Abstract
Zinc (Zn) deficiency caused by inadequate dietary intake is a global nutritional problem, particularly in developing countries. Therefore, zinc biofortification of wheat and other cereal crops is being urgently addressed and highly prioritized as a research topic. A field study was planned to evaluate the influence of zinc application on grain yield, grain zinc content, and grain phytic acid concentrations of wheat cultivars, and the relationships between these parameters. Three wheat cultivars, C1 = Faisalabad-2008, C2 = Punjab-2011, and C3 = Millet-2011 were tested with five different methods of zinc application: T1 = control, T2 = seed priming, T3 = soil application, T4 = foliar application, and T5 = soil + foliar application. It was found that grain yield and grain zinc were positively correlated, whereas, grain phytic acid and grain zinc were significantly negatively correlated. Results also revealed that T5, T3, and T4 considerably increased grain yield; however, T2 only slightly enhanced grain yield. Grain zinc concentration increased from 33.1 and 33.7 mg kg−1 in T1 to 62.3 and 63.1 mg kg−1 in T5 in 2013–2014 and 2014–2015, respectively. In particular, T5 markedly decreased grain phytic acid content; however, maximum concentration was recorded in T1. Moreover, all the tested cultivars exhibited considerable variation in grain yield, grain zinc, and grain phytic acid content. In conclusion, T5 was found to be most suitable for both optimum grain yield and grain biofortification of wheat.
Keywords: zinc deficiency, zinc application methods, grain zinc contents, grain phytic acid, biofortification
Introduction
Zinc (Zn) is an essential micronutrient in biological metabolism, and is receiving growing attention around the globe because of increasing reports of zinc deficiency in food crops as well as in humans (Alloway, 2004; Hotz and Brown, 2004; Cakmak, 2008). Zinc is required for normal growth and development of humans and plants (Hafeez et al., 2013). Moreover, it affects multiple aspects of the immune system (Shankar and Prasad, 1998) and is required for normal development and proper function of cell mediating immunity, neutrophils, and natural killer cells (Prasad, 2008). Similarly, in plants, zinc plays a crucial role in enzymatically driven metabolism (Tisdale et al., 1984). It also makes a notable contribution toward gene expression, stress tolerance (Cakmak, 2000), and pollen tube formation (Pandey et al., 2006).
Zinc deficiency is among the top five micronutrient deficiencies and severely affects one-third of the world’s population, especially rural communities (Hotz and Brown, 2004; Stein, 2010). Inadequate intake of food low in zinc content is a major contributor to the prevalence of zinc deficiency in humans. As one of the commonest cereal crops, wheat contributes to the provision of daily calories, proteins, and bioavailable micronutrients. In many developing nations, wheat provides over 50% of the daily calorific intake (Cakmak, 2008).
An excessive intake of monotonous wheat products is a major reason for zinc malnutrition in humans because wheat is inherently low in zinc content and high in phytate, which further limits zinc bioavailability (Welch and Graham, 2004; Cakmak et al., 2010b). Different reports are available indicating that more than 50% of wheat around the globe is cultivated on zinc-deficient soils (Alloway, 2004; Cakmak, 2008), which further lowers grain zinc content. The adoption of high-yielding cultivars seems to have aggravated this problem (Zhao and McGrath, 2009; Cakmak et al., 2010b; Stein, 2010). Furthermore, wheat processing after harvesting markedly decreases grain zinc and micronutrients such as iron, which enhances the chance of zinc deficiency in humans (Cakmak, 2008; Zhang et al., 2010b; Kutman et al., 2011). Hence, there is an urgent challenge and dire need to increase grain zinc content and bioavailability in developing countries (Welch and Graham, 2004; Cakmak, 2008; Zhao and McGrath, 2009).
In response to the aforementioned problem, different approaches have been suggested and applied in developing nations (Bouis, 2003; Pfeiffer and McClafferty, 2007), where the biofortification of cereals with important micronutrients is receiving a great deal of attention (Cakmak, 2008; Zhao and McGrath, 2009; Bouis and Welch, 2010). Key tools in biofortification include breeding and agronomic techniques such as fertilizer application. Breeding techniques are prime, and there are long-term strategies to deal with micronutrient malnutrition through evolving new genotypes with higher grain nutrient content (Welch and Graham, 2004; Bouis et al., 2011). However, breeding techniques take time and are costly, so agronomic techniques may provide a quicker solution to the micronutrient malnutrition problem. Agronomic techniques involve fertilizer application by seed priming or soil and foliar application. Moreover, the fertilization approach is a quick and complementary strategy, which maintains and builds a pool of zinc for translocation and uptake (Cakmak, 2008). Zinc has moderate phloem mobility (Haslett et al., 2001), so its application as a foliar feed alone or as a combination of soil plus foliar application markedly increases grain zinc content (Cakmak, 2008). Furthermore, grain zinc concentration is severely affected by the availability of a physiological pool of zinc in vegetative tissues as a result of foliar application (Cakmak et al., 2010a); the latter can substantially increase zinc concentration in wheat endosperm (Cakmak et al., 2010a; Zhang et al., 2010a). On the other hand, soil application of zinc is less effective in increasing grain zinc concentration because of poor zinc mobility and its rapid absorption in alkaline calcareous soils (Alloway, 2008). Furthermore, zinc application substantially reduces grain phytic acid concentration, which is widely used as an indicator of zinc bioavailability in diets (Erdal et al., 2002; Cakmak et al., 2010b). Therefore, agronomic biofortification through fertilization is the most valuable approach for combatting zinc malnutrition.
