-mails to the related organizations using specifically designed data collection forms. The findings showed that the electronic prescription system was used at the national, state, local, and area levels in the studied countries and covered the whole prescription process or part of it. There were capabilities of creating electronic prescription, decision support, electronically transmitting prescriptions from prescriber systems to the pharmacies, retrieving the electronic prescription at the pharmacy, electronic refilling prescriptions in all studied countries. The patient, prescriber, and dispenser were main human actors, as well as the prescribing and dispensing providers were main system actors of the Electronic Prescription Service. The selected countries have accurate, regular, and systematic plans to use electronic prescription system, and health ministry of these countries was responsible for coordinating and leading the electronic health. It is suggested to use experiences and programs of the leading countries to design and develop the electronic prescription systems.
KEY WORDS: Comparative study, developed countries, electronic prescription system
INTRODUCTION
Over the years, hand-written prescription has been a preferred communication method for physicians in decisions relating to medication therapy and for pharmacists to distribute medications. It is also considered as a valuable resource for the patients on how to use the medicine to achieve the maximum benefit.[1]
In the last decade, electronic prescription is always considered as an interested subject among other electronic health solutions to process the health-related data.[1,2,3,4]
In fact, electronic prescription is a broad term that means using the computer devices to enter, modify, review, and generate or transmit medicine prescriptions that prepare two-way transmissions between the point of care and the dispenser. This form of technology would safely transmit prescription or prescription-related information between stakeholders (prescribers, dispensers, pharmacies, health plans, and health insurers) either directly or through an intermediary (including an electronic prescription network) using electronic media.[2,3,4,5,6,7,8]
The electronic prescription system connects to information systems in health-care organizations such as hospitals, health-care centers, and pharmacies. The implementation of it can overcome many problems of paper prescribing process and will bring benefits, including cost savings, reducing prescription errors, increasing prescription legibility, improving medication therapy outcomes, reducing redundant paperwork, electronically accessing to updated pharmacopeia information, and patient medication history.[9,10,11,12,13,14,15,16,17]
Electronic prescription has been discussed in many experts' reports and public national plans, and it is tested, implemented, or are implementing in several European and the United States countries.[18,19,20,21] Electronic prescription systems have been designed and implemented according to the domestic needs of each country, and different standards are also established to make it better every year.[22,23,24,25] Implementation of electronic prescription systems is an irreversible intervention in the prescribing process. This system is a representative of the multidisciplinary sociotechnical information systems that has a wide scope, different specialties, numerous complexities, various actors and subsystems, distinct implementation process, and specific technical solutions in each country. Medicine is the most important part of therapy supported by the governments of many nations. In addition, the process of medication prescription and consumption is one of the important pillars of the health system in each country.[7,26,27]
Today, the necessity of using electronic prescription systems can be felt more than ever due to their many benefits. Therefore, study on this system as a systematic or comprehensive review has been done.[12,28,29,30,31] However, since many countries, especially developing countries, still use manual traditional prescription systems, using the experiences of leading countries is very necessary and useful to develop and design the electronic prescription system in the other countries. Taking into account the above considerations, the aim of the present study is to identify and compare national electronic prescription system in the selected countries.
METHODS
This review study was done in the period of 2013–2015. Countries with the electronic prescription system were selected. The selection process of the countries was as follows:
Since electronic prescription has been fully implemented only in a few European countries and the United States, it has been adopted as a part of the national electronic-health strategy in the European Union (EU) countries.[32,33] EU countries (27 countries) and the United States were selected in the first stage
At this stage, the definition of electronic prescription was applied for countries selection. The range of the definitions was broad from creating electronic prescription to electronic transmission and processing. There was no generally agreed and same definition for electronic prescription.[34,35] Therefore, three key features of the electronic prescription definitions were used for selection of the countries included: creating electronic prescription, electronically sending electronic prescription to the pharmacy, and two-way transmissions between the point of care and the pharmacy. As a result of this step, eight countries (Denmark, Finland, Germany, New Zealand, the Netherlands, Sweden, England, and the United States) were selected due to the possibility of creating electronic prescriptions[23,26,36,37,38,39,40]
In the final stage, the national prescription system in each country was studied in terms of the capability of electronically transmitting prescriptions (ETPs) to the pharmacies and two-way electronic communication between the prescriber and dispenser. In the end, five countries (Denmark, Finland, Sweden, England, and the United States) were selected which were approved based on the other criteria of the existing studies review in this field.
A form of data collection was designed and developed based on the main components of the prescription system model[4,6,8,39] to collect data from the selected countries, including as follows:
Legal infrastructure of the electronic prescription systems (adoption of the electronic prescription law, adoption of the electronic prescription transmission law, issuing the electronic prescriptions for controlled medicines, issuing a paper prescription if needed, legality of the prescription electronic signature, patient satisfaction to participate in this service, and patient satisfaction to access stakeholders to needed information)
Accepting prescriptions from other countries (dispensing fax, paper and electronic prescriptions, approving dispensation laws, constraints)
Electronic prescription system architecture (architecture type, national electronic prescription database, patient demographic database, national medicine database, national electronic health record, personal electronic medicine profile, the Internet, dedicated national health network, national electronic prescription network, national electronic health portal)
Responsibility of the electronic prescription system (coordination and leading electronic health, financing, creating and maintaining national electronic prescription database, establishing a dedicated health network, implementing a national health portal)
Electronic prescription system identifiers (national patient identifier, medicine identifier, physician identifier, technology of confirming the identification of the prescriber and dispenser, prescription unique identifier (PUID), pharmacy identifier)
The process of electronic prescription system (electronic prescription, requesting the needed information, capabilities of decision support, providing the required information, choosing the pharmacy by the patient, electronic dispensation of medication, ETPs to the pharmacies, electronically retrieving the prescription at the pharmacies, electronic dispensing, storing electronic prescription in national prescription database, informing the prescribing physician of the dispensation (fill) status, transferring the dispensed prescription into the national electronic prescription database, electronic submission of reimbursement claims, electronically repeating or refilling the prescription).
