Abstract
Summary: In addition to strategies designed to decrease amyloid beta (Aβ) levels, it is likely that successful Alzheimer’s disease (AD) therapeutic regimens will require the concomitant application of neuroprotective agents. Elucidation of pathophysiological processes occurring in AD and identification of the molecular targets mediating these processes point to potential high-yield neuroprotective strategies. Candidate neuroprotective agents include those that interact specifically with neuronal targets to inhibit deleterious intraneuronal mechanisms triggered by Aβ and other toxic stimuli. Strategies include creating small molecules that block Aβ interactions with cell surface and intracellular targets, down-regulate stress kinase signaling cascades, block activation of caspases and expression of pro-apoptotic proteins, and inhibit enzymes mediating excessive tau protein phosphorylation. Additional potential neuroprotective compounds include those that counteract loss of cholinergic function, promote the trophic state and plasticity of neurons, inhibit accumulation of reactive oxygen species, and block excitotoxicity. Certain categories of compounds, such as neurotrophins or neurotrophin small molecule mimetics, have the potential to alter neuronal signaling patterns such that several of these target actions might be achieved by a single agent.
Keywords: Alzheimer, neuroprotection, amyloid, stress kinase, neurotrophin
Potential impacts of neuroprotection on AD
This review will focus on strategies targeted to neurons and designed to decrease their vulnerability to neurodegenerative mechanisms occurring in Alzheimer’s disease (AD). Potential therapies intended to decrease amyloid burden1,2 or inflammatory processes3,4 have been covered in recent reviews. Implicit in neuroprotection is the concept of delaying onset or slowing progression of AD. The late onset of symptomatic impairment in the majority of AD cases creates a particularly high-impact opportunity for neuroprotective strategies that achieve even modest delays in disease onset. Delaying disease onset by only 2 years would have a marked impact on reducing prevalence and a 5-year delay would reduce AD prevalence by half.5,6 Delaying onset by 10 years would, for the majority of individuals, eliminate symptomatic AD as a significant factor in advanced age. Once AD is present, delaying losses of independent activities of daily living or nursing home placement would markedly decrease costs associated with caregiver stress and nursing home care.
Will neuroprotection play a critical role in AD therapeutics?
Given the substantial body of evidence suggesting that the accumulation of amyloid beta (Aβ) is a major and early causative process in AD7 it can be argued that treatments decreasing levels or availability of toxic forms of Aβ will constitute high-priority, first-tier treatment strategies while neuroprotective strategies focused on non-Aβ targets might play a supportive, less critical role. Promising approaches for decreasing Aβ levels include inhibition of Aβ generation, reduction of soluble Aβ levels and enhancement of Aβ clearance from the CNS.1,8 While development of Aβ-based treatments follows logically from known Aβ mechanisms, a number of factors might limit the effectiveness of such treatments if applied in isolation. First, the degree to which Aβ levels need to be reduced to delay onset or slow progression of AD is unknown. If Aβ levels are several-fold above those capable of causing maximum rates of neural degeneration, a large proportionate reduction in levels by a “successful” drug candidate might be insufficient to slow degeneration. Second, the normal physiological functions of Aβ, including its possible role as an antioxidant9 remain unknown; disruption of critical functions might prove toxic. For example, in vitro studies suggest that excessive depletion of endogenously produced Aβ from culture medium leads to neuronal death.10 Third, the ideal scenario would include the application of Aβ-based drugs in early stages of Aβ accumulation, i.e., years before onset of symptoms. This approach would require drugs of exceptionally low toxicity administered with difficult-to-achieve high compliance rates years before clinical manifestations begin. Fourth, Aβ-based therapies alone are unlikely to improve function or plasticity of damaged but still surviving neurons. Finally, although the bulk of current evidence points to Aβ accumulation as a critical primary causative factor in sporadic AD, a number of other potential mechanisms might constitute important causative factors.11 Such non-Aβ mechanisms might play even larger roles, or perhaps synergistic roles, as the disease progresses. Thus, it is likely that parallel application of neuroprotective strategies will play a vital role in delaying AD onset and slowing AD progression.
Neurodegenerative mechanisms point to potential neuroprotective strategies
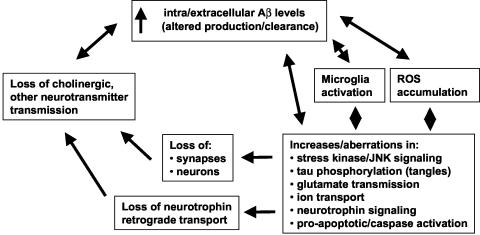
Neurodegenerative mechanisms likely involved in AD are outlined in FIG. 1. While AD mechanisms are often outlined in linear terms of one pathophysiological process leading to the next, a more biological perspective might include multiple cycles and subcycles of self-amplifying neurodegenerative steps. Moreover, the pattern of relative contributions of different pathological cycles is likely to change as the disease progresses. This perspective encourages the view that one or more neuroprotective strategies, applied in parallel, will be required to successfully slow AD progression. Neuronal targets can be viewed from the perspective of those known to directly interact with Aβ, or alternatively, those found to be affected in AD and not necessarily interacting directly with Aβ. Many of these targets offer potential sites for therapeutic small molecules (Table 1).
