Abstract
Objective
Several haemostatic agents are available for clinical use. Ankaferd Blood Stopper® (ABS), a mixture of five medicinal plant extracts, has been used historically as a haemostatic agent. The aim of this in vivo study was to investigate the effects of ABS on early bone healing using a rat tibia defect model.
Material and Methods
Sixteen male Wistar rats were randomized into two groups of 8 animals each. After deep anesthesia with ketamine, bone defects (3 mm diameter and 2 mm deep) were created in the right and left tibiae of all animals and either treated with 1 cc of ABS (Group 1) or left untreated (Group 2; control). Surgical areas were closed primarily. The animals were sacrificed on the 7th postoperative day and bone samples were collected from the tibias. The samples were examined histopathologically for infection, necrosis, fibrosis, new bone formation and foreign body reaction. The histomorphometric results were analyzed statistically by the chi square test, with the level of significance set at p<0.05.
Results
Significant differences were found in both groups in terms of inflammation, necrosis and new bone formation (p=0.001, p=0.0001, p=0.001). No foreign body reaction was observed in the experimental group. ABS application decreased fibrosis in the experimental group, but there were no statistically significant differences from the control group.
Conclusions
Histopathologically, it was observed that the application of ABS decreased the occurrence of inflammation and necrosis, while increasing new bone formation in early bone healing period. Further in vitro and in vivo studies are necessary for evaluating the benefits and possible adverse effects of the application of this herbal product on wound healing.
Keywords: Ankaferd Blood Stopper® (ABS), Thymus vulgaris, Glycyrrhiza glabra, Vitis vinifera, Alpinia officinarum, Urtica dioica, Herbal medicines, Bone healing
INTRODUCTION
Bleeding can cause significant morbidity and mortality in clinical settings. Several haemostatic agents have been investigated for their role in haemostasis6,9,11,13.
Ankaferd Blood Stopper® (ABS; Ankaferd Health Products Ltd., Istanbul, Turkey) is a traditional folk medicinal plant extract product that has been approved in the management of external hemorrhage and dental surgery bleedings in Turkey. ABS comprises a standardized mixture of the plants Thymus vulgaris, Glycyrrhiza glabra, Vitis vinifera, Alpinia officinarum and Urtica dioica. Several studies have shown that each of these plants has some effect on the endothelium, blood cells, angiogenesis, cellular proliferation, vascular dynamics and cell mediators2,3,7,8,10,12.Göoker, et al.5 (2008) investigated the haemostatic effects of ABS and reported its therapeutic potential to be used for the management of haemorrhage5.
Although clinical and in vivo studies have been reported about different haemostatic agents that are commonly used for the management of hemorrhage in clinical dentistry6,13, there is no evidence about the effects of ABS in vivo experimental models. The aim of this in vivo study was to investigate the effects of Ankaferd Blood Stopper® on early bone healing using a rat tibia defect model.
MATERIAL AND METHODS
Animals and Surgery
The study was carried out in the Istanbul University, Faculty of Dentistry, Department of Oral & Maxillofacial Surgery and Institute of Oncology, Department of Tumor Pathology & Cytology. Treatment of the experimental animals was approved by the Istanbul University Animal Research and ethics Committee. experimental animals were obtained from The Laboratory of experimental Animals, DeTAM, Istanbul, Turkey.
A total of 16 twenty-week-old male Wistar rats weighing 250 to 300 g were used in this study and randomly assigned to two groups of 8 animals each. Prior to surgery, the animals were anesthetized with a 0.7 mL intramuscularly injection of a solution containing xylazine hydrochloride (Rompun®, Bayer, Leverkusen, Germany) and ketamine hydrochloride (Ketalar; Pfizer, New York, NY USA) at 1/0.5 proportion, 0.1 mL/100 g body weight. Surgery was performed under sterile conditions.