Data were extracted by combination search of the keywords pertaining to electronic prescription with AND/OR operators in the search engines and databases of Google, Yahoo, Google Scholar, PubMed, ProQuest, and Iranian National Library of Medicine without time limitation: “Eprescri*” OR “e-prescri*” OR “electronic prescri*” OR “e-Rx” OR “electronically transmitting prescription (ETP)” OR “Medical order entry systems” OR “eDispensing” OR “electronic dispensing” OR “two-way electronic order system” OR “Computerized Physician Order Entry (CPOE)” OR “Prescription routing services” AND the name of each five selected countries. All retrieved papers, research projects, theses, directories, and progress reports in English were scrutinized. In addition, authorized organizations' web sites, national health-care networks, and national central databases concerned with electronic prescription system of each country were also visited and their available documentations were studied. To clarify the ambiguity in the electronic prescription system of the studied countries, an E-mail was sent to the related organizations (MedCom and sundhed in Denmark, Kela and KanTa in Finland, Apotekens Service and lakemedeletjanster in Sweden, NHS CFH in England, and Surescripts in the United States). Data collection form was completed according to the retrieved information sources.
Then, the similarities and differences of the electronic prescription system models in the selected countries were shown in comparative tables.
RESULTS
The results of the comparative review of the electronic prescription in the studied countries are listed in Tables 1–4. In Table 1, the legal infrastructures of the electronic prescription system have been investigated and compared. Based on the results, prescriber's electronic signature was legal in these countries and patient's satisfaction was necessary for stakeholders to access to the required information.
Table 1.
Comparison of the legal infrastructure of the electronic prescription system in the selected countries
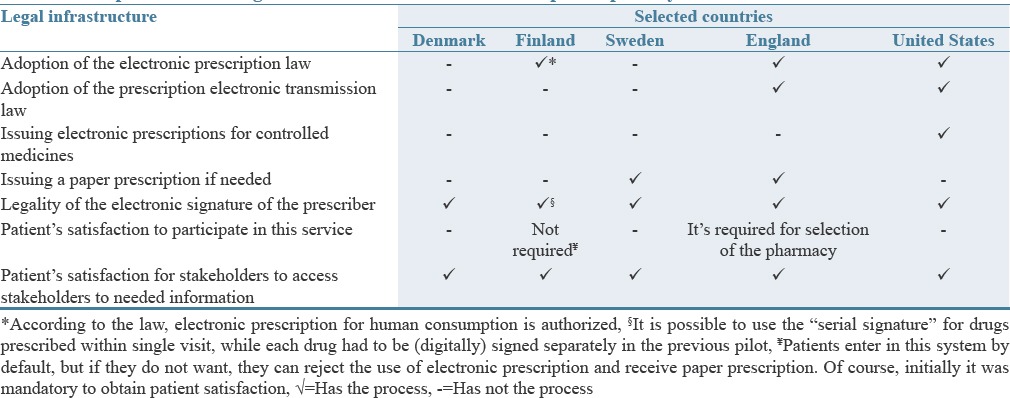
Table 4.