Zinc is an active nutrient and has antagonisms [phosphorus (Mousavi, 2011), copper (Imtiaz et al., 2003), and cadmium (Moustakas et al., 2011) and synergisms [iron (Mousavi, 2011) and boron (Rengel et al., 1998)]. Higher phosphorus levels in soil reduce zinc concentrations in plant aerial parts and also reduce total zinc content; similarly, phosphorus exerts P-Zn antagonism in plants (Singh et al., 1986). Phytic acid binds nutritionally important minerals such as zinc and impairs their biological utilization. Thus, a high concentration of phytic acid in cereal-based foods is a major cause of zinc deficiency in humans (Gibson et al., 1997). To combat this, the application of zinc substantially reduces grain phytic acid content and increases zinc bioavailability, as shown in soybean after enhanced zinc supply (Raboy and Dickinson, 1984). In most cases, there is an inverse relationship between grain yield and grain zinc concentration (Garvin et al., 2006; McDonald et al., 2008) with higher grain zinc concentrations being most commonly associated with lower yielding genotypes (Oury et al., 2006; Fan et al., 2008; McDonald et al., 2008). Moreover, some studies reveal that grain yield increases simultaneously, along with a remarkable increase in grain zinc concentration, as shown in Pakistan (Zou et al., 2012), China (Karim et al., 2012), and Turkey (Yilmaz et al., 1997). Thus, this study aimed to address the following questions: (1) what is the influence of zinc application method on grain yield, grain zinc concentration, and grain phytic acid concentration of wheat, (2) what is the relationship between grain zinc concentration and grain yield, and (3) what is the relationship between grain zinc and grain phytic acid content?
Materials and Methods
Experimental Site and Planting Material
The experiment was conducted at the Agronomic Research Farm, University of Agriculture, Faisalabad, Pakistan, during the winter seasons (November to April) of 2013–2014 and 2014–2015. The temperature of this region ranges between −1°C in January and 48°C in June, with a mean annual rainfall of around 200–250 mm. The prevailing conditions during both years are presented in Tables 1A,B. Seeds from three wheat cultivars, Faisalabad-2008, Punjab-2011, and Millet-2011 were obtained from the Wheat Research Institute, Ayub Agricultural Research Institute, Faisalabad, Pakistan.
Table 1A.
Prevailing climatic conditions for the experimental site during crop growing seasons for the years 2013–2014.
| Months | Rainfall (mm) | Monthly mean maximum temperature (°C) | Monthly mean minimum temperature (°C) | Monthly average temperature (°C) | Relative humidity (%) |
|---|---|---|---|---|---|
| November-13 | 0.5 | 26.1 | 11.8 | 19 | 59.4 |
| December-13 | 0 | 20.5 | 8.4 | 8.2 | 66.5 |
| January-14 | 0 | 19.1 | 6.1 | 12.6 | 63.8 |
| Feburary-14 | 14.3 | 20 | 8.9 | 14.4 | 65 |
| March-14 | 41.7 | 24.7 | 13.6 | 19.2 | 60.1 |
| April-14 | 28.2 | 32.2 | 18.6 | 25.4 | 52.2 |
Table 1B.
Prevailing climatic conditions for the experimental site during crop growing seasons for the years 2014–2015.
| Months | Rainfall (mm) | Monthly mean maximum temperature (°C) | Monthly mean minimum temperature (°C) | Monthly average temperature (°C) | Relative humidity (%) |
|---|---|---|---|---|---|
| November-14 | 10 | 26.3 | 11.5 | 18.9 | 61.7 |
| December-14 | 0 | 18.5 | 5.9 | 12.2 | 75 |
| January-15 | 12.2 | 16.6 | 6.9 | 11.7 | 75.3 |
| Feburary-15 | 20.5 | 22 | 11.1 | 16.5 | 66 |
| March-15 | 67.9 | 24.5 | 13.6 | 19.1 | 64 |
| April-15 | 32.8 | 33.2 | 20.7 | 27 | 43.9 |
Treatments and Crop Husbandry
The experiment included three different wheat cultivars, C1 = Faisalabad-2008, C2 = Punjab-2011, and C3 = Millet-2011, and five zinc application protocols: T1 = control, T2 = seed priming, T3 = soil application, T4 = foliar application, and T5 = soil + foliar application. The source of zinc was “Naya Zinc,” which is 98% pure containing 21% zinc as ZnSO4⋅7H2O. For T1, no zinc was applied, while in T2, seeds were soaked in 0.3% ZnSO4 solution; for T3, ZnSO4⋅7H2O was applied at the rate of 50 kg ZnSO4 per ha; for T4, ZnSO4⋅7H2O was applied at the rate of 0.5% at two growth stages (booting and milking); and in T5, zinc was applied in both the soil and as a foliar feed. Furthermore, for T2, seeds were initially soaked in 0.3% ZnSO4 solution and subsequently given three surface washings with distilled water, then dried close to the original moisture level with forced air, after which they were sealed in polythene bags and stored in a refrigerator at 7 ± 1°C until use. In T3, ZnSO4⋅7H2O was applied to the soil surface and after that incorporated into soil prior to sowing. For T4, each application of an aqueous solution of ZnSO4⋅7H2O was sprayed in the late afternoon until most leaves were wet.
