Abstract
Mild palladium-catalyzed aminations of aryl tosylates and the first aminations of heteroaryl tosylates are described. In the presence of the combination of L2Pd(0) (L=P(o-tol)3) and the hindered Josiphos ligand CyPF-t-Bu, a variety of primary alkylamines and arylamines react with both aryl and heteroaryl tosylates at room temperature to form the corresponding secondary arylamines in high yields with complete selectivity for the monoarylamine. These reactions at room temperature occur in many cases with catalyst loadings of 0.1 mol % and 0.01 mol % in one case, constituting the most efficient aminations of aryl tosylates by nearly two orders of magnitude. This catalyst is make practical by the development of a convenient method to synthesize the L2Pd(0) precursor. This complex is stable to air as a solid. In contrast to conventional relative rates for reactions of aryl sulfonates, the reactions of aryl tosylates are faster than parallel reactions of aryl triflates, and the reactions of aryl tosylates are faster than parallel or competitive reactions of aryl chlorides.
Graphical abstract
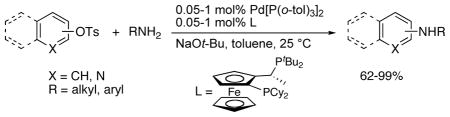
Palladium-catalyzed aminations of aryl halides occur under mild conditions with many catalysts,1–3 but aminations of aryl sulfonates have been more challenging. The coupling of amines with aryl triflates has been known for many years,1 but aryl triflates are expensive to prepare and less stable to hydrolysis than other aryl sulfonates. Aryl tosylates are less expensive to prepare and are stable, crystalline solids. However, they are also less reactive. Although Suzuki and Kumada couplings of aryl tosylates at room temperature have been reported,4,5a only one coupling of amines with aryl tosylates at room temperature or with low catalyst loadings has been reported.5 Moreover, the coupling of amines with heteroaryl tosylates has not been reported, and the couplings of primary amines with aryl tosylates are rare.
We previously reported the oxidative addition of aryl tosylates at room temperature,5a but this fast addition did not lead to a general coupling with amines under similar conditions. Here, we report a new method to create a catalyst that couples aryl tosylates with primary alkylamines, arylamines, and N-H imines with fast rates and high turnover numbers under mild conditions. This process was achieved by combining the hindered Josiphos ligand CyPF-t-Bu (Table 1) with the unconventional Pd(0) precursor Pd[P(o-tol)3]2, which we show can be made in a practical manner.
Table 1.
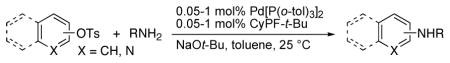
Coupling of Aryl and Heteroaryl Tosylates with Primary Alkylamines and Arylamines Catalyzed by Pd[P(o-tol)3]2 and CyPF-t-Bu (1:1)a
| ||||||
|---|---|---|---|---|---|---|
| entry | substrate | R | cat. (%) | time (h) | yield (%)b | |
| 1 |
|
X = OTs | octyl | 0.1 | 24 | 94 |
| 2c | OTs | octyl | 0.1 | 10 min | 97 | |
| 3 | OTf | octyl | 0.1 | 48 | 29 | |
| 4 | OMs | octyl | 0.1 | 24 | -d | |
| 5 | OSO2Ph-4-F | octyl | 0.1 | 48 | 76 | |
| 6 |
|
R1 = H | i Bu | 0.1 | 24 | 87 |
| 7 | H | sBu | 0.2 | 24 | 83 | |
| 8 | H | c Hex | 0.1 | 24 | 72 | |
| 9 | H | Bn | 0.1 | 24 | 90 | |
| 10 | H | 1- methylbenzyle | 1.0 | 24 | 95f | |
| 11 | H | PhNHC2H4NH2g | 0.5 | 24 | 96h | |
| 12 | H | Ph2C=NH | 0.1 | 48 | 91 | |
| 13 | Me | sBu | 0.2 | 24 | 99 | |
| 14 |
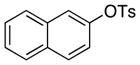
|
octyl | 0.05 | 24 | 97 | |
| 15c | octyl | 0.01 | 48 | 84 | ||
| 16 |
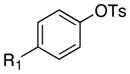
|
R1 = CN | octyl | 0.5 | 24 | 99 |
| 17i | CO2Et | octyl | 1.0 | 24 | 99 | |
| 18 | CO2tBu | octyl | 1.0 | 24 | 88 | |
| 19 | OMe | Bn | 0.5 | 24 | 87 | |
| 20 |
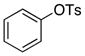
|
4-Me-C6H4 | 0.1 | 24 | 87 | |