Comparison of the electronic prescription system processes in the selected countries
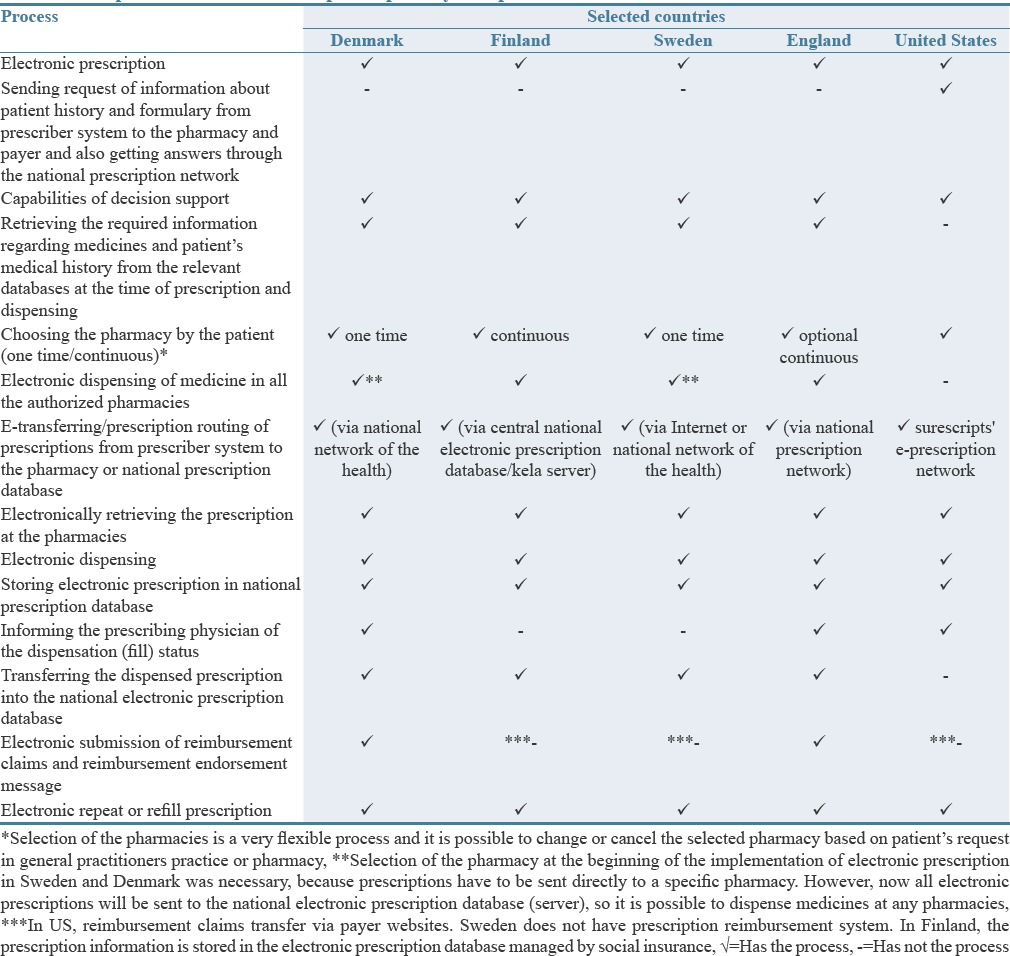
The overall comparison of the selected countries in terms of accepting prescriptions from other countries showed that none of the electronic prescription system of these countries accept electronic prescription from other countries (nonnational), although dispensation acts for these prescriptions have been approved in Sweden, Finland, and Denmark. However, a number of local pilot projects to accept the nonnational electronic prescriptions are in progress in Denmark.[41,42] In addition, dispensing fax or paper prescriptions relevant to northern Europe in four European studied countries is possible, but there are constraints for these prescriptions such as type of the medicines, type of the prescriptions (paper, fax and…), accuracy, and validity of the prescriptions. Rejection of the nonnational electronic prescription in the selected countries is due to the lack of standardization and legislation in this area, legal prohibition, verification and authentication problems, concerns about privacy and security of electronic prescriptions, and poor interaction and communication between health systems.[22,23,25,43,44,45] However, established in 2008, the European Patient Smart Open Services project is intended to provide concrete cross-border services that ensure safe, secure, and efficient medical treatment for citizens when traveling across Europe. Two specific areas were identified: a shared patient summary for EU citizens and an Electronic Prescription Service (including e-Dispensing).[46]
Table 2 compares the electronic prescription system architecture in the selected countries from ten important dimensions. Based on the results, the electronic prescription system in four European studied countries had centralized architecture and national database. However, the architecture of the US applied was diffused and decentralized. Furthermore, the patient, prescriber, and dispenser were main human actors, as well as the prescribing and dispensing providers were main system actors of the Electronic Prescription central prescription server provider was a system actor of the Electronic Prescription Service in Denmark, Finland, Sweden, and England.
Table 2.
Comparison of the electronic prescription system architecture in the selected countries
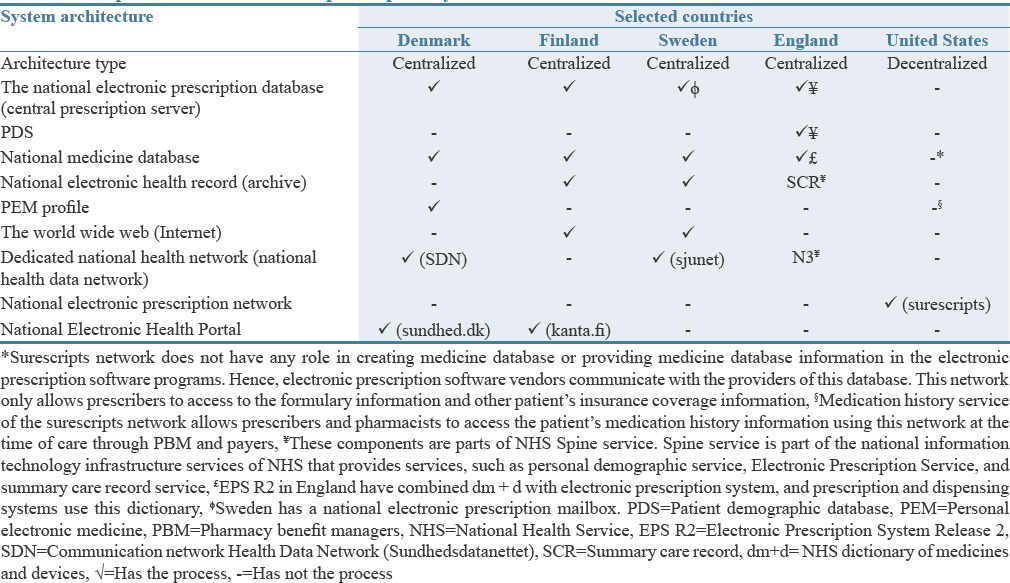
Based on comparing the responsibility of the electronic prescription system in the selected countries, the system of the United States does not have a unit coordinator and leader to create and maintain a national electronic prescription database.[47,48,49] Financial investment in the technology needed for electronic prescription is done in England in partnership with the private sector (such as system users, pharmacies, hospitals, and physician).[22,23,25,45] Furthermore, governmental or communal resources were used to set up electronic prescriptions' systems in Denmark, Finland, Sweden, and England.[23]
Table 3 compares the identifiers of the electronic prescription system in the selected countries. The United States, Sweden, and Denmark did not have PUID at the time of creating the electronic prescription. In addition, the only electronic prescription system of the United States lacks a national patient ID.