The seeds were sown on November, 19 in 2013–2014 and November, 23 in 2014–2015. In both growing seasons, wheat cultivars were planted in rows 22.5 cm apart using a hand drill and a seed rate of 125 kg ha−1. Nitrogen, phosphorus, and potassium were applied at a rate of 100:50:50 (N:P:K) kg ha−1. Nitrogen, phosphorus, and potash were applied in the form of urea (46% N), single super phosphate (14% P), and sulfate of potash (50% K), respectively. Nitrogen was applied in three splits, one-third as a basal dose and the remaining two-thirds in two equal splits at the tillering and booting stages. All the potash and phosphorus were applied as basal doses. During crop growth, field water conditions were managed by flood irrigation.
Soil Analysis
To determine the physicochemical properties of experimental soil, composite soil samples were taken from the top (0–30 cm) soil layer of the experimental site prior to sowing. Collected samples were analyzed using the protocols described by Homer and Pratt (1961). The soil was loamy containing sand (41.23%), silt (39.35%), and clay (19.42%) particles, having a bulk density of 1.36 g cm-3, pH 7.8, EC 1.03 dSm−1, organic matter 0.81%, available nitrogen 0.031%, available phosphorus 22 ppm, available potassium 121 ppm, and available zinc 29 ppm.
Sampling and Measurements
At maturity, the crop was harvested and tied into bundles for determination of yield. The individual plots were threshed using a mini thresher. Grain weight for each treatment was recorded by digital balance in kilograms and later expressed in tons per hectare (t ha−1). The harvested grain was stored for determination of grain zinc and phytic acid concentration.
Sample Preparation and Analysis
Samples of wheat grain were dried in a drying oven at 60°C for 48 h (Liu et al., 2006). Dried samples were ground in a mill (IKA Werke, MF 10 Basic, Staufen, Germany) fitted with a stainless steel chamber and blades. Subsequently, finely ground 1.0 g samples of wheat flour were placed in a conical flask and kept overnight after adding a di-acid (HNO3:HClO4 ratio of 2:1) digestion mixture (Jones and Case, 1990). After 24 h, samples were digested on a hot plate at 150°C until all the material was digested. After digestion, the material was cooled and diluted to 50 ml by adding de-ionized water. Digesta was then filtered with Whatman filter paper No. 42 and stored in air tight plastic bottles. Zinc concentration in the digested samples was determined by atomic absorption spectrophotometer (PerkinElmer, 100 AAnalyst, Waltham, MA, USA). Phytic acid in the extract was measured by an indirect method that uses absorption of the pink color developed by un-reacted Fe (III) with 2,2′-bi-pyridine (Haug and Lantzsch, 1983) at 519 nm with a spectrophotometer (Shimadzu, UV-1201, Kyoto, Japan). All samples for zinc and phytic acid determinations were prepared and analyzed in duplicate.
Experimental Design and Statistical Analysis
The experiment was laid out in a randomized complete block design in a factorial arrangement with three replications. Data were statistically analyzed using Statistix 8.1 (Analytical, Tallahassee, FL, USA), while the least significant difference (LSD) test at 5% probability was used to compare treatment means. Graphs for experimental and climatic data were prepared using Microsoft Excel 2007.
Results
Zinc application methods significantly (p ≤ 0.05) affected economic yield, grain zinc, and grain phytic acid concentrations (see Table 2). Maximum improvement in grain yield, 24.27 and 24.06%, was recorded with T5 in 2013–2014 and 2014–2015, respectively, and the minimum improvement in grain yield was recorded under T1. The overall trend of zinc application methods regarding grain yield was: T5 > T3 > T4 > T2 > T1. Similarly, zinc application via different methods markedly (p ≤ 0.05) influenced grain zinc and phytic acid concentrations. As for grain zinc concentration, a maximum increase of 50.08 and 46.59% was observed in T5, followed by 47.81 and 46.59% increase in T4 during both years. T5 appeared to be an excellent strategy to increase grain zinc concentration, whereas minimum increase was observed with T2 and T1 (Table 2). Grain phytic acid concentration was also significantly (p ≤ 0.05) reduced by zinc application (Table 2). During 2013–2014 and 2014–2015, a reduction of 29.05 and 28.69% in grain phytic acid was recorded under T5 followed by T4 and T3 (Table 2); minimum reduction in grain phytic acid content was recorded with T2 and T1.
Table 2.
The effect of zinc application methods on grain yield, grain zinc, and grain phytic acid concentrations of wheat cultivars.
| Zinc application method | Grain yield (t ha−1) |
Grain zinc concentration (mg kg−1) |
Grain phytic acid concentration (mg g−1) |
|||
|---|---|---|---|---|---|---|
| 2013–2014 | 2014–2015 | 2013–2014 | 2014–2015 | 2013–2014 | 2014–2015 | |
| No zinc | 3.59 e | 3.66 e | 33.1 e | 33.7 e | 11.68a | 11.53a |
| Seed priming | 3.90 d | 3.99 d | 38.4 d | 40.5 d | 11.37a | 11.18a |
| Soil | 4.61 b | 4.69 b | 44.4 c | 47.6 c | 9.68b | 9.56b |
| Foliar | 4.37 c | 4.13 c | 59.6 b | 60.7 b | 8.74c | 8.66c |
| Soil + foliar | 5.10 a | 5.18 a | 62.3 a | 63.1 a | 8.28d | 8.19c |
| LSD (p ≤ 0.05) | 0.024 | 0.050 | 2.06 | 2.27 | 0.43 | 0.51 |
| Cultivars | ||||||
| Faisalabad-2008 | 3.77 c | 3.88 c | 41.8 c | 43.1 c | 10.90a | 10.88a |
| Punjab-2011 | 4.80 a | 4.89 a | 54.4 a | 55.6 a | 9.73b | 9.53b |
| Millat-2011 | 4.34 b | 4.40 b | 46.5 b | 48.6 b | 9.21c | 9.11c |
| LSD (p ≤ 0.05) | 0.031 | 0.039 | 1.59 | 1.78 | 0.33 | 0.39 |
LSD values were shown as bold in order to differentiate from data values.