| 21 | 4-OMe-C6H4 | 0.1 | 24 | 77 | ||
| 22 | 2-Me-C6H4 | 0.2 | 24 | 75 | ||
| 23j | 2-pyridyl | 1.0 | 24 | 71 | ||
| 24 | 3-OMe-5-CF3-C6H4 | 1.0 | 48 | 62(88k) | ||
| 25 |
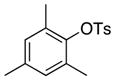
|
4-Me-C6H4 | 0.1 | 24 | 99 | |
| 26 |
|
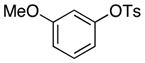
2-Me-C6H4 | 0.2 | 24 | 70 | |
| 27 |
|
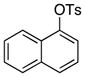
4-Me-C6H4 | 0.2 | 24 | 96 | |
| 28 |
|
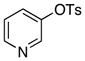
octyl | 0.5 | 24 | 90 | |
| 29 | 4-OMe-C6H4 | 1.0 | 48 | 63 | ||
| 30 |
|
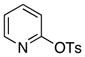
octyl | 1.0 | 24 | 80 | |
| 31 |
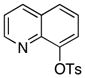
|
octyl | 1.0 | 24 | 82 | |
| 32 |
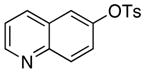
|
octyl | 0.2 | 24 | 91 | |
| 33 | i Bu | 0.2 | 24 | 97 | ||
| 34 | Bn | 0.2 | 24 | 87 | ||
| 35 | 4-Me-C6H4 | 1.0 | 24 | 78 | ||
Reactions conducted with a 1:1 ratio of metal to ligand 1 mmol Ar-OTs, 1.2 equiv amine and 1.4 equiv NaOt-Bu in 1 mL toluene.
Isolated yields are an average of two runs.
At 80 °C.
Phenol was formed in 23% yield.
From phenethylamine that is stated to be 99% ee.
99% ee.
1.5 equiv amine was used.
N,N′-diphenylethylenediamine was obtained as a single product.
Reaction conducted with 1.2 equiv K3PO4 at 100 °C.
At 110 °C.
2.0 mol % catalyst was used.
The difference between the rates of oxidative addition and the catalytic coupling stems from the difficulty in accessing the (CyPF-t-Bu)Pd intermediate under mild conditions. Typical catalyst precursors include Pd(II) acetate or chloride complexes, which require reduction to the true Pd(0) catalyst. To avoid the need for reduction to Pd(0), Pdn(dba)m is often used, but Pd(CyPF-t-Bu)(dba) generated from CyPF-t-Bu and Pd2(dba)3 dissociates dba particularly slowly, presumably because the strongly-donating bisphosphine leads to strong backbonding into the electron-poor dba ligand. Thus, an alternative precatalyst was needed to couple aryl tosylates with amines under mild conditions.
Instead of using the combination of CyPF-t-Bu and typical catalyst precursors, we sought a practical synthesis of Pd(CyPF-t-Bu)[P(o-tol)3] (1), which oxidatively adds aryl tosylates at room temperature.5a Pd(0) catalyst 1 is generated in solution at room temperature within minutes from Pd[P(o-tol)3]2 (2) and CyPF-t-Bu (3),6 but 2 has rarely been used in catalytic chemistry because of its lack of commercial availability and difficult synthesis.7 Thus, we sought a more convenient method to prepare 1 independently or a convenient method to prepare precursor 2.
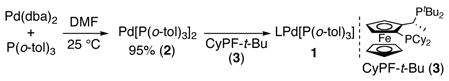 |
(1) |
Our efforts to isolate 1 in high yield were hampered by the high solubility of this species in organic solvents. Thus, we sought to simplify the synthesis of Pd(0) complex 2 and to use 1 generated in situ. Based on the most efficient synthesis of Pd[P(t-Bu)3]2,8 we prepared 2 in 95% isolated yield from the reaction of P(o-tol)3 and Pd(dba)2 in DMF.9 Complex 2 precipitated after 1 h at room temperature without the need for excess of P(o-tol)3. Complex 2 is stable for > 1 week in air as a solid at room temperature.10 Because CyPF-t-Bu is also stable in air, both catalyst components can be stored outside of a drybox, and a solid mixture of the two materials can mimic a single-component catalyst (vide infra).11
Examples of the reactions of aryl tosylates and heteroaryl tosylates with various alkylamines are summarized in Table 1. Reactions with 0.05–1.0 mol % of the combination of 2 and 3 in toluene at room temperature produced the corresponding monoary-lamines in excellent yields. Most reactions were complete at room temperature within 24 h. However, similar yields could also be obtained after short (<10 min) times at 80 °C.