Table 3.
Comparison of the electronic prescription system identifiers in the selected countries
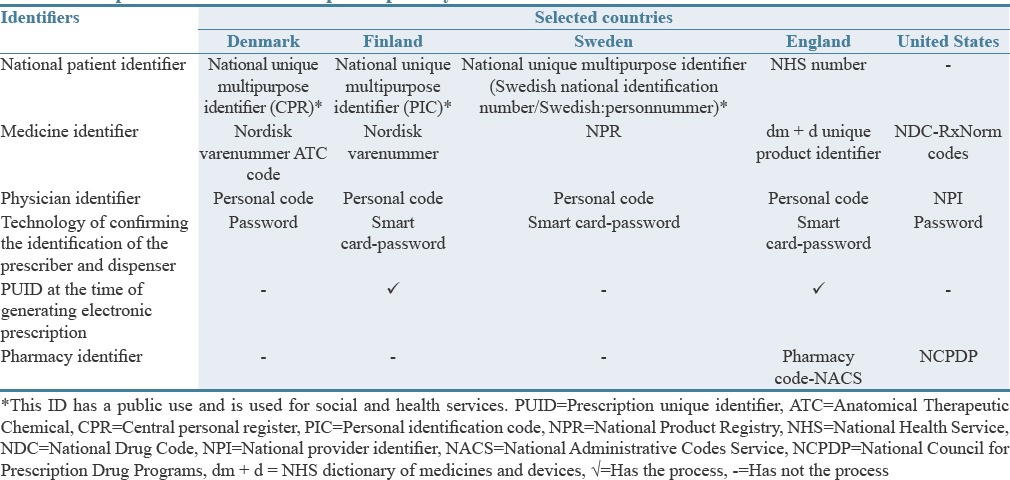
Table 4 compares the processes of the electronic prescription system in the selected countries. Only the United States has a process of sending requests of information about patient's history and formulary from prescriber systems to the pharmacies and the payers.
DISCUSSION
Comparing features of the electronic prescription system in the selected countries (the United States, England, Sweden, Finland, and Denmark) indicates that this system is used routinely or as a pilot at the state, local, or area levels and covers the whole process of prescription or major part of it. Electronic prescription system is used only in Denmark and Sweden routinely at the national level and covers the entire prescription process. Furthermore, the studied countries use different classification, vocabulary, terminology, and data interchange standards in their electronic prescription system. Results of the other studies indicate that each national system selects different approaches toward electronic prescription (regarding the field and starting point, the process of implementation, and technical solutions) and its development. It could be due to differences in their health-care and insurance systems. Differences in approaches to implement this system had an impact on the adoption of the electronic prescription in different countries.[50,51,52,53,54,55,56,57,58,59] Thus, the countries were at different levels and stages of implementation of the electronic prescription.[37,38,39,40,41,42,43,44,45,47,48,49,50,51,52] In addition, electronic prescription systems and models in different countries and also even within a country were not similar.[45,49,50,51,53]
A variety of electronic prescription standards in the countries with this system has been mentioned in various sources.[37,52,53,54,55] It could be due to different standard trustee organizations in each of the studied countries. Using uniform standards and vocabularies and centralized knowledge bases can prevent from repetitive tasks of the care providers and suppliers.[56]
Electronic prescription in all the studied countries except England had started from primary health-care centers. In addition, according to an international study that was done in six countries (Australia, New Zealand, England, Northern Ireland, Scotland, and Denmark) titled “EPrescribing and Electronic Transfer of Prescriptions: An International Review,” all of them similarly emphasized on electronic prescription and dispensation in outpatient centers and public pharmacies. The similarity of the work processes of pharmacists and general physicians with their other peers have caused to their further support for the computerization of this process. In contrast, pharmaceutical management processes of the hospital is often more complex, so its standardization and computerization is more complex.[38]
Patient's satisfaction for stakeholders' access to the required information and legality of the prescriber's electronic signature were necessary in the legal infrastructure of the electronic prescription system of the studied countries. Electronic prescription law was approved in the United States, England, and Finland. However, the study of Stroetmann et al. with the aim of determining the legal challenges on the road toward interoperable ehealth solutions in Europe showed that some of these countries did not use ePescription in primary care due to national legislation forbidding or not addressing the electronic transmission of prescriptions and the use of electronic signatures.[45] Therefore, further efforts are necessary in these countries to adopt the electronic prescription laws, the electronic transmission of the prescriptions, issuing electronic prescription for controlled medicines, and accepting electronic prescriptions from other countries. Stroetmann et al.'s research also determined legal requirements of the countries about electronic prescription that include authentication, electronic signature of the patient's satisfaction, and access to the paper prescription.[45]
Comparing the architecture of the electronic prescription system in the selected countries showed that the architecture type and components of this system are more similar to each other in four European countries (England, Sweden, Finland, and Denmark), because these countries have a centralized architecture and a national electronic prescription database.[25,36,40,42,43,48,51,52,57,58,59,60,61] However, the United States used diffused and decentralized architecture and had only a national electronic prescription network (Surescripts).[40,49,62] Dedicated national health networks such as Sjunet in Sweden[63] and SDN in Denmark[41,42] play an increased role in successful implementation of the electronic prescription system because it provides communication between different system stakeholders and transmission of electronic prescriptions.