Similarly, all wheat cultivars differed significantly for grain yield, grain zinc, and phytic acid concentrations (Table 2). Wheat cultivar Punjab-2011 had a higher grain yield and grain zinc concentration followed by Millet-2011 and Faisalabad-2008 for both study years. Minimum grain yield and grain zinc concentrations were recorded in Faisalabad-2008 (Table 2). However, for grain phytic acid, considerable variation was observed among the wheat cultivars. Punjab-2011 had the lowest grain phytic acid content, followed by Millet-2011. However, Faisalabad-2008 performed poorly and had a higher grain phytic acid content when compared to Punjab-2011 and Millet-2011 (Table 2).
Interactions between zinc application methods and wheat cultivars were found to be significant for grain zinc concentration but not for grain yield or grain phytic acid concentration (see Table 3). For the interactive effect of grain zinc concentration and wheat cultivars, Punjab-2011 registered the highest values for grain zinc concentration at T5 in the first (71.8 mg kg−1) and second (70.6 mg kg−1) year, respectively. However, Faisalabad-2008 registered the lowest value of grain zinc concentration under T1 (Table 3). There was a significant positive correlation between grain yield and grain zinc during both years of study (Figures 1A,B); an increase in grain zinc concentration substantially enhanced grain yield. Similarly, and interestingly, a strong negative correlation was found between grain zinc and grain phytic acid concentration (Figures 1C,D); it was found that zinc enriched seeds had a lower phytic acid content than seeds with lower zinc content.
Table 3.
Interactive effect of zinc application methods and wheat cultivar on grain yield, grain zinc, and phytic acid concentrations.
| Zinc application method | Cultivars | Grain yield (t ha−1) |
Grain zinc concentration (mg kg−1) |
Grain phytic acid concentration (mg kg−1) |
|||
|---|---|---|---|---|---|---|---|
| 2013–2014 | 2014–2015 | 2013–2014 | 2014–2015 | 2013–2014 | 2014–2015 | ||
| No zinc | Faisalabad-2008 | 3.10 | 3.17 | 30.0i | 31.3i | 12.8 | 12.6 |
| Punjab-2011 | 4.11 | 4.19 | 36.9 fg | 36.0gh | 10.8 | 10.6 | |
| Millat-2011 | 3.56 | 3.61 | 32.5hi | 33.9 hi | 11.5 | 11.3 | |
| Seed priming | Faisalabad-2008 | 3.32 | 3.41 | 34.3gh | 35.3h | 12.2 | 12.0 |
| Punjab-2011 | 4.43 | 4.56 | 42.8d | 46.2 d | 10.7 | 10.5 | |
| Millat-2011 | 3.94 | 4.01 | 38.2f | 39.9fg | 11.2 | 11.0 | |
| Soil | Faisalabad-2008 | 4.16 | 4.26 | 38.8 ef | 41.7 ef | 10.6 | 10.5 |
| Punjab-2011 | 5.11 | 5.16 | 52.2 c | 56.2c | 8.9 | 8.8 | |
| Millat-2011 | 4.56 | 4.63 | 42.3 de | 45.0 de | 9.5 | 9.3 | |
| Foliar | Faisalabad-2008 | 3.85 | 3.92 | 51.8 c | 52.5 c | 9.7 | 9.6 |
| Punjab-2011 | 4.83 | 4.92 | 68.4 a | 69.1 a | 8.0 | 8.0 | |
| Millat-2011 | 4.42 | 4.45 | 58.6 b | 60.3 b | 8.5 | 8.4 | |
| Soil + foliar | Faisalabad-2008 | 4.51 | 4.63 | 53.9 c | 55.0 c | 9.3 | 9.2 |
| Punjab-2011 | 5.55 | 5.62 | 71.8 a | 70.6 a | 7.6 | 7.6 | |
| Millat-2011 | 5.24 | 5.3 | 61.2 b | 63.7 b | 7.9 | 7.8 | |
| LSD (p ≤ 0.05) | NS | NS | 3.57 | 3.93 | NS | NS | |
LSD values were shown as bold in order to differentiate from data values.
FIGURE 1.