In the mid tibia of rats, a 5-mm long straight longitudinal skin incision was done on the front skin and, after muscle splitting (plane-by-plane muscle dissection), the periosteal membrane was stripped away to expose bone surface. A standardized ellipsoid round bone defect (5 mm in length, 1 mm in height, 1 mm in depth) was created at the anterior portion of the diaphysis of bilateral tibias, 6 mm below the knee joint using a round carbide bur (SS White, Lakewood, NJ, USA). The defect size was confirmed by a surgical stainless steel stent with the corresponding dimensions. The surgical stent was placed in the defect and confirmed visually by checking congruity to the defect wall.
Group 1 received 1 cc of Ankaferd Blood Stopper® at the time of surgery, while Group 2 received no treatment and served as the control. The muscles were sutured with 4/0 catgut (Doğsan, Istanbul, Turkey), and the flaps were carefully repositioned and sutured with 3/0 black silk sutures (Doğsan, Istanbul, Turkey). Antibiotic (Sefazol, Mustafa Nevzat, Turkey) was given to the animals as an intramuscular injection intraoperatively and during 3 days postoperatively. No postoperative complications were noticed during the postsurgical course. All animals survived throughout the study period.
The rats of each group were housed into separate cages with two or three animals under climate-controlled conditions (12 h light/12 h dark; thermostatically regulated room temperature) without any restriction of mobilization. The animals of each group were sacrificed with an overdose of ketamine hydrochloride (50 mg/kg) on the 7th day after surgery, and the defects together with surrounding bone were immediately removed for histopathological analysis.
Tissue Preparation and histopathological Examination
The specimens were fixed in 10% neutral buffered formalin overnight at 4°C, rinsed in phosphate buffered saline and decalcified in 20% formic acid solution (Merck, Darmstadt, Germany) for 10 days. The decalcified specimens were embedded in paraffin and cut into 20 semi-serial sections using a microtome (Leica Microsystemic, Germany), and routine hematoxylin and eosin (He) staining and Mallory Trichrome staining were performed. The sections were examined with light microscope under 40, 100 and 200x magnification (Nikon eclipse e600, Japan). A histomorphological review was performed by a single blinded oral pathologist to evaluate the presence of infection, necrosis, fibrosis, new bone formation, and foreign body reaction. The scores for infection, necrosis, fibrosis and new bone formation scores were determined by counting the associated cells and their ratio to the total cell count in a standardized area at 40x magnification. The ratio of cells between 0-25% was scored as none, 25-50% as slight, 5075% as moderate, and 75-100% as advanced.