Reactions of linear primary amines were fast and occurred in high yield with 0.05–0.1 mol % catalyst (entries 1, 6, 9, and 14). The reaction of octylamine with 2-naphthyl tosylate was particularly efficient and occurred at 80 °C in 84% isolated yield with only 0.01 mol % of catalyst (entry 15). This turnover number of 8,400 is the highest for any type of coupling of an aryl tosylate of which we are aware.12–14 The reactions of hindered α-branched primary amines, such as cyclohexylamine and sec-butylamine, occurred in high yields at room temperature with just 0.1 to 0.2 mol % of catalyst (entries 7–8, and 13). Both electron-rich and electron-poor aryl tosylates reacted in high yield with primary amines without formation of any diarylamine. These reactions occurred with aryl tosylates containing ortho substituents, as well as aryl tosylates containing cyano and carboalkoxy groups.
Because this new catalyst does not require reduction to a palladium(0) species or dissociation of dba, primary arylamines also couple with aryl tosylates at room temperature. Although these reactions were slightly slower than those of alkylamines, reactions of electron-rich arylamines (entries 20–21, 25, and 27), including an ortho-substituted arylamine (entries 22 and 26), occurred in good-to-excellent yield with complete selectivity for monoarylation. Reactions of electron-deficient primary aryl- and heteroarylamines also occurred. Although these reactions required higher catalyst loadings than the reactions of electron-rich primary arylamines (entries 23–24), they still occurred at elevated temperatures or with extended reaction times at room temperature.
The scope of the couplings of this catalyst also includes the first aminations of heteroaryl tosylates (entries 28–35). These reactions occurred under the same conditions we developed for the amination of aryl tosylates. Pyridyl and quinolinyl tosylates underwent reaction with primary alkyl and aryl amines in good-to-excellent yields. The reactions of 2-, and 3-pyridyl and 6-, and 8-quinolyl tosylate also occurred, although 0.5 and 1.0 mol % catalyst was needed in some cases.
Consistent with the high selectivity for reactions of primary amines, the reactions of secondary amines were slower. The reactions of aryl tosylates with morpholine, dibutylamine, and N-methylaniline gave no coupled products or low yield of products at 110 °C with 1 mol % catalysts.
A comparison of these reactions to those of aryl tosylates conducted with other precatalysts showed the value of initiating reactions with P(o-tol)3-ligated 2. No reaction of octylamine with phenyl tosylate catalyzed by 0.1 mol % Pd(dba)2 and 3 occurred at room temperature. The same reaction catalyzed by 0.1 mol % of Pd(OAc)2 and 3 at room temperature for 48 h gave the product in 6% yield, as determined by GC, and this reaction catalyzed by 0.1 mol % PdCl2(CyPF-t-Bu) occurred in 65% isolated yield. Indicating the importance of Josiphos ligand 3, the reaction of phenyl tosylate with octylamine catalyzed by the combination of 0.1 mol % Pd(OAc)2, Pd(dba)2, or Pd[P(o-tol)3]2 as precursor and 0.1 to 0.25 mol % Q-phos,15 X-phos,16,17 SIPr,3 DPPF, or BINAP as ligand gave no coupled products or very low yield of products, even in toluene at 110 °C (see supporting information for details).
Finally, a solid mixture of the two catalyst components catalyzes the reaction with efficiency equal to that of the catalyst generated from the two separate solids. Although expected, this procedure does allow the use of the combination of 2 and 3 as if it were an air-stable single-component catalyst.
A comparison of the reactions of different types of aryl sulfonates revealed some unusual trends (Table 1, entries 1–5). Most striking, reactions of aryl tosylates were faster than those of aryl triflates when conducted as separate reactions (c.f. Table 1, entry 1 vs entry 3). Reactions of octylamine with a 1:1 ratio of the two aryl sulfonates formed mostly phenol (14%) with only 4% yield of the coupled product in the presence of 0.1 mol % catalyst. Reactions with Cs2CO318 did not improve the yield from reactions of triflates. Second, aryl tosylates reacted faster than aryl chlorides. The reaction of 3- or 4-chlorophenyl tosylate led to coupling at the tosylate group (Eq. 2). These relative rates contrast those catalyzed by complexes of dialkyl-o-biarylphosphines,19 but parallel those catalyzed by complexes of secondary phosphine oxides.13
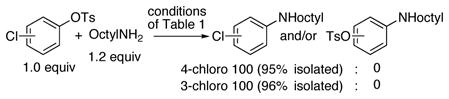 |
(2) |
In summary, we have developed a highly efficient catalyst system for the amination of aryl tosylates at room temperature, as well as the first examples of the Pd-catalyzed amination of heteroaryl tosylates. The use of an unusual precatalyst now prepared in a practical fashion enabled us to achieve this high activity.
Supplementary Material
Acknowledgments
We thank the NIH (GM-55382) for support of this work, Johnson-Matthey for a gift of PdCl2, and Solvias for a gift of CyPF-t-Bu. TO thanks JSPS for fellowship.