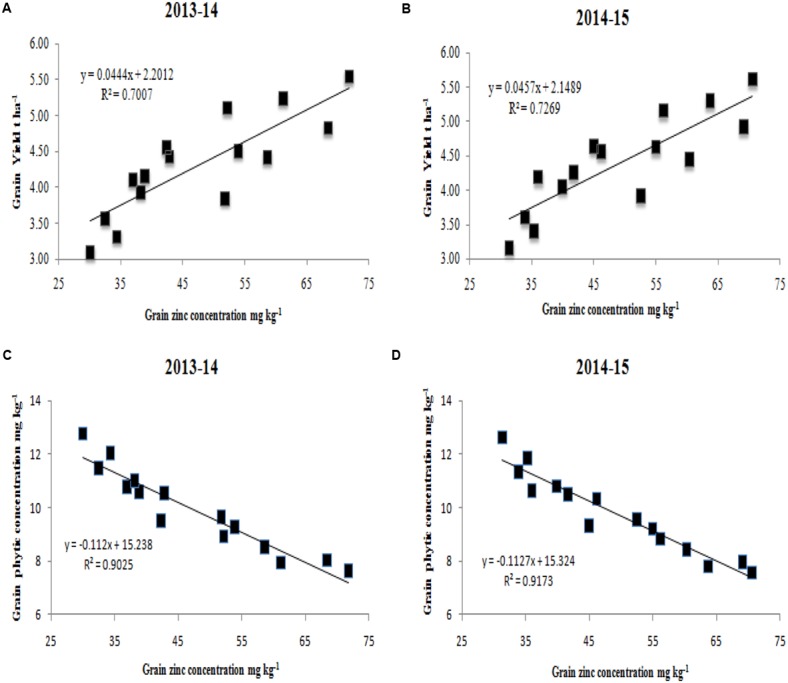
Relationships between grain zinc concentration and grain yield (A,B), and grain zinc and phytic acid concentration (C,D) during the years 2013–2014 and 2014–2015. mg kg−1, milligram per kilogram; t ha−1, tons per hectare.
Discussion
Zinc is essential for all biological systems in humans, animals, and plants. Low zinc availability and zinc fixation resulted in greater reduction of grain yield and grain zinc content; further, it also enhanced grain phytic acid content (Table 2).
Zinc application improves yield and yield components through various mechanisms, for example, it improves chlorophyll content and triggers photosynthetic activity and auxin synthesis which lead to better growth and development of the crop, thus effectively amplifying yield and yield components (Rakesh and Jitendra, 2014). Seed priming is a cheap source of zinc application, which can increase the yield of various crops (Harris et al., 2008); however, in the present study, T2 was unable to fulfill the zinc requirement of the wheat crop for optimum yield (Table 2). The slight improvement in grain yield with T2 could be explained by the fact that zinc synchronizes stand establishment and also helps in increasing the range of temperature during germination, which ultimately enhances wheat grain yield (Farooq et al., 2008). For the other application methods, T5 markedly enhanced grain yield as compared to T3 and T4 (Table 2). These results agree with previous literature (Torun et al., 2001; Zorita et al., 2001) where it is reported that foliar feeding of zinc ensures the increased availability of zinc at anthesis and grain filling stages, while Khan et al. (2009) also states that soil application substantially improves the translocation of nutrients from soil, which leads to better stand establishment and grain yield. Variation in grain yield, grain zinc, and phytic acid concentration among wheat cultivars might be due to their genetic makeup and their response toward zinc uptake.
Wheat, inherently, has a lower grain zinc concentration, especially when grown on zinc-deficient soils. Wheat cultivars are mostly zinc deficient and unable to fulfill human zinc requirements. For a measurable impact on human health, agronomic biofortification should enhance grain zinc content from 35 to 45 mg kg−1 (Pfeiffer and McClafferty, 2007; Cakmak, 2008). In our study T5, T4, and T3 significantly increased grain zinc content as compared to T2 and T1. The improvement in grain zinc concentration in T5 could be due to the improved availability of nutrients and maintenance of a greater zinc pool within plant tissues during the later growth stages. However, T4 was superior to T3 for improving grain zinc concentration even though just a small amount of zinc was applied in T4 compared to T3 (Erdal et al., 2002; Cakmak et al., 2010a). On the other hand, T3 was less effective as compared with T5 and T4 because of poor mobility and rapid adsorption of zinc in soil (Alloway, 2008). This explains why better results were obtained regarding grain zinc concentration from T5 (Table 2). Soil application was less effective for several reasons. Mostly, wheat roots and applied zinc have different soil distribution profiles, which reduces the uptake of zinc by plant roots (Holloway et al., 2010). In addition, top soil is mostly dry during the reproductive stages, meanwhile root activity is generally reduced due to lower allocation of photo-assimilates. Thus, zinc uptake from soil or zinc fertilizers is usually reduced during the reproductive stages, a factor that substantially decreases zinc accumulation in grains. Zinc accumulation in wheat grain largely depends on re-translocation of zinc from vegetative tissue during the reproductive stages (Cakmak, 2008; Cakmak et al., 2010a). Foliar feeding of zinc maintains a high concentration of zinc in vegetative tissues during re-translocation periods and contributes significantly to zinc biofortification of wheat grain under field conditions.
Phytate is a major phosphorus storing compound in cereal grains and acts as a metal chelator in the human intestine; it therefore hinders the absorption of dietary zinc and other metals into the blood (Bohn et al., 2008). According to Rengel and Graham (1995b), soil zinc deficiency enhances plant phosphorus uptake and reduces zinc availability. Zinc application decreased grain phytic acid concentrations (Table 2), and this may be attributed to the inhibitory effect of zinc on root uptake and the accumulation of phosphorus in plant shoots (Erdal et al., 2002). In the present study, T5 substantially reduced grain phytic acid concentration followed by T4 (Table 2). These results agree with previous findings of Mabesa et al. (2013). On the other hand, foliar application of zinc is useful for increasing grain zinc concentration and decreasing phytic acid concentration, which ultimately increase zinc bioavailability in both whole wheat grain and in wheat flour (Cakmak et al., 2010a; Kutman et al., 2011).