FIG. 1.
Overview of pathophysiological processes occurring in AD. A perspective emphasizing the many mutually reinforcing pathological processes in AD suggests that neuroprotective strategies, inhibiting as many of these process as possible, will likely be required for successful therapy in AD in parallel to therapies reducing Aβ accumulation.
TABLE 1.
Candidate Neurodegenerative Mechanisms in AD and Corresponding Therapeutic Neuroprotective Approaches
| Target Mechanism | Examples of Corresponding Therapeutic or Potential Therapeutic Development |
|---|---|
| Aβ interaction with binding targets | Neurotrophin small molecule mimetics binding to p75NTR might block Aβ-p75NTR mediated toxicity |
| Activation of stress kinase/JNK signaling | CEP-1347 inhibitor of stress kinase activation in clinical trials for Parkinson’s disease |
| Neurotrophin signaling blocks stress kinase signaling | |
| Excessive tau phosphorylation and microtubule instability | GSK-3 inhibitors under development |
| Valproate in AD trial underway | |
| Microtubule stabilizing drugs under development | |
| Caspase activation | Minocycline caspase inhibitor in trials for ALS |
| Loss of synapses, neuronal death | Trial underway in which NGF-secreting fibroblasts are grafted to basal forebrain |
| Neurotrophin mimetics under development | |
| Loss of cholinergic function | ACIs in clinical use |
| M1 agonists such as talsaclidine in clinical trials | |
| Neurotrophin mimetics under development | |
| Generation of ROS | Vitamin E in trials for MCI |
| Various antioxidants in MCI/AD trials | |
| Clioquinol metal chelator completed phase II trial | |
| Glutamate excitotoxicity | Memantine NMDA uncompetitive antagonist in use in Europe for AD with FDA approval in US pending |
| Other NMDA modulators under development |
Neuronal targets of Aβ
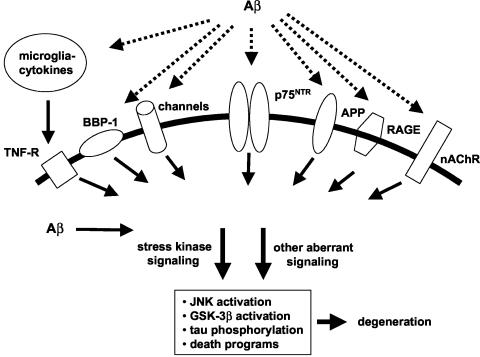
Evidence that either extracellular12 or intracellular13,14 accumulation of Aβ results in neuronal degeneration has encouraged identification of direct neuronal targets of Aβ that serve as candidates for mediating its toxicity (FIG. 2). Aβ has been reported to bind with relatively high affinity to a number of neuronal targets,15 including the α7 nicotinic acetylcholine receptor (α7nAChR), the neurotrophin p75 (p75NTR) receptor, cell surface amyloid precursor protein (APP), the receptor for advanced glycation end products (RAGE), and BBP-1, a G protein-coupled receptor. Except for α7nAChR, Aβ binding to these receptors leads to neuronal death. Intracellular binding targets of Aβ identified thus far include the endoplasmic reticulum Aβ-binding dehydrogenase (ERAB). A non-receptor based mechanism by which Aβ might affect neurons is suggested by its ability to form Ca2+-permeable channels or to modulate ion-conducting channels, especially K+ channels.16 Application of compounds that block Aβ binding or that inhibit at proximal steps deleterious Aβ-induced signaling are potential neuroprotective approaches. Limitations of these approaches include the possibility that Aβ toxicity is mediated via multiple targets or that critical physiological functions of the target receptors or other proteins might be impaired. The ability of a given small molecule to confer neuronal protection by blocking such interactions will depend on the extent to which Aβ interaction with the intended target contributes to the degenerative process. A critical current challenge is to determine whether the predominant neurotoxic effects of Aβ can be narrowed to one or two pharmacologically accessible Aβ binding targets. Of the above candidate targets, Kawasumi et al.15 have argued that interaction with p75NTR is likely to play the predominant role in Aβ toxicity.
FIG. 2.
Aβ binding targets and candidate associated neurodegenerative mechanisms. Extracellular Aβ interacts with a number of neuronal and glial cell surface receptors. Evidence suggests that many of these interactions promote stress kinase and other signaling triggering neurodegenerative processes. Intracellular Aβ is also likely to bind to one or more targets to contribute to neurodegenerative signaling.