Statistical analysis
The statistical differences between the control and test groups were compared by chi square test using the GraphPad Prisma V.3 (GraphPad Software, Inc., USA) and the critical level of significance was P < 0.05.
RESULTS
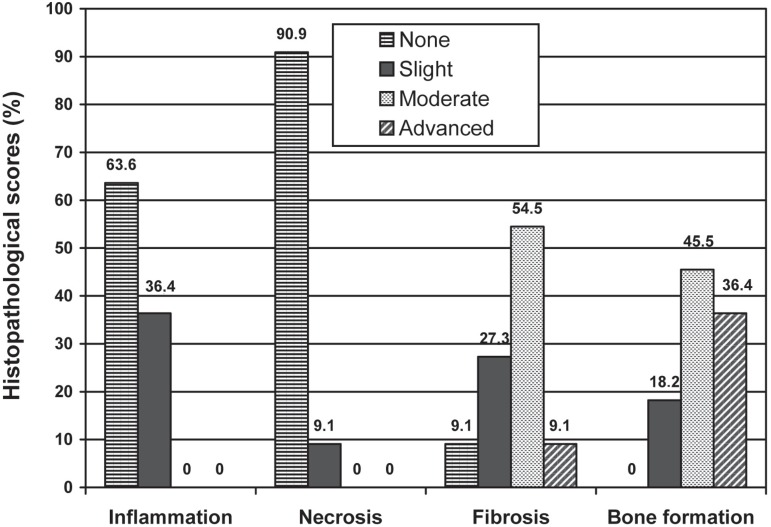
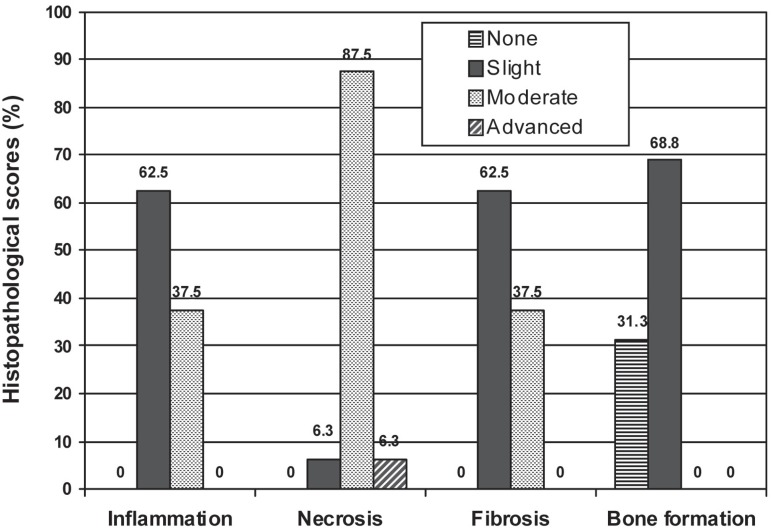
The scores and percentages of inflammation, necrosis, fibrosis, and new bone formation in both groups are presented in Table 1 and illustrated in Figures 1 and 2. Comparisons between the test and control groups indicate a significant variability in the scores of inflammation, necrosis and new bone formation (p<0.001, p<0.0001, p<0.001, respectively). No foreign body reactions were seen in either of the groups.
Table 1.
Inflammation, necrosis, fibrosis and bone formation scores in the test and control groups
| Test group | Control group | |||
|---|---|---|---|---|
| Inflammation | % | n | % | n |
| None | 63.6 | 7 | 0.0 | 0 |
| Slight | 36.4 | 4 | 62.5 | 10 |
| Moderate | 0.0 | 0 | 37.5 | 6 |
| Advanced | 0.0 | 0 | 0.0 | 0 |
| X2:15.16 p=0.001 | ||||
| Test group | Control group | |||
| Necrosis | % | n | % | n |
| None | 90.9 | 10 | 0.0 | 0 |
| Slight | 9.1 | 1 | 6.3 | 1 |
| Moderate | 0.0 | 0 | 87.5 | 14 |
| Advanced | 0.0 | 0 | 6.3 | 1 |
| X2:24.92 p=0.0001 | ||||
| Test group | Control group | |||
| Fibrosis | % | n | % | n |
| None | 9.1 | 1 | 0.0 | 0 |
| Slight | 27.3 | 3 | 62.5 | 10 |
| Moderate | 54.5 | 6 | 37.5 | 6 |
| Advanced | 9.1 | 1 | 0.0 | 0 |
| X2:5.01 p=0.171 | ||||
| Test group | Control group | |||
| Bone formation | % | n | % | n |
| None | 0.0 | 0 | 31.3 | 5 |
| Slight | 18.2 | 2 | 68.8 | 11 |
| Moderate | 45.5 | 5 | 0.0 | 0 |
| Advanced | 36.4 | 4 | 0.0 | 0 |
| X2:19.99 p=0.0001 | ||||
Figure 1.
Comparison of inflammation, necrosis, fibrosis and bone formation scores in test group in percentage
Figure 2.
Comparison of inflammation, necrosis, fibrosis and bone formation scores in control group in percentage
In the control group, 62.5% and 37.5% of the specimens showed slight and moderate inflammation, respectively. In the test group, 36.4% of the specimens showed slight inflammation, while 63.6% of them were free of inflammation. Statistically significant differences (p<0.001) were found between the test and control groups.
There was statistically significant difference between the groups as for the necrosis scores (p<0.0001). In the test group, 90.9% of the specimens did not show necrosis, while in the control group slight, moderate and advanced necrosis was observed in 6.3%, 87.5% and 6.3% of the specimens, respectively.