The United States, England, and Sweden had centers for leading of the electronic health that were responsible for coordinating activities related to health information technology at the national level. In the studied countries, financial investment for the electronic prescription system was mainly done by government and public resources, but in England, part of the cost was financed by the private sector and also in Denmark and Sweden the cost of system development was paid by the actors (service providers and pharmacies). Mäkinen et al. in their article similarly expressed that the government, society, or nonuser resources were responsible for financing the electronic prescription infrastructure in the EU countries.[23] They stated that financing the costs of prescription is an obstacle to use this system at the national level and resulting in slow adoption of the electronic prescription in the future.[63] Hence, the actors should participate directly in financing the electronic prescription. In addition to financial support of the government or similar organizations, according to Protti et al., support also plays important roles in the adoption of electronic prescription in Denmark, England, and Scotland.[64]
In the electronic prescription system of all the selected countries, a unique identifier was used for physicians and medicines. As well as, patients in the studied European countries (England, Finland, Sweden, and Denmark) had a multipurpose and health unique national ID. The social security number was used as the unique patient's identifier in the United States, and based on many resources, the unique identifier of the patient, medication, physician, prescriptions, and pharmacies is necessary to implement the electronic prescription system.[40,65,66]
Comparing the process of the electronic prescription system showed that some important capabilities exist in the system of the selected countries, including electronic prescription, decision support, selecting the pharmacy by the patient, ETPs both directly or by virtual interface to a pharmacy, retrieving the electronic prescription in the pharmacies, electronic dispensing, and electronically refilling prescriptions. Previous studies about analysis of the work flow and electronic prescription process also mentioned to these activities.
CONCLUSION
Based on the results of this study, it is suggested to create a central national prescription database to store the electronic medication prescriptions and pharmacy dispensing documentation. The past experiences from the failures to implement this system indicate that it should be part of the national health-care infrastructure to facilitate safe and secure electronic transmission of prescription between the prescribers and dispensers. The government should also provide legal and financial incentives (preparing hardware and software, offering free technical support and making allowance for implementation) for better acceptance of this system among relevant stakeholders. It is also recommended that message transmission standards and interoperability framework are expanded to support the ETP data between the stakeholders' organizations. In addition, uniform standards and terminology should be applied in this system to provide an appropriate background for achieving higher levels of electronic prescription system in the future.
AUTHORS' CONTRIBUTION
Mahnaz Samadbeik designed the study, reviewed the literatures, analysed the data, drafted the article and supervised the study. Maryam Ahmadi collected the data, redrafted the article and revised the article. Farahnaz Sadoughi reviewed the article. Ali Garavand revised the article.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
ACKNOWLEDGMENTS
This study was part of a PhD thesis supported by the Tehran University of Medical Sciences (Grant No.: TUMS/SHMIS-1390/672).
REFERENCES
- 1.Hellström L, Waern K, Montelius E, Astrand B, Rydberg T, Petersson G. Physicians' attitudes towards ePrescribing – Evaluation of a Swedish full-scale implementation. BMC Med Inform Decis Mak. 2009;9:37. doi: 10.1186/1472-6947-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell DS, Cretin S, Marken RS, Landman AB. A conceptual framework for evaluating outpatient electronic prescribing systems based on their functional capabilities. J Am Med Inform Assoc. 2004;11:60–70. doi: 10.1197/jamia.M1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Computerized Prescribing and Policy Development Workgroup. E-prescribing and the Role of Electronic Health Networks: Electronic Health Information Exchange, Task Force to Study Electronic Health Records. 2006. [Last accessed on 2014 Feb 10]. Available from: http://www.mhcc.dhmh.maryland.gov/hit/ehr/Documents/sp.mhcc.maryland.gov/hit/Resources/Presentations/eprescribingehlth.pdf .
- 4.A Cinician's Guide to Electronic prescribing. Washington, DC: 2008. [Last accessed on 2015 Feb 19]. eHealth initiative. Available from: http://www.ama-assn.org/ama1/pub/up-load/mm/472/electronic-e-prescribing.pdf . [Google Scholar]
- 5.American Health Information Management Association (AHIMA). The intersections between e-prescribing and him. Practical tools for seminar learning. Chicago, Illinois [Online] 2009. [Last accessed on 2015 Jan 15]. Available from: http://campus.ahima.org/audio/2009/RB051909.pdf .