Neuroprotection via modulation of stress-activated protein kinase signaling
An alternative approach to neuroprotection that would not rely on identification and modulation of individual Aβ-target interactions is the identification and modulation of primary signaling pathways mediating Aβ toxic effects. Interestingly, studies of human AD brain, AD mouse model brain, and in vitro models of AD all point to the stimulation of stress-activated protein kinases, particularly c-Jun N-terminal kinase (JNK), as a critical early event in AD-associated neuronal degeneration.17,18 Application of Aβ to cultured cortical neurons is associated with JNK activation and with subsequent downstream activation of caspases and expression of pro-apoptotic genes such as bax.19,20 Immunostaining reveals JNK activation in degenerating neurons in AD brain,21,22 including in association with intraneuronal Aβ accumulation and in association with tangle-like inclusions in entorhinal cortex before Aβ deposition.23,24 Interestingly, evidence suggests that JNK activation also contributes to tau phosphorylation (discussed below). These findings, along with evidence in vitro that inhibition of JNK activation inhibits Aβ toxicity20,25–28 and blocks caspase activation,17,20 point to small molecule targets modulating stress kinase signaling and JNK activation as a high priority area for AD therapeutics. CEP-1347 is an example of a compound that inhibits stress kinase signaling and partially blocks Aβ induced neuronal degeneration.17,29 CEP-1347 is currently undergoing clinical trials in Parkinson’s disease and is a candidate agent for AD trials (Cephalon Inc., West Chester, PA; http://www.cephalon.com/research).
Neuroprotection via inhibition of aberrant tau phosphorylation
Excessive phosphorylation of the tau microtubule-associated protein in AD is thought to cause formation of insoluble tau filaments with resulting neurofibrillary tangles, disruption of microtubules, and subsequent neuronal dysfunction.30 Although the mechanisms responsible for aberrant tau phosphorylation remain to be fully established, their elucidation has begun to point to novel protective strategies. Evidence that glycogen synthase kinase-3β (GSK-3β) phosphorylates tau has engendered considerable interest in GSK-3β inhibitors as neuroprotective agents.31,32 The finding that valproate, a well-established epilepsy and mood-stabilizing medication, inhibits GSK-3β33 has led to proposal that this drug might improve symptoms of or slow progression of AD.34 Interestingly, lithium, another well-established mood stabilizer, has also been shown to inhibit GSK-3β.31 Evidence that JNK contributes to tau phosphorylation suggests that inhibition of JNK activation might promote the parallel beneficial effects of inhibiting tau phosphorylation along with cell death signaling.23,35 The large number of kinases found to be capable of phosphorylating tau, including extracellular signal-regulated kinase (ERK) and cyclin-dependent kinase 5 (Cdk5), among others,32 introduce a number of potential small molecule targets. The presence of multiple candidate targets raises the important questions of which kinases play a critical pathophysiological role in AD, and if more than one is involved, how many would have to be targeted to prevent pathological tau phosphorylation. Another recent neuroprotective approach emerging from microtubule studies is the development of small molecules that stabilize microtubules and prevent Aβ-induced cytoskeletal disruption and toxicity.36
Neuroprotection via inhibition of caspase activation
A number of observations have raised the possibility that caspases contribute to neuronal degeneration in AD, although the actual extent to which caspase-mediated cell degeneration or death occurs in AD remains to be established.37 In AD brain, activated caspases are found in association with neurofibrillary tangles.38 As described above, activation of JNK promotes caspase activation.20,29,39 In vitro studies demonstrate that application of Aβ induces caspase activation and that caspase inhibitors can block Aβ-induced cell death.40,41 Consistent with a role for caspases in relatively distal steps of cell death pathways, caspase inhibitor-protected cells have been found to survive in an atrophic and metabolically compromised state in which there is decreased protein synthesis, glucose uptake, and mitochondrial activity.29,42 An analysis of neuronal death induced by nerve growth factor (NGF) withdrawal from sympathetic neurons demonstrated that caspase inhibition resulted in neurons that were more atrophic and had decreased metabolic function compared with those rescued via blocking of stress kinase activation using CEP-1347.29 These authors concluded that caspase inhibitors are unlikely to constitute an effective therapy in chronic neurodegenerative settings since caspase inhibitor rescue of neurons might result in neurons surviving but in a dysfunctional state. In contrast to this view, the finding of caspase activation in early stages of AD and in association with neurofibrillary tangles suggests that caspases might serve as a link between senile plaques and tangles and contribute to early as well as terminal steps of cell death.38 Minocycline, a tetracycline-type antibiotic known to inhibit caspases, is currently under trials for ALS (NINDS) and might be considered for trials in AD.
Ligand-receptor mechanisms promoting neuroprotective signaling
A number of growth factors and other ligands acting via known receptors, as well as peptides acting via unknown targets, have been found to protect neurons from Aβ toxicity and other deleterious mechanisms relevant to AD. These factors include neurotrophins (discussed below), insulin-like growth factor-1,26,43 basic fibroblast growth factor,44 estrogen,45 activity-dependent neurotrophic factor,46 and Humanin.47 Given the long-standing, large body of work assessing neurotrophins in the context of AD, the recent entry of neurotrophins into clinical trials, and the space limitations of the present review, we will focus primarily on neurotrophins.