Both groups showed similar range of fibrosis scores with no statistically significant difference (p= 0.171) between them.
The results showed that bone formation scores were significantly higher in the test group than in the control group (p<0.0001). In the group treated with ABS, slight, moderate and advanced bone formation was observed in 18.2%, 45.5% and 36.4% of the specimens, respectively.
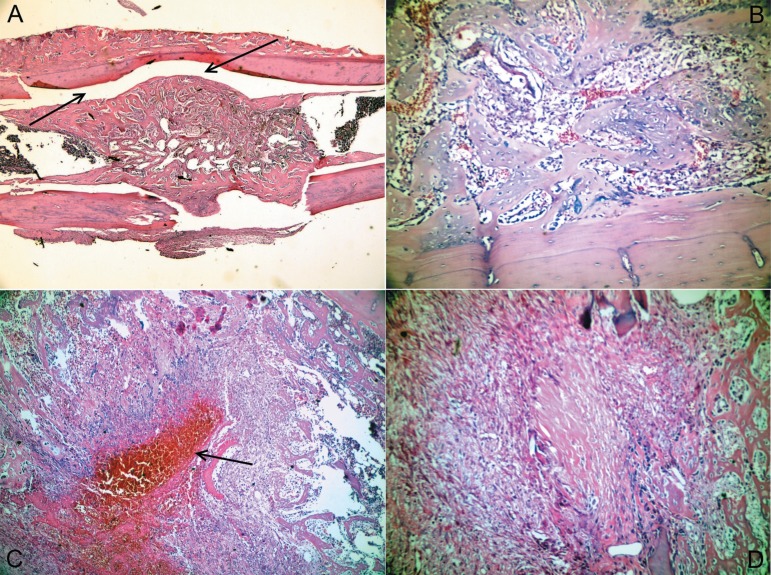
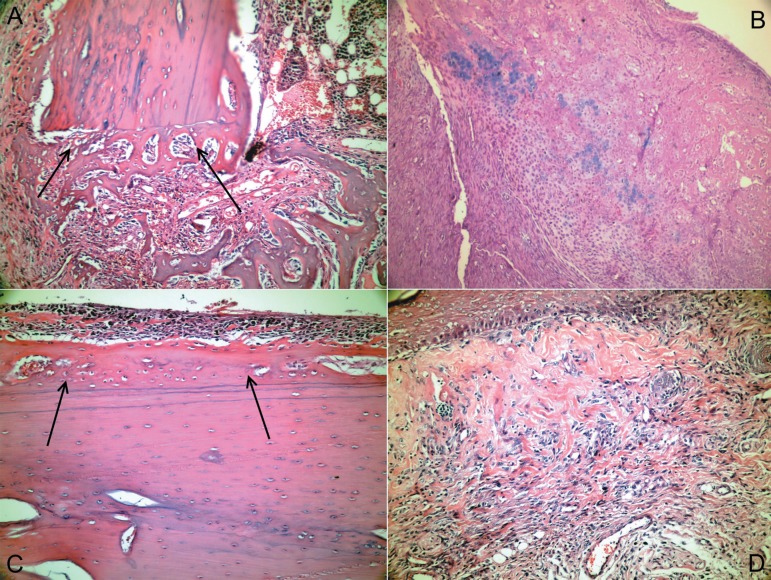
Representative histological sections of various specimens in the test group that showed decreased inflammation and necrosis, and increased new bone formation in early bone healing period are illustrated in Figures 3 and 4.
Figure 3.
a) Non-remodeled newly formed bone tissue covering the defect area and filling the medullar space (Hematoxylin- Eosin (HE) x40). (b) Numerous new bone trabeculae in vessel-rich loose connective tissue at the medullar space (H&E x200). (c) New bone trabeculae surrounding an organizing hematoma (HE x100). (d) New bone formation areas and mild lymphocyte infiltration in an active fibrous tissue formed by mesenchymal cells (HE x200)
Figure 4.