In most previous cases, authors report an inverse relationship between grain yield and grain zinc concentration (Garvin et al., 2006; McDonald et al., 2008). However, our results indicated that grain yield and grain zinc were positively correlated, resulting in a substantial yield increase (Figures 1A,B). These results are not consistent with previous studies of Oury et al. (2006) and McDonald et al. (2008) who reported an inverse relation between grain yield and grain zinc concentration. However, our findings support the results of Zou et al. (2012) in Pakistan, Karim et al. (2012) in China, and Yilmaz et al. (1997) in Turkey, who reported a simultaneous increase in grain yield and grain zinc concentrations with applied zinc. Considering the ever-growing global demand for food and widespread occurrence of zinc malnutrition, increasing grain Zn concentration in high-yielding wheat cultivars is important (Graham et al., 2007). In the current study, a negative correlation was also found between grain zinc and grain phytic acid content (Figures 1C,D). The decreasing effect of applied zinc on phytic acid content could be explained by the fact that zinc inhibits root uptake and shoot accumulation of phosphorus. It is well-reported that zinc deficiency increases the potential of plants for phosphorus uptake; however, zinc supply to zinc-deficient plants decreases phosphorus uptake and accumulation (Rengel and Graham, 1995a). Therefore, the substantial reduction in grain phytic acid content seen in Table 2 can be attributed to zinc application reducing the uptake and accumulation of phosphorus.
Conclusion
Zinc application via different methods substantially improved grain yield; however, seed priming had a marginal influence on grain yield. A combined application of soil + foliar zinc gave a higher grain yield on zinc-deficient soil. Similarly, maximum grain zinc concentration and lowest values for grain phytic acid were recorded in the same treatment. Therefore, the soil + foliar application of zinc was a more successful agronomic practice for achieving optimum yields, as well as grain biofortification. This study has also reported that grain yield and grain zinc were positively correlated, while grain zinc and grain phytic acid content were significantly negatively correlated.
Author Contributions
MC (1st author) and IK designed the experiment and wrote the manuscript. MH performed the experiment. MBC and AM analyzed the data. MC (6th author), MN, and MS contributed reagents/materials/analysis tools. MK and SK revised statistical analysis and manuscript. All the authors read and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer HA declared a shared affiliation, though no other collaboration, with one of the authors MUC to the handling Editor, who ensured that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
We wish to thank Mr. Wajid Ishque, Senior Scientist at the Nuclear Institute for Agriculture and Biology, Faisalabad, Pakistan, for his generous input and institutional support during this research and preparation of the manuscript.
Footnotes
Funding. University of the Punjab may pay funding for publishing this manuscript. They need acceptance letter for the application of funding.
References
- Alloway B. J. (2004). Zinc in Soils and Crop Nutrition. Brussels: IZA Publications. [Google Scholar]
- Alloway B. J. (2008). Zinc in Soils and Crop Nutrition, 2nd Edn. Brussels: IZA and IFA, 23–26. [Google Scholar]
- Bohn L., Meyer A., Rasmussen S. (2008). Phytate: impact on environment and human nutrition. A challenge for molecular breeding. J. Zhejiang Univ. Sci. B 9 165–191. 10.1631/jzus.B0710640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouis H. E. (2003). Micronutrient fortification of plants through plant breeding: can it improve nutrition in man at low cost? Proc. Nutr. Soc. 62 403–411. 10.1079/PNS2003262 [DOI] [PubMed] [Google Scholar]
- Bouis H. E., Hotz C., McClafferty B., Meenakshi J. V., Pfeiffer W. H. (2011). Biofortification: a new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 32(1 Suppl.), S31–S40. 10.1177/15648265110321S105 [DOI] [PubMed] [Google Scholar]
- Bouis H. E., Welch R. M. (2010). Biofortification–a sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop. Sci. 50 S20–S32. 10.2135/cropsci2009.09.0531 [DOI] [Google Scholar]
- Cakmak I. (2000). Role of zinc in protecting plant cells from reactive oxygen species. New Phytol. 146 185–205. 10.1046/j.1469-8137.2000.00630.x [DOI] [PubMed] [Google Scholar]
- Cakmak I. (2008). Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302 1–17. 10.1007/s11104-007-9466-3 [DOI] [Google Scholar]
- Cakmak I., Kalayci M., Kaya Y., Torun A. A., Aydin N., Wang Y. (2010a). Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 58 9092–9102. 10.1021/jf101197h [DOI] [PubMed] [Google Scholar]