Neuroprotection via neurotrophins
The extensive overlap in signaling pathways regulated by neurotrophins and those likely to be involved in AD degeneration along with the expression of neurotrophin receptors by neurons undergoing degeneration point to neuroprotective applications for neurotrophins. The major role of neurotrophins in synapse stabilization and function48 along with emerging evidence that synaptic failure is a critical early process in AD49 further adds to interest in neurotrophins as candidate therapeutic agents. In the context of AD, NGF and brain-derived neurotrophic factor (BDNF) have been of particular interest. Neurotrophins each bind to a dual receptor system, consisting of p75NTR along with one of the Trk tyrosine kinase receptors. NGF binds to TrkA and BDNF to TrkB.50,51 Neurotrophin receptors are expressed by neuronal populations particularly vulnerable in early stages of AD. p75NTR, TrkA, and TrkB are each expressed by basal forebrain cholinergic neurons, hippocampal pyramidal neurons and layer V cortical neurons.50,52–56
Neurotrophin signaling is directly relevant to the aberrations in core signaling mechanisms, likely contributing to neuronal dysfunction and degeneration in AD. Neurotrophin binding to Trk receptors activates at least three fundamental pathways, including phosphatidylinositol-3-kinase (PI3K)/Akt kinase, mitogen-activated protein (MAP) kinase, and phospholipase Cγ (PLCγ) signaling.50,51 Activation of the PI3K/Akt pathway inactivates SEK1 and ASK1, two key activators of JNK and other stress kinases, suppresses JNK activation, inhibits pro-apoptotic members of the Bcl-2 family, activates CREB and IκB kinase/NF-κB signaling to promote survival and protects cultured neurons from Aβ toxicity.26,29,39,57 NGF-induced MAP kinase signaling blocks formation of reactive oxygen species (ROS) (see discussion below).58 p75NTR acts in coordination with, and independently of, Trk receptors to modulate neurite integrity, neuronal size, and neuronal survival. In the context of different ligands or different cell types, p75NTR signaling promotes either neuronal survival or death.59,60 p75NTR-ligand interactions provide a good example of the principle that different ligands acting at a given receptor can differentially modulate its function; for example, binding of different neurotrophins to p75NTR can elicit different patterns of signaling.50,61 Recent observations that levels of “pro-NGF,” a precursor form of NGF, are doubled in parietal cortex harvested from AD patients,62 along with the finding that pro-NGF might bind with greater affinity to p75NTR compared to NGF and promote death via binding to p75NTR,63 add additional complexity to potential mechanisms by which p75NTR signaling regulates trophic status.
In paradigms in which ligand-induced activation of p75NTR prevents neuronal death, evidence suggests that p75NTR signaling prevents death via either the PI3K/Akt pathway and/or NF-kB activation.59,60 In view of these signaling studies and evidence that different ligands can differentially affect p75NTR signaling, it is of interest that synthetic mimetics of the loop 1 region of the NGF protein were found to prevent death of dorsal root ganglia sensory neurons.64 Preliminary data in our laboratory suggest that application of NGF loop 1 mimetics to cultured hippocampal neurons results in activation of PI3K/Akt signaling and prevents cell death. In earlier studies, addition of NGF to cultured E18 rat hippocampal neurons was found to up-regulate p75NTR expression and potentiate Aβ neurotoxicity.12 Current studies will determine if NGF loop 1 mimetics prevent rather than promote Aβ toxicity.
Within the neurotrophin protein family, NGF has been the most extensively studied with respect to its ability to confer neuroprotection in in vivo models of AD. NGF administration to multiple models of basal forebrain cholinergic neuron atrophy, including post-cholinergic neuron axotomy, trisomy 16 mice, aged rodents, and aged primates demonstrates a potent effect in reversing atrophy, up-regulating cholinergic function, reversing age-related cognitive impairment and increasing density of cortical cholinergic innervation.50,65 Interestingly, in vitro studies point to the possibility that NGF might promote non-amyloidogenic secretory processing of APP.66 In AD brain, NGF levels in the basal forebrain are reduced, while levels in the hippocampus target region have been reported as either unchanged or increased.67 This shift in NGF distribution, along with direct evidence of impaired retrograde transport of NGF in a mouse model of AD,68 suggests an impairment of retrograde transport of NGF. These findings raise the possibility of a degenerative cycle in which degeneration leads to a critical lack of NGF reaching the neuronal soma with deficiency of somal NGF leading to further degeneration.69 Interestingly, a transgenic mouse model in which chronic NGF deficiency is created via the expression of NGF antibodies demonstrates degeneration of basal forebrain cholinergic neurons, tau hyperphosphorylation associated with neurofibrillary pathology, and accumulation of Aβ plaques.70 This model points further to a potential degenerative cycle incorporating accumulation of Aβ, neuronal degeneration and loss of neurotrophin function, and further Aβ accumulation. Augmentation of neurotrophin function might serve to both decrease Aβ accumulation and render neurons less vulnerable to Aβ toxicity.