(a) New bone tissue layer separated from the defect wall by apposition lines, which is located in the loose connective tissue with mild inflammatory cell infiltration at the apex of the defect fragment (Hematoxylin-Eosin (HE) x200). (b) Areas of endochondral ossification in an active connective tissue at the defect area (HE x200). (c) Subperiostal ossification and osteoprogenitor cells that are proliferated and transforming into osteoblasts underneath the periosteum, which is traumatized near the defect area (HE x200). (d) Vessel-rich active connective tissue formed by fusiform cells underneath the surface epithelium (HE x200).
DISCUSSION
Bleeding can cause significant morbidity and mortality in any clinical setting. Bleeding management has been studied extensively and various haemostatic agents are available for clinical use6,13.
ABS is a folkloric medicinal plant extract product, which has historically been used in Turkish traditional medicine as a haemostatic agent. It is a standardized mixture of the plants T. vulgaris, G. glabra, V. vinifera, A. officinarum and U. dioica, each of which has some effect on hematological and vascular parameters, and cellular proliferation2,3,7,8,10,12. each ingredient of this mixture has specific characteristics. G. glabra inhibits angiogenesis, decreases vascular endothelial growth factor production and cytokineinduced neovascularization. G. glabra also has antiinflammatory, anti-thrombin, antiplatelet, antioxidant, anti-atherosclerotic, and antitumor activities10. T. vulgaris has been shown to exhibit varying levels of anti-oxidant activity, which may help to prevent in vivo oxidative damage, such as lipid peroxidation, associated with atherosclerosis7. Inoculation experiments on detached leaves of V. vinifera exhibited enhanced resistance towards pathogens2,3. V. vinifera also has anti-atherosclerotic and antitumor effects14,15. A. officinarum inhibits nitric oxide production in lipopolysaccharide activated mouse peritoneal macrophages8. U. dioica can produce hypotensive responses through a vasorelaxation effect mediated by the release of endothelial nitric oxide and the opening of potassium channels, and through a negative inotropic action12.
Goker, et al.5 (2008) showed that the ABSinduced network formation is related to the functions of blood proteins and red blood cells. The basic mechanism of action for ABS appears to be the formation of an encapsulated protein network that provides focal points for erythrocyte aggregation. Blood cells (erythrocytes and platelets) also aggregated and participated in the network formation, with the erythrocytes forming a mass. exposure to ABS seems to provide a tissue oxygenation as well as a physiological haemostatic process without affecting any individual clotting factor. This unique mechanism of action provides ABS with an advantage over other haemostatically active plant extracts1,4.
The histopathological results of the present study showed that over sixty percent of the defects treated with ABS were free of inflammation, which is probably related to the antiinflammatory activity of some components of the haemostatic agent. Although the occurrence of fibrosis was statistically similar in both groups, the ABS-treated group showed lower fibrosis rate than the non-treated control group, which may be attributed to the increased speed of healing in the test group.
The defects treated with ABS also showed more intense new bone formation and less occurrence of necrosis, which may be related to the increased speed of healing and decreased inflammation which is associated with antioxidant activity of the components of the ABS.
CONCLUSION
Within the limitations of this study, the following conclusions were drawn: 1. ABS decreased the inflammation and necrosis process; 2. ABS increased the new bone formation in early bone healing period; 3. No foreign body reaction to ABS was observed; 4. Further in vitro and in vivo studies are necessary to assess benefits and possible adverse effects of the application of Ankaferd Blood Stopper® on wound healing.