- Cakmak I., Pfeiffer W. H., McClafferty B. (2010b). Biofortification of durum wheat with zinc and iron. Cereal Chem. 87 10–20. 10.1094/CCHEM-87-1-0010 [DOI] [Google Scholar]
- Erdal I., Yilmaz A., Taban S., Eker S., Torun B., Cakmak I. (2002). Phytic acid and phosphorus concentrations in seeds of wheat cultivars grown with and without zinc fertilization. J. Plant Nutr. 25 113–127. 10.1081/PLN-100108784 [DOI] [Google Scholar]
- Fan M., Zhao F., Fairweathertait S., Poulton P., Dunham S., McGrath S. (2008). Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol. 22 315–324. 10.1016/j.jtemb.2008.07.002 [DOI] [PubMed] [Google Scholar]
- Farooq M., Basra S. M. A., Rehman H., Saleem B. A. (2008). Seed priming enhances the performance of late sown wheat (Triticum aestivum L.) by improving chilling tolerance. J. Agron. Crop Sci. 194 55–60. 10.1111/j.1439-037X.2007.00287.x [DOI] [Google Scholar]
- Garvin D. F., Welch R. M., Finley J. W. (2006). Historical shifts in the seed mineral micronutrient concentration of US hard red winter wheat germplasm. J. Sci. Food Agric. 86 2213–2220. 10.1002/jsfa.2601 [DOI] [Google Scholar]
- Gibson R. S., Donovan U. M., Heath A. L. M. (1997). Dietary strategies to improve the iron and zinc nutriture of young women following a vegetarian diet. Plant Foods Hum. Nutr. 51 1–16. 10.1023/A:1007966104442 [DOI] [PubMed] [Google Scholar]
- Graham R. D., Welch R. M., Saunders D. A., Ortiz-Monasterio I., Bouis H. E., Bonierbale M., et al. (2007). Nutritious subsistence food systems. Adv. Agron. 92 1–74. 10.1016/S0065-2113(04)92001-9 [DOI] [Google Scholar]
- Hafeez B., Khanif M., Saleem M. (2013). Role of zinc in plant nutrition: a review. Am. J. Exp. Agric. 3 374–391. 10.9734/AJEA/2013/2746 [DOI] [Google Scholar]
- Harris D., Rashid A., Miraj G., Arif M., Yunas M. (2008). ‘On-farm’ seed priming with zinc in chickpea and wheat in Pakistan. Plant Soil 306 3–10. 10.1007/s11104-007-9465-4 [DOI] [Google Scholar]
- Haslett B. S., Reid R. J., Rengel Z. (2001). Zinc mobility in wheat: uptake and distribution of zinc applied to leaves or roots. Ann. Bot. 87 379–386. 10.1006/anbo.2000.1349 [DOI] [Google Scholar]
- Haug W., Lantzsch H.-J. (1983). Sensitive method for the rapid determination of phytate in cereals and cereal products. J. Sci. Food Agric. 34 1423–1426. 10.1002/jsfa.2740341217 [DOI] [Google Scholar]
- Holloway R. E., Graham R. D., McBeath T. M., Brace D. M. (2010). The use of a zinc efficient wheat cultivar as an adaptation to calcareous subsoil: a glasshouse study. Plant Soil 336 15–24. 10.1007/s11104-010-0435-x [DOI] [Google Scholar]
- Homer D. C., Pratt P. F. (1961). Methods of Analysis for Soils, Plants and Waters. Davis: University of California, Davis. [Google Scholar]
- Hotz C., Brown K. H. (2004). Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 25 S91–S204. [PubMed] [Google Scholar]
- Imtiaz M., Alloway B. J., Shah K. H., Siddiqui S. H., Memon M. Y., Aslam M., et al. (2003). Zinc nutrition of wheat: II: interaction of zinc with other trace elements. Asian J. Plant Sci. 2 156–160. 10.3923/ajps.2003.156.160 [DOI] [Google Scholar]
- Jones J. R., Case V. W. (1990). “Sampling, handling, and analysing plant tissue samples,” in Soil Testing and Plant Analysis, ed. Westerman R. L. (Madison, WI: Soil Science Society of America; ), 389–428. [Google Scholar]
- Karim M. R., Zhang Y. Q., Zhao R. R., Chen X. P., Zhang F. S., Zou C. Q. (2012). Alleviation of drought stress in winter wheat by late foliar application of zinc, boron, and manganese. J. Plant Nutr. Soil Sci. 175 142–151. 10.1002/jpln.201100141 [DOI] [Google Scholar]
- Khan R., Gurmani A. R., Khan M. S., Gurmani A. H. (2009). Residual, direct and cumulative effect of zinc application on wheat and rice yield under rice wheat system. Soil Environ. 28 24–28. [Google Scholar]
- Kutman U. B., Yildiz B., Cakmak I. (2011). Improved nitrogen status enhances zinc and iron concentrations both in the whole grain and the endosperm fraction of wheat. J. Cereal Sci. 53 118–125. 10.1016/j.jcs.2010.10.006 [DOI] [Google Scholar]
- Liu Z., Wang H., Wang X. E., Zhang G. P., Chen P. D., Liu D. J. (2006). Genotypic and spike positional difference in grain phytase activity, phytate, inorganic phosphorus, iron, and zinc contents in wheat (Triticum aestivum L.). J. Cereal Sci. 44 212–219. 10.1016/j.jcs.2006.06.001 [DOI] [Google Scholar]
- Mabesa R. L., Impa S. M., Grewal D., Johnson-Beebout S. E. (2013). Contrasting grain-Zn response of biofortification rice (Oryza sativa L.) breeding lines to foliar Zn application. Field Crops Res. 149 223–233. [Google Scholar]
- McDonald G. K., Genc Y., Graham R. D. (2008). A simple method to evaluate genetic variation in Zn grain concentration by correcting for differences in grain yield. Plant Soil 306 49–55. 10.1016/j.fcr.2013.05.012 [DOI] [Google Scholar]