- 6.A Cinician's Guide to Electronic Prescribing. Arlington: 2011. [Last accessed on 2015 Aug 18]. The Center for Improving Medication Management. Available from: http://www.mgma.com/Libraries/Assets/Government Affairs/Issues/Health Information Technology/E-prescribing/2011-Clinicians-Guide-to-e-prescribing.pdf . [Google Scholar]
- 7.Phillips DP, Christenfeld N, Glynn LM. Increase in US medication-error deaths between 1983 and 1993. Lancet. 1998;351:643–4. doi: 10.1016/S0140-6736(98)24009-8. [DOI] [PubMed] [Google Scholar]
- 8.Oregon Health Authority Office of Health IT. E-prescribing Toolkit. A Practical Resource for Providers. Oregon Health Authority Office of Health IT. 2011. [Last accessed on 2014 April 0]. Available from: https://www.oregon.gov/oha/OHPR/HITOC/Stakeholder_Materials/eRx/E-PrescribeProviderResource9_11.pdf .
- 9.Byrne CM, Mercincavage LM, Pan EC, Vincent AG, Johnston DS, Middleton B. The value from investments in health information technology at the U.S. Department of Veterans Affairs. Health Aff (Millwood) 2010;29:629–38. doi: 10.1377/hlthaff.2010.0119. [DOI] [PubMed] [Google Scholar]
- 10.Devine EB, Hansen RN, Wilson-Norton JL, Lawless NM, Fisk AW, Blough DK, et al. The impact of computerized provider order entry on medicine errors in a multispecialty group practice. J U S Med Inf Assoc. 2010;17:78–84. doi: 10.1197/jamia.M3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohn LT. GAO-11-159. Washington, DC: DIANE Publishing; 2011. [Last cited on 2015 Feb 17]. Elec tronic Pres cribing: CMS Should Address Inconsistencies in Its Two Incentive Programs That Encourage the Use of Healthy Information Technology. Available from: http://www.gao.gov/assets/320/315811.pdf . [Google Scholar]
- 12.Samadbeik M, Ahmadi M, Hosseini Asanjan SM. A theoretical approach to electronic prescription system: Lesson learned from literature review. Iran Red Crescent Med J. 2013;15:e8436. doi: 10.5812/ircmj.8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor JA, Loan LA, Kamara J, Blackburn S, Whitney D. Medicine administration variances before and after implementation of computerized physician order entry in a neonatal intensive care unit. Pediatrics. 2008;121:123–8. doi: 10.1542/peds.2007-0919. [DOI] [PubMed] [Google Scholar]
- 14.Varkey P, Aponte P, Swanton C, Fischer D, Johnson SF, Brennan MD. The effect of computerized physician-order entry on outpatient prescription errors. Manag Care Interface. 2007;20:53–7. [PubMed] [Google Scholar]
- 15.Wang CJ, Patel MH, Schueth AJ, Bradley M, Wu S, Crosson JC, et al. Perceptions of standards-based electronic prescribing systems as implemented in outpatient primary care: A physician survey. J Am Med Inform Assoc. 2009;16:493–502. doi: 10.1197/jamia.M2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weingart SN, Simchowitz B, Shiman L, Brouillard D, Cyrulik A, Davis RB, et al. Clinicians' assessments of electronic medication safety alerts in ambulatory care. Arch Intern Med. 2009;169:1627–32. doi: 10.1001/archinternmed.2009.300. [DOI] [PubMed] [Google Scholar]
- 17.Yu F, Salas M, Kim Yi, Menachemi N. The relationship between computerized physician order entry and pediatric adverse medicine events: A nested matched case-control study. Pharmacoepidemiol Med Saf. 2009;18:751–5. doi: 10.1002/pds.1777. [DOI] [PubMed] [Google Scholar]
- 18.Costa AL, de Oliveira MM, de Oliveira Machado R. An information system for medicine prescription and distribution in a public hospital. Int J Med Inf. 2004;73:371–81. doi: 10.1016/j.ijmedinf.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Ekedahl A. Problem prescriptions in Sweden necessitating contact with the prescriber before dispensing. Res Social Adm Pharm. 2010;6:174–84. doi: 10.1016/j.sapharm.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Smith JA., Jr Role of computerized physician order entry systems in facilitating medication errors. J Urol. 2005;174(4 Pt 1):1400–1. [PubMed] [Google Scholar]
- 21.Ahmadi M, Samadbeik M, Sadoughi F. Modeling of outpatient prescribing process in Iran: A gateway toward electronic prescribing system. Iran J Pharm Res. 2014;13:725–38. [PMC free article] [PubMed] [Google Scholar]
- 22.Mäkinen M. Delivery of European Cross-Border Health Care and the Relevance and Effects of EU Regulations and Judicial Processes with Reference to Delivery of Medicines and Blood Donor Information Material. Turk: TURUN YLIOPISTO; 2008. [Last cited on 2015 Dec 18]. Available from: http://www.gao.gov/assets/320/315811.pdf . [Google Scholar]
- 23.Mäkinen M, Rautava P, Forsström J, Aärimaa M. Electronic prescriptions are slowly spreading in the European Union. Telemed J E Health. 2011;17:217–22. doi: 10.1089/tmj.2010.0111. [DOI] [PubMed] [Google Scholar]