Application of NGF protein as a therapeutic agent for AD faces a number of critical limitations typical of protein ligands, including limited blood brain barrier and intraparenchymal tissue penetration and a short half-life. Moreover, NGF interaction with its dual p75NTR/TrkA receptor system elicits a wide range of biological actions beyond those preventing neural degeneration including sprouting of sympathetic fibers and up-regulation of pain transmission.71 In the limited clinical experience assessing NGF actions in AD patients, NGF was administered to three patients via intraventricular infusion over a period of up to three months.72 No significant improvement in cognition was detected and patients experienced back pain and weight loss. In an ongoing phase I trial, NGF is being delivered to the basal forebrain via intraparenchymal grafting of autologous fibroblasts engineered to secrete NGF.65 Another neurotrophin-based therapeutic approach is the development of NGF small molecule mimetics.73–75 Such mimetics acting selectively at p75NTR64 or TrkA76 receptors have been shown to prevent neuronal degeneration in vitro and activate partially distinct patterns of intracellular signaling cascades, compared with those activated by NGF. These findings point to the possibility of creating compounds with preferred pharmacological properties and bioavailability that are capable of preventing neuronal degeneration without stimulation of the entire range of NGF effects.
Cholinergic strategies for neuroprotection
An early pathological process in AD consists of degeneration of basal forebrain cholinergic neurons along with their projections to hippocampal, cortical, and limbic targets.77 Losses in certain other neurotransmitter systems might occur as a secondary result of reduced cholinergic innervation and function. Strategies involving application of acetylcholinesterase inhibitors (ACIs) and cholinergic agonists have largely been focused on improving cognitive and neurobehavioral symptoms in AD rather than slowing underlying neuronal degeneration. It is of interest to note, however, that recent clinical observations, along with emerging insight into reciprocal interactions between Aβ production and cholinergic function, suggest that cholinergic-based therapies might in fact have neuroprotective effects.
Results of ACI trials have raised the question of whether ACIs might slow disease progression.78–80 Patients initially placed in placebo groups appeared to have lost cognitive function that was not restored after starting ACIs in open-label extension phases. The open-label design of the extension phase of these trials, however, would preclude any formal conclusion regarding slowing of progression. In a study of cognitive function of patients who had dropped out of an ACI trial, individuals who had initiated and then discontinued ACI treatment demonstrated less subsequent cognitive loss compared with placebo-treated patients.81 These findings again raise the interesting scenario of slowed disease progression; however, a critical caveat is that patients discontinuing the medication cannot be assumed to represent a random sample from the total study population. Detection of slowed disease progression requires study designs that incorporate strategies such as delayed treatment with subsequent blinded follow-up or random discontinuation of drug with adequate washout periods. In the case of ACIs, their recognition as a standard of care in AD limits such trial designs. Current trials of ACIs applied to mild cognitive impairment (MCI) will determine whether ACIs can delay progression to AD, although distinguishing between symptomatic versus disease-slowing effects will remain a challenge.
There are several potential mechanisms by which ACIs or cholinergic agonists might slow underlying disease progression including decreased Aβ production and/or reduction of neuronal vulnerability to Aβ toxicity.66,82 M1 muscarinic acetylcholine receptors (mAChR) are primarily localized postsynaptically to cortical cholinergic nerve terminals. M1 mAChR signaling activates protein kinase C (PKC), increases secretory processing of APP and down-regulates production of Aβ.83–86 Cholinergic stimulation has also been found to: reduce tau phosphorylation;87 protect neurons from Aβ88 and promote neurotrophin release.89,90 Chronic M1 agonist treatment in clinical trials has been shown to reduce Aβ levels in cerebrospinal fluid of AD patients.91,92 Current goals in the development of cholinergic-based strategies in neuroprotection include the development of M1 agonists with adequate bioavailability, potency, and receptor selectivity.82
The known inhibitory effects of Aβ on the synthesis and release of Ach and on cholinergic signaling point to the possibility of a degenerative feedback loop in which Aβ impairs Ach release, which leads to altered APP processing, increased Aβ levels and disrupted neurotrophin regulation.16,66,77 These processes in turn lead to further increases in Aβ production, further loss of neuronal function and further decline in Ach release. Given a potential neurotrophic role of cholinergic neurons on target hippocampal and cortical neurons and the effect of cholinergic input on APP regulation, it has been proposed that cholinergic degeneration might lead to secondary degeneration in a wide range of non-cholinergic target systems.90 From the perspective of these mechanisms, it is plausible that drug strategies designed to up-regulate cholinergic function might slow degeneration.