- Mousavi S. R. (2011). Zinc in crop production and interaction with phosphorus. Aust. J. Basic Appl. Sci. 5 1503–1509. [Google Scholar]
- Moustakas N. K., Akoumianaki A. I., Barouchas P. E. (2011). The effects of cadmium and zinc interactions on the concentration of cadmium and zinc in pot marigold (Calendula officinalis L.). Aust. J. Crop Sci. 5 277–282. 10.1007/s11104-008-9555-y [DOI] [Google Scholar]
- Oury F. X., Leenhardt F., Remesy C., Chanliaud E., Duperrier B., Balfourier F., et al. (2006). Genetic variability and stability of grain magnesium, zinc and iron concentrations in bread wheat. Eur. J. Agron. 25 177–185. 10.1016/j.eja.2006.04.011 [DOI] [Google Scholar]
- Pandey N., Pathak G. C., Sharma C. P. (2006). Zinc is critically required for pollen function and fertilisation in lentil. J. Trace Elem. Med. Biol. 20 89–96. 10.1016/j.jtemb.2005.09.006 [DOI] [PubMed] [Google Scholar]
- Pfeiffer W. H., McClafferty B. (2007). HarvestPlus: breeding crops for better nutrition. Crop Sci. 47 S88–S105. 10.2135/cropsci2007.09.0020IPBS [DOI] [Google Scholar]
- Prasad A. S. (2008). Clinical, anti inflammatory and antioxidant role of zinc. Exp. Gerintol. 43 370–377. 10.1016/j.exger.2007.10.013 [DOI] [PubMed] [Google Scholar]
- Raboy V., Dickinson B. D. (1984). Effect of phosphorus and zinc nutrition on soybean seed phytic acid and zinc. Plant Physiol. 75 1094–1098. 10.1104/pp.75.4.1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh K., Jitendra J. S. (2014). Effect of NPKS and Zn application on growth, yield, economics and quality of baby corn. Arch. Agron. Soil Sci. 60 1193–1206. 10.1080/03650340.2013.873122 [DOI] [Google Scholar]
- Rengel Z., Graham R. D. (1995a). Wheat genotypes differ in zinc efficiency when grown in the chelate-buffered nutrient solution. II. Nutrient uptake. Plant Soil 176 317–324. 10.1007/BF00011796 [DOI] [Google Scholar]
- Rengel Z., Graham R. D. (1995b). Importance of seed Zn content for wheat growth on Zn-deficient soil. Plant Soil 173 267–274. 10.1007/BF00011464 [DOI] [Google Scholar]
- Rengel Z., Romheld V., Marschner H. (1998). Uptake of zinc and iron by wheat genocatypes differing in tolerance to zinc deficiency. J. Plant Physiol. 142 433–438. 10.1016/S0176-1617(98)80260-5 [DOI] [Google Scholar]
- Shankar A. H., Prasad A. S. (1998). Zinc and immune infection: the biology of altered resistance to infection. Am. J. Clin. Nutr. 68 447–463. [DOI] [PubMed] [Google Scholar]
- Singh J. P., Karamonas R. E., Stewart J. W. B. (1986). Phosphorus-induced zinc deficiency in wheat on residual phosphorus plots. Agron. J. 78 668–675. 10.2134/agronj1986.00021962007800040023x [DOI] [Google Scholar]
- Stein A. J. (2010). Global impacts of human mineral malnutrition. Plant Soil 335 133–154. 10.1007/s11104-009-0228-2 [DOI] [Google Scholar]
- Tisdale S. L., Nelson W. L., Beaten J. D. (1984). Zinc in Soil Fertility and Fertilizers, 4th Edn. New York, NY: Macmillan Publishing Company, 382–391. [Google Scholar]
- Torun A., Gültekin I. G. A., Kalayci M., Yilmaz A., Eker S., Cakmak I. (2001). Effects of zinc fertilization on grain yield and shoot concentrations of zinc, boron, and phosphorus of 25 wheat cultivars grown on a zinc-deficient and boron-toxic soil. J. Plant Nutr. 24 1817–1829. 10.1081/PLN-100107314 [DOI] [Google Scholar]
- Welch R. M., Graham R. D. (2004). Breeding for micronutrients in staple food crops from a human nutrition perspective. J. Exp. Bot. 55 353–364. 10.1093/jxb/erh064 [DOI] [PubMed] [Google Scholar]
- Yilmaz A., Ekiz H., Torun B., Gultekin I., Karanlik S., Bagci S. A., et al. (1997). Effect of different zinc application methods on grain yield and zinc concentration in wheat grown on zinc-deficient calcareous soils in Central Anatolia. J. Plant Nutr. 20 461–471. 10.1080/01904169709365267 [DOI] [Google Scholar]
- Zhang Y., Shi R., Rezaul K. M., Zhang F., Zou C. (2010a). Iron and zinc concentrations in grain and flour of winter wheat as affected by foliar application. J. Agric. Food Chem. 58 12268–12274. 10.1021/jf103039k [DOI] [PubMed] [Google Scholar]
- Zhang Y., Song Q., Yan J., Tang J., Zhao R., Zhang Y. (2010b). Mineral element concentrations in grains of Chinese wheat cultivars. Euphytica 174 303–313. 10.1007/s10681-009-0082-6 [DOI] [Google Scholar]
- Zhao F. J., McGrath S. P. (2009). Biofortification and phytoremediation. Curr. Opin. Plant Biol. 12 373–380. 10.1016/j.pbi.2009.04.005 [DOI] [PubMed] [Google Scholar]
- Zorita M. D., Canigia M. V. F., Grosso G. A. (2001). Applications of foliar fertilizers containing glycinebetaine improve wheat yields. J. Agron. Crop Sci. 186 209–215. 10.1046/j.1439-037X.2001.00469.x [DOI] [Google Scholar]
- Zou C. Q., Zhang Y. Q., Rashid A., Savasli E., Arisoy R. Z., Ortiz-Monasterio I., et al. (2012). Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil 361 119–130. 10.1007/s11104-012-1369-2 [DOI] [Google Scholar]