- 24.Qureshi NA, Al-Dossari DS, Al-Zaagi IA, Al-Bedah AM, Abudalli AN, Koenig HG. Electronic Health Records, Electronic Prescribing and Medication Errors: A Systematic Review of Literature, 2000-2014. British J Med Med Res. 2015;6:672–704. [Google Scholar]
- 25.Salmivalli L, Hilmola OP. Business pluralism of electronic prescriptions: State of development in Europe and the USA. Int J Electron Healthc. 2006;2:132–48. doi: 10.1504/IJEH.2006.008828. [DOI] [PubMed] [Google Scholar]
- 26.Dean B, Schachter M, Vincent C, Barber N. Causes of prescribing errors in hospital inpatients: A prospective study. Lancet. 2002;359:1373–8. doi: 10.1016/S0140-6736(02)08350-2. [DOI] [PubMed] [Google Scholar]
- 27.Oliven A, Michalake I, Zalman D, Dorman E, Yeshurun D, Odeh M. Prevention of prescription errors by computerized, on-line surveillance of drug order entry. Int J Med Inform. 2005;74:377–86. doi: 10.1016/j.ijmedinf.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed Z, Barber N, Jani Y, Garfield S, Franklin BD. Economic impact of electronic prescribing in the hospital setting: A systematic review. Int J Med Inform. 2016;88:1–7. doi: 10.1016/j.ijmedinf.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Ammenwerth E, Schnell-Inderst P, Machan C, Siebert U. The effect of electronic prescribing on medicine errors and adverse medicine events: A systematic review. J U S Med Inf Assoc. 2008;15:585–600. doi: 10.1197/jamia.M2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kannry J. Effect of e-prescribing systems on patient safety. Mt Sinai J Med. 2011;78:827–33. doi: 10.1002/msj.20298. [DOI] [PubMed] [Google Scholar]
- 31.Qureshi NA, Al-Dossari DS, Al-Zaagi IA, Al-Bedah AM, Abudalli AN, Koenig HG. Electronic health records, electronic prescribingand medicine errors: A systematic review of literature, 2000-2014 systematic review. Br J Med Med Res. 2015;5:672. [Google Scholar]
- 32.eHealth Initiative. Electronic prescribing: Toward maximum value and rapid adoption: Recommendations for optimal design and implementation to improve care, increase efficiency and reduce costs in ambulatory care. Washington: The Electronic Prescribing Initiative; 2004. [Google Scholar]
- 33.Walker J, Pan E, Johnston D, Milsten J, Bates D, Middleton B. The value of computerized provider order entry in ambulatory settings. Boston, MA: Center for Information Technology Leadership; 2004. [Google Scholar]
- 34.Johnston D, Pan E, Walker J. The value of CPOE in ambulatory settings. J Healthc Inf Manag. 2004;18:5–8. [PubMed] [Google Scholar]
- 35.Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, et al. Medicine errors and adverse medicine events in pediatric inpatients. JAMA. 2001;285:2114–20. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- 36.Westerling A. Information technology development needs in community pharmacies: A strategic approach. Finland: Yliopistopaino University Press; 2011. [Last cited on 2015 Feb 12]. Available from: https://helda.helsinki.fi/bitstream/handle/10138/28196/informat.pdf . [Google Scholar]
- 37.Puustjärvi J, Puustjärvi L. The Challenges of Electronic Prescription Systems Based on Semantic Web Technologies. European Conference on eHealth, Proceedings of the ECEH'06; October 12-13, 2006; Fribourg, Switzerland. [Google Scholar]
- 38.Deetjen U. Europesn E-Prescriptions: Benefits and Success Factors. Oxford: University of Oxford; 2016. [Last cited on 2016 Jan 24]. (Working paper series No 5). Available from: http://www.politics.ox.ac.uk/materials/publications/15224/workingpaperno5ulrikedeetjen.pdf . [Google Scholar]
- 39.eHealth Observatory. eHealth Observatory ePrescribing Workflow Handbook v3.0. 2011. [Last accessed on 2016 Apr 19]. Available from: http://www.ehealth.uvic.ca/resources/tools/WorkflowModeling/2011.02.18-ePrescribing_Workflow_Handbook-v3.0.pdf .
- 40.Castro D. Explaining International Application Leadership: Health IT. Washington: Information Technology and Innovation Foundation; 2009. [Google Scholar]
- 41.DG INFSO. MedCom, Denmark – Danish Health Data Network. Denmark: European Commission Information Society and Media; 2006. [Google Scholar]
- 42.Doupi P, Renko E, Giest S, Dumortier J. Health. San Francisco: 2010. Oct, Country Brief: Denmark. [Google Scholar]
- 43.The RIDE Project. A Roadmap for Interoperability of eHealth Systems in Support of COM 356 with Special Emphasis on Semantic Interoperability. European Commission within the Sixth Framework Programme, IFOMIS. 2007. [Last cited on 2015 Feb 09]. Available from: http://www.srdc.com.tr/metu-srdc/projects/ride/
- 44.European Commission. Knowledge of the Progress of Health Professionals Cards in Europe, Deliverable 3: Mapping of Smart Cards, Identifiers and Frames of Reference of Health Professionals in the Member States. WP2, Project of Health Professional Card. 2009. [Last cited on 2015 Oct 15]. Available from: http://www.hprocard.eu/images/20091012-hpc-wp2-deliverable3.pdf .