Oxidative stress and antioxidant neuroprotective strategies
The role of oxidative stress, an imbalance between the production and detoxification of oxidative reaction products, continues to be the subject of extensive research in AD.93,94 While oxidation products accumulate to some degree in normal brain, their levels increase with age and are substantially greater in AD, including in its early stages.95 Excessive levels of hydrogen peroxide and ROS such as hydroxyl free radical and superoxide lead to formation of oxidization products including oxidized proteins, lipid peroxides, advanced glycosylation end products (AGEs), and DNA adducts. Protein and lipid oxidation leads to loss of critical enzyme functions, including those regulating glutamate transport, which results in excitotoxicity due to excessive extracellular glutamate, and to the loss of ion-transporting ATPases, causing a disruption of calcium ion homeostasis and impaired mitochondrial function.96 Oxidative stress triggers degenerative signaling, including activation of stress kinases (including JNK) and caspases.25,28 Sources of oxidative stress in AD include impaired mitochondrial metabolism and Aβ-associated sources.97,98 Addition of vitamin E to neuronal cultures inhibits Aβ-induced toxicity, protein oxidation, and JNK and p38MAPK activation.28,93 Studies in AD transgenic mice (Tg2576) revealed elevated peroxidation occurring several months before detectable Aβ accumulation and amyloid plaque formation.99 Further supporting a causal role for oxidative stress in amyloid-induced pathology, administration of the antioxidant curcumin to these mice led to reduced oxidative stress and amyloid pathology.100 Aβ itself, in particular when binding Cu2+ or Fe3+ and forming certain types of aggregates, may be a primary source of ROS.98 Cu2+ and Zn2+ promote aggregation of human Aβ and chelation of these metals renders the structure of Aβ aggregates less compact and less resistant to turnover. Clioquinol (CQ), a retired hydrophobic antibiotic with brain-penetrating and Cu/Zn-chelating properties, is a potential agent for inhibiting Aβ accumulation and decreasing ROS production.98,101 In the Tg2576 transgenic mouse model, CQ led to reduced amyloid accumulation and improved behavioral scores.102 Clinical phase II efficacy testing of this agent is currently completed, though the results are not yet published.101
In human antioxidant studies, vitamin E is one of the most extensively studied antioxidant agents. Data from cross-sectional and longitudinal studies assessing the relationship between vitamin E consumption and AD risk have led to conflicting results. Two prospective epidemiological cohort studies of AD found that diets containing higher levels of vitamin E were associated with lower odds of developing AD.103,104 A surprising finding was that neither study was able to identify an association between AD incidence and use of vitamin E supplements. In commentary on these studies, Foley and White105 pointed to the limitations of observational studies and raised the possibilities that clinical status before the onset of dementia might have influenced diet, recollection of diet, and/or supplement use. In addition, it is possible that predicted vitamin E content of food might have served as a marker for the presence of other compounds that actually conferred the neuroprotective effects. In a prospective cohort study, Luchsinger et al.106 were unable to detect an association between antioxidant vitamin use and AD risk. Finally, a pioneering trial with vitamin E supplementation of 2000 IU per day for moderate-stage AD patients led to a small but significant delay in reaching the endpoints of institutionalization, loss of major activities of daily living, or death, but did not delay loss of cognitive performance.107 These findings have encouraged current trials in which vitamin E is being given to individuals with MCI with the hope that antioxidant therapy administered at earlier stages of disease might have a greater impact on outcomes.108 Other antioxidant compounds have been studied in terms of delaying AD onset, slowing progression, or improving cognitive function.99 European trials in small numbers of AD patients with idebenone, a centrally active antioxidant and analog of coenzyme Q, suggested improved cognitive scores, with efficacy similar to ACIs.109 To date, there is no clear body of definitive data, derived from adequately controlled prospective trials of sufficient size and duration, that suggests a given antioxidant compound delays the onset of cognitive loss or slows its progression in AD.
Modulation of NMDA receptor function
Oxidative stress, accumulation of Aβ and other mechanisms lead to neuronal energy deficits in AD which in turn can result in excessive neuronal depolarization with a subsequent excess of extracellular glutamate, evoking further depolarization. Persistent depolarization leads to activation of NMDA receptors and deleterious increases in intracellular Ca2+.110,111 Olney et al.112 proposed that an early event in AD pathophysiology consists of increased sensitivity to glutamate-induced excitotoxicity secondary to effects of Aβ accumulation, oxidative stress, and/or energy metabolic dysfunction. Aβ has been shown to inhibit glutamate uptake by synaptosomes and glia.16 The observation that free-radical scavengers block these effects is consistent with a model in which Aβ inhibits glutamate uptake via oxidative damage.113 The potentially synergistic multiple effects of Aβ on glutamate function, including enhancing its release, preventing its uptake, and increasing neuronal vulnerability, along with the degenerative feedback cycle of excess glutamate, excess depolarization and intracellular Ca2+, with subsequent further glutamate release and loss of multiple neuronal functions, points to a prominent role for excitotoxicity in AD.
These findings suggest that modulation of glutamate receptors might serve as a neuroprotective strategy in AD. Memantine is an NMDA channel uncompetitive antagonist that preferentially blocks channel opening and neuronal death due to excessive exposure to glutamate while allowing physiological activation required for long-term potentiation (LTP).114,115 Administration of memantine to rats has been found to block neuronal degeneration caused by injection of Aβ1–40 raising the possibility that memantine can protect against Aβ-induced degeneration.116 In a recent clinical trial, a comparison of AD patients treated with memantine versus placebo showed that after 26 weeks, patients treated with memantine demonstrated a significantly reduced decline in scores measuring overall clinical impression of change and activities of daily living.117 Determining whether these effects were due to an actual slowing of underlying disease progression or were merely the result of symptomatically improved function will require further clinical and animal studies.