Footnotes
Supporting Information Available: All experimental procedures and spectroscopic data of new compounds. This material is free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Hartwig JF. Acc Chem Res ASAP. doi: 10.1021/ar800098p. [DOI] [Google Scholar]; (b) Hartwig JF. In: Modern Arene Chemistry. Astruc D, editor. Wiley-VCH; Weinheim: 2002. p. 107. [Google Scholar]; (c) Hartwig JF. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E, editor. Wiley-Interscience; New York: 2002. p. 1051. [Google Scholar]; (b) Muci AR, Buchwald SL. Top Curr Chem. 2002;219:131. [Google Scholar]
- 2.For a recent review of aminations conducted with o-biaryl diarylphosphine ligands, see: Surry DS, Buchwald SL. Angew Chem Int Ed. 2008;47:6338. doi: 10.1002/anie.200800497.
- 3.For a recent report of aminations conducted with N-heterocyclic carbenes, see: Marion N, Navarro O, Mei J, Stevens ED, Scott NM, Nolan SP. J Am Chem Soc. 2006;128:4101. doi: 10.1021/ja057704z.
- 4.(a) Tang ZY, Hu QS. J Am Chem Soc. 2004;126:3058. doi: 10.1021/ja038752r. [DOI] [PubMed] [Google Scholar]; (b) Tang ZY, Spinella S, Hu QS. Tetrahedron Lett. 2006;47:2427. [Google Scholar]; (c) Kobayashi Y, Mizojiri R. Tetrahedron Lett. 1996;37:8531. [Google Scholar]; (d) Limmert ME, Roy AH, Hartwig JF. J Org Chem. 2005;70:9364. doi: 10.1021/jo051394l. [DOI] [PubMed] [Google Scholar]; (e) Fürstner A, Leitner A, Mendez M, Krause H. J Am Chem Soc. 2002;124:13856. doi: 10.1021/ja027190t. [DOI] [PubMed] [Google Scholar]; (f) Fürstner A, Leitner A. Angew Chem Int Ed. 2002;41:609. [Google Scholar]
- 5.Roy AH, Hartwig JF. J Am Chem Soc. 2003;125:8704. doi: 10.1021/ja035835z.for two examples of the amination of aryl tosylates at elevated temperatures with a related catalyst, see: Hamann BC, Hartwig JF. J Am Chem Soc. 1998;120:7369.for recent work on the amination of aryl mesylates, at elevated temperatures, see: So CM, Zhou Z, Lau CP, Kwong FY. Angew Chem Int Ed. 2008;47:6402. doi: 10.1002/anie.200802157.
- 6.Ligand 3 is commercially available from Johnson-Matthey.
- 7.(a) Paul F, Patt J, Hartwig JF. Organometallics. 1995;14:3030. [Google Scholar]; (b) Böhm VPW, Herrmann WA. Chem Eur J. 2001;7:4191. doi: 10.1002/1521-3765(20011001)7:19<4191::aid-chem4191>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Dai C, Fu GC. J Am Chem Soc. 2001;123:2719. doi: 10.1021/ja003954y. [DOI] [PubMed] [Google Scholar]
- 9.Pd(0) complex 2 is now commercially available from Johnson-Matthey.
- 10.Samples left in air as a solid for one week were unchanged, as judged by 1H NMR and 31P NMR spectroscopy.
- 11.Naber JR, Buchwald SL. Adv Synth Catal. 2008;350:957. [Google Scholar]
- 12.For reactions of activated aryl tosylates with 0.5 mol % palladium, see references 13 and 14.
- 13.Ackermann L, Althammer A. Org Lett. 2006;8:3457. doi: 10.1021/ol061116o. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Meng T, Wu J. J Org Chem. 2007;72:9346. doi: 10.1021/jo7019064. [DOI] [PubMed] [Google Scholar]
- 15.Shelby Q, Kataoka N, Mann G, Hartwig JF. J Am Chem Soc. 2000;122:10718. [Google Scholar]
- 16.Huang X, Anderson KW, Zim D, Jiang L, Klapars A, Buchwald SL. J Am Chem Soc. 2003;125:6653. doi: 10.1021/ja035483w. [DOI] [PubMed] [Google Scholar]
- 17.Reaction with 2 mol % Pd(OAc)2 and 5 mol % X-phos at 110 °C for 18 h occurred to 84% conversion.
- 18.Åhman J, Buchwald SL. Tetrahedron Lett. 1997;38:6363. [Google Scholar]
- 19.Nguyen HN, Huang X, Buchwald SL. J Am Chem Soc. 2003;125:11818. doi: 10.1021/ja036947t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