- 45.Stroetmann KA, Artmann J, Stroetmann VN, Protti D, Dumortier J, Giest S, et al. European countries on their journey towards national eHealth infrastructures. Luxembourg: Office for Official Publications of the European Communities; 2011. [Google Scholar]
- 46.Thorp J. Europe's e-health initiatives. J AHIMA. 2010;81:56–8. [PubMed] [Google Scholar]
- 47.Christie T, Alami KM, King NE. Process implications of e-prescribing information integration models: United States versus a Middle East approach. E Serv J. 2008;5:15–38. [Google Scholar]
- 48.Friedman MA, Schueth A, Bell DS. Interoperable electronic prescribing in the United States: A progress report. Health Aff (Millwood) 2009;28:393–403. doi: 10.1377/hlthaff.28.2.393. [DOI] [PubMed] [Google Scholar]
- 49.King NE, Christie T, Alami KM. Process implications of e-prescribing information integration models: United States versus a Middle East approach. E Serv J. 2007;5:15–38. [Google Scholar]
- 50.Hyppönen H, Salmivalli L, Suomi R, editors. Organizing for a national infrastructure project: The case of the Finnish electronic prescription. System Sciences, 2005 HICSS’05 Proceedings of the 38th Annual Hawaii International Conference on; 2005. IEEE. [Google Scholar]
- 51.Gider Ö, Ocak S, Top M. Evaluation of electronic prescription implications in Turkey: An investigation of the perceptions of physicians. Worldviews Evid Based Nurs. 2015;12:88–97. doi: 10.1111/wvn.12082. [DOI] [PubMed] [Google Scholar]
- 52.Schectman JM, Schorling JB, Nadkarni MM, Voss JD. Determinants of physician use of an ambulatory prescription expert system. Int J Med Inform. 2005;74:711–7. doi: 10.1016/j.ijmedinf.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Medicare and Medicaid Services (CMS) E-Prescribing Measure. 2012. [Last accessed on 2015 Feb 15]. Available from: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ERxIncentive/E-Prescribing_Measure.html .
- 54.Kernan B. A Medicines and Medicines Reference Catalogue for e-Prescribing in Ireland. [master's thesis] Dublin: University of Dublin; 2011. [Google Scholar]
- 55.Suna T. Finnish national archive of health information (KanTa): General concepts and information model. FUJITSU Sci Tech J. 2011;47:49–57. [Google Scholar]
- 56.Barbarito F, Pinciroli F, Mason J, Marceglia S, Mazzola L, Bonacina S. Implementing standards for the interoperability among healthcare providers in the public regionalized healthcare information system of the Lombardy Region. J Biomed Inform. 2012;45:736–45. doi: 10.1016/j.jbi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 57.NHS Connecting for Health (NHS CFH). ePrescribing Functional Specification for NHS Trusts. Baselined v 1/0: Crown. 2007. [Last cited on 2015 Jan 17]. p. 142. Available from: www.connectingforhealth.nhs.uk/systemsandservices/eprescribing/baselinefunctspec.pdf .
- 58.Doupi P, Renko E, Hämäläinen P, Mäkelä M, Giest S, Dumortier J. Country Brief: Finland, e-Health Strategies Study. Finland: 2010. [Google Scholar]
- 59.Salmivalli L, Hypponen H, Nykanen P, Ruotsalainen P, Pajukoski M. Testing a theoretical framework for interdisciplinary IT evaluation: The case of finnish electronic prescription. Int J Healthcare Technol Manage. 2007;8:42–65. [Google Scholar]
- 60.Hibberd R, Barber N, Cornford T, Lichtner V. The evaluation of the electronic prescription service in primary care: Interim report on the findings from the evaluation in early implementer sites. UCL School of Pharmacy, The University of Nottingham, London School of Economics and Political Science. 2012. [Last cited on 2015 Feb 09]. Available from: http://www.birmingham.ac.uk/Documents/college-mds/haps/projects/cfhep/projects/004/CfHEP-004-Final-Report-27-Jan-2014.pdf .
- 61.Grepstad M, Kanavos P. A comparative analysis of coverage decisions for outpatient pharmaceuticals: Evidence from Denmark, Norway and Sweden. Health Policy. 2015;119:203–11. doi: 10.1016/j.healthpol.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 62.The National Progress Report on E-prescribing and Interoperable Healthcare. Virginia: Surescripts LLC; 2011. [Last cited 2015 Feb 11]. Surescripts. Available from: http://www.surescripts.com/docs/default-source/national-progress-reports/surescripts_2013_national_progress_report.pdf?sfvrsn=2 . [Google Scholar]
- 63.Inera. Sjunet – Quality Assured Communications. 2013. [Last cited 2012 Feb 12]. Available from: http://www.inera.se/Infrastrukturtjanster/Sjunet/
- 64.Protti D, Wright G, Treweek S, Johansen I. Primary care computing in England and Scotland: A comparison with Denmark. Inform Prim Care. 2006;14:93–9. doi: 10.14236/jhi.v14i2.619. [DOI] [PubMed] [Google Scholar]
- 65.Bell DS, O'Neill S, Reynolds K, Schoeff D. TR-941-CMS. Santa Monica, CA: Rand Corporation; 2011. [Last cited on 2015 Oct 15]. Evaluation of RxNorm in Ambulatory Electronic Prescribing. Available from: http://www.rand.org/pubs/technical_reports/TR941 . [PMC free article] [PubMed] [Google Scholar]
- 66.Mundy D, Chadwick DW, editors. A system for secure electronic prescription handling. Proceeding of the nd International Conference on the Management of Healthcare and Medical Technology.2002. [Google Scholar]