REFERENCES
- 1.Adachihara A. Oral treatment of hemophilia A using traditional kanpo medicine, Huang-lienchieh-tu-tang (plant extract) Haemostasis. 1983;13:78–82. doi: 10.1159/000214707. [DOI] [PubMed] [Google Scholar]
- 2.Barka EA, Belarbi A, Hachet C, Nowak J, Audran JC. Enhancement ofin vitrogrowth and resistance to gray mould of Vitis vinifera co-cultured with plant growth-promoting rhizobacteria. FEMS Microbiol Lett. 2000;186:91–95. doi: 10.1111/j.1574-6968.2000.tb09087.x. [DOI] [PubMed] [Google Scholar]
- 3.Barka EA, Gognies S, Nowak J, Audran JC, Belarbi A. Inhibitory effect of endophyte bacteria on Botrytis cinerea and its influence to promote the grapevine growth. Biol Control. 2002;24:135–142. [Google Scholar]
- 4.Gao J, Hooker BS, Anderson DB. Expression of functional human coagulation factor XIII A-domain in plant cell suspensions and whole plants. Protein Expr Purif. 2004;37:89–96. doi: 10.1016/j.pep.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Goker H, Haznedaroglu IC, Ercetin S, Kirazli S, Akman U, Ozturk Y, et al. Haemostatic actions of the folkloric medicinal plant extract Ankaferd Blood Stopper(r) J Int Med Res. 2008;36:163–170. doi: 10.1177/147323000803600121. [DOI] [PubMed] [Google Scholar]
- 6.Kozen BG, Kircher SJ, Henao J, Godinez FS, Johnson AS. An alternative hemostatic dressing: comparison of CeLOX, HemCon, and QuikClot. Acad emerg Med. 2008;15:74–81. doi: 10.1111/j.1553-2712.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Umano K, Shibamoto T, Lee KG. Identification of volatile components in basil ( Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L. ) and their antioxidant properties. Food Chem. 2007;91:131–137. [Google Scholar]
- 8.Matsuda H, Ando S, Kato T, Morikawa T, Yoshikawa M. Inhibitors from the rhizomes of Alpinia officinarum on production of nitric oxide in lipopolysaccharide-activated macrophages and the structural requirements of diarylheptanoids for the activity. Bioorg Med Chem. 2006;14:138–142. doi: 10.1016/j.bmc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Pusateri AE, Modrow He, Harris RA, Holcomb JB, Hess JR, Mosebar RH, et al. Advanced hemostatic dressing development program: animal model selection criteria and results of a study of nine hemostatic dressings in a model of severe large venous hemorrhage and hepatic injury in Swine. J Trauma. 2003;55:51826–51826. doi: 10.1097/01.TA.0000075336.92129.27. [DOI] [PubMed] [Google Scholar]
- 10.Sheela ML, Ramakrishna MK, Salimath BP. Angiogenic and proliferative effects of the cytokine VEGF in ehrlich ascites tumor cells is inhibited by Glycyrrhiza glabra. Int Immunopharmacol. 2006;6:494–498. doi: 10.1016/j.intimp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Sondeen JL, Pusateri AE, Coppes VG, Gaddy CE, Holcomb JB. Comparison of 10 different hemostatic dressings in an aortic injury. J Trauma. 2003;54:280–285. doi: 10.1097/01.TA.0000037431.19185.B4. [DOI] [PubMed] [Google Scholar]
- 12.Testai L, Chericoni S, Calderone V, Nencioni G, Nieri P, Morelli I, et al. Cardiovascular effects of Urtica dioica L. (Urticaceae) roots extracts:in vitro andin vivo pharmacological studies. J Ethnopharmacol. 2002;81:105–109. doi: 10.1016/s0378-8741(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 13.Wedmore I, McManus JG, Pusateri Ae, Holcomb JB. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60:655–658. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 14.Yamakoshi J, Kataoka S, Koga T, Ariga T. Proanthocyanidinrich extract from grape seeds attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 1999;142:139–149. doi: 10.1016/s0021-9150(98)00230-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Wang J, Chen Y, Agarwal R. Anti-tumor promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation-promotion protocol and identification of procyanidin B5-3'-gallate as the most effective antioxidant constituent. Carcinogenesis. 1999;20:1737–1745. doi: 10.1093/carcin/20.9.1737. [DOI] [PubMed] [Google Scholar]