Summary
Given the many potential limitations of isolated Aβ-based therapies, it is likely that effective AD therapeutics will include parallel strategies that confer neuroprotection against deleterious forms of Aβ and other agents and processes causing neuronal dysfunction and degeneration. Mechanisms underlying the onset and progression of AD are likely to consist of a number of interacting events including the following: excessive accumulation of Aβ, oxidative stress, deleterious stress kinase/JNK signaling, aberrant tau phosphorylation, excitotoxicity, disruption of neurotrophin signaling, loss of synapses, neurites and neurons, and loss of cholinergic and other neurotransmitter function. Given the many layers of potential integration and the mutually reinforcing nature of these processes, it seems unlikely that clinically achievable modulation of a single process will prevent onset or significantly slow AD.
These candidate underlying mechanisms of neuronal degeneration point to a number of therapeutic strategies currently at various stages of development. A number of agents with the potential to provide neuroprotective effects (including ACIs, memantine, and antioxidants) are already clinically available; however, results of additional clinical testing will be required to determine if any of these are capable of delaying onset or slowing underlying disease progression. Other compounds not in widespread clinical use but undergoing clinical trials in AD and/or other neurodegenerative disorders include nerve growth factor, valproate and other GSK inhibitors, various nicotinic agonists, the CEP-1347 stress kinase inhibitor, minocycline as a caspase inhibitor, and metal chelators. Some compounds, such as neurotrophin small molecule mimetics, might prove successful in addressing multiple underlying disease mechanisms in parallel. Taken together, the work reviewed here points to a promising emerging picture of successful, mechanism-based, neuroprotective strategies for AD.
Acknowledgments
Work by the authors is supported by NIA R01AG09873 (F. L.), the Alzheimer’s Association (F. L.), the Institute for the Study on Aging (F. L. and S. M.), and the Veterans Administration (S. M.).
REFERENCES
- 1.Golde TE. Alzheimer disease therapy: can the amyloid cascade be halted? J Clin Invest 111: 11–18, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid-based therapies. Annu Rev Pharmacol Toxicol 43: 545–584, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Golde TE. Inflammation takes on Alzheimer disease. Nat Med 8: 936–938, 2002. [DOI] [PubMed] [Google Scholar]
- 4.McGeer PL, McGeer EG. Local neuroinflammation and the progression of Alzheimer’s disease. J Neurovirol 8: 529–538, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health 88: 1337–1342, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hake AM, Scherer P. On the brink of the pandemic: epidemiology and risk factors for Alzheimer’s. Paper presented at the World Alzheimer’s Congress, Washington, D.C., July 9–18, 2000.
- 7.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297: 353–356, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Holtzman DM, Bales KR, Paul SM, DeMattos RB. Aβ immunization and anti-Aβ antibodies: potential therapies for the prevention and treatment of Alzheimer’s disease. Adv Drug Delivery Rev 54: 1603–1613, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Smith MA, Casadesus G, Joseph JA, Perry G. Amyloid-β and τ serve antioxidant functions in the aging and Alzheimer brain. Free Radic Biol Med 9: 1194–1199, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Plant LD, Boyle JP, Smith IF, Peers C, Pearson HA. The production of amyloid β peptide is a critical requirement for the viability of central neurons. J Neurosci 23: 5531–5535, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yankner BA. The pathogenesis of Alzheimer’s disease. Is amyloid β-protein the beginning of the end? Ann NY Acad Sci 924: 26–28, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Yankner BA, Caceres A, Duffy LK. Nerve growth factor potentiates the neurotoxicity of β-amyloid. Proc Natl Acad Sci USA 87: 9020–9023, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H et al. Intraneuronal Alzheimer Aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Path 161: 1869–1879, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, McLaughlin R, Goodyer C, LeBlanc A. Selective cytotoxicity of intracellular amyloid β peptide1–41 through p53 and Bax in cultured primary human neurons. J Cell Biol 156: 519–529, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawasumi M, Hashimoto Y, Chiba T, Kanekura K, Yamagishi Y, Ishizaka M et al. Molecular mechanisms for neuronal cell death by Alzheimer’s amyloid precursor protein-relevant insults. Neurosignals 11: 236–250, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Tran MH, Yamada K, Nabeshima T. Amyloid β-peptide induces cholinergic dysfunction and cognitive deficits: a minireview. Peptides 23: 1271–1283, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Bozyczko-Coyne D, O’Kane TM, Wu ZL, Dobrzanski P, Murthy S, Vaught JL, Scott RW. CEP-1347/KT-7515, as an inhibitor of SAPK/JNK pathway activation, promotes survival and blocks multiple events associated with Aβ-induced cortical neuron apoptosis. J Neurochem 77: 849–863, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Bozyczko-Coyne D, Saporito MS, Hudkins RL. Targeting the JNK pathway for therapeutic benefit in CNS disease. Curr Drug Target CNS Neurol Disord 1: 31–49, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Flavell R, Davis RJ, Shirasaki Y, Greenberg ME. β-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci 21: 7551–7560, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fogarty MP, Downer EJ, Campbell V. A role for c-Jun N-terminal kinase 1 (JNK1), but not JNK2, in the β-amyloid-mediated stabilization of protein p53 and induction of the apoptotic cascade in cultured cortical neurons. Biochem J 371: 789–798, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoji M, Iwakami N, Takeuchi S, Waragai M, Suzuki M, Kanazawa I, Lippa CF, Ono S, Okazawa H. JNK activation is associated with intracellular β-amyloid accumulation. Brain Res Mol Brain Res 85: 221–233, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Raina AK, Rottkamp CA, Aliev G, Peggy G, Boux H, Smith MA. Activation and redistribution of c-Jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer’s disease. J Neurochem 76: 435–441, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winbald B, Cowburn RF. Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer’s disease brains at different stages of neurofibrillary degeneration. J Alzheimers Dis 3: 41–48, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Savage MJ, Lin YG, Ciallella JR, Flood DG, Scott RW. Activation of c-Jun N-terminal kinase and p38 in an Alzheimer’s disease model is associated with amyloid deposition. J Neurosci 22: 3376–3385, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troy CM, Rabacchi SA, Xu Z, Maroney AC, Connors TJ, Shelanski ML, Greene LA. β-Amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J Neurochem 77: 157–164, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Wei W, Wang X, Kusiak JW. Signaling events in amyloid β-peptide-induced neuronal death and insulin growth factor I protection. J Biol Chem 277: 17649–17656, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Marques CA, Keil U, Bonert A, Steiner B, Hass C, Muller WE, Eckert A. Neurotoxic mechanisms caused by the Alzheimer’s disease-linked Swedish APP mutation: oxidative stress, caspases and JNK pathway. J Biol Chem 278: 28294–28302, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Tamagno E, Robino G, Obbili A, Bardini P, Aragno M, Parola M et al. H2O2 and 4-hydroxynonenal mediate amyloid β-induced neuronal apoptosis by activating JNKs and p38MAPK. Exp Neurol 180: 144–155, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Harris CA, Deshmukh M, Tsui-Pierchala B, Maroney AC, Johnson EM. Inhibition of the c-Jun N-terminal kinase signaling pathway by the mixed lineage kinase inhibitor CEP-1347 preserves metabolism and growth of trophic factor-deprived neurons. J Neurosci 22: 103–113, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trojanowski JQ, Lee VM. The role of tau in Alzheimer’s disease. Med Clin North Am 86: 615–627, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez G, Munoz-Montano JR, Satrustegui J, Avila J, Bogonez E, Diaz-Nido J. Regulation of tau phosphorylation and protection against β-amyloid-induced neurodegeneration by lithium. Possible implications for Alzheimer’s disease. Bipolar Disorder 4: 153–165, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Gozes I. Tau as a drug target in Alzheimer’s disease. J Mol Neurosci 19: 337–338, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem 72: 1327–1330, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Loy R, Tariot PN. Neuroprotective properties of valproate: potential benefit for AD and tauopathies. J Mol Neurosci 19: 303–307, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Sato S, Tatebayashi Y, Akagi T, Chui DH, Murayama M, Miyasaka T et al. Aberrant tau phosphorylation by glycogen synthase kinase-3β and JNK3 induces oligomeric tau fibrils in COS-7 cells. J Biol Chem 277: 42060–42065, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Michaelis ML, Chen Y, Hill S, Reiff E, Georg G, Rice A, Audus K. Amyloid peptide toxicity and microtubule-stabilizing drugs. J Mol Neurosci 19: 101–105, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Roth KA. Caspases, apoptosis, and Alzheimer disease: causation, correlation, and confusion. J Neuropathol Exp Neurol 60: 829–838, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Rohn TT, Rissman RA, Head E, Cotman CW. Caspase activation in the Alzheimer’s disease brain: tortuous and torturous. Drug News Perspect 15: 549–557, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature 407: 802–809, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Ivins KJ, Thornton PL, Rohn TT, Cotman CW. Neuronal apoptosis induced by β-amyloid is mediated by caspase-8. Neurobiol Dis 6: 440–449, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Allen JW, Eldadah BA, Huang X, Knoblach SM, Faden AI. Multiple caspases are involved in β-amyloid-induced neuronal apoptosis. J Neurosci Res 65: 45–53, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Deshmukh M, Vasilakos J, Deckwerth TL, Lampe PA, Shivers BD, Johnson EM Jr. Genetic and metabolic status of NGF-deprived sympathetic neurons saved by an inhibitor of ICE family proteases. J Cell Biol 135: 1341–1354, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dore S, Kar S, Quirion R. Insulin-like growth factor I protects and rescues hippocampal neurons against β-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci 94: 4772–4777, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mattson MR, Tomaselli K, Rydel RE. Calcium-destabilizing and neurodegenerative effects of aggregated β-amyloid peptide are attenuated by basic FGF. Brain Res 1993a; 621: 35–49, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Fitzpatrick JL, Mize AL, Wade CB, Harris JA, Shapiro RA, Dorsa DM. Estrogen-mediated neuroprotection against β-amyloid toxicity requires expression of estrogen receptor α or β and activation of the MAPK pathway. J Neurochem 82: 674–682, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Gozes I, Brenneman DE. A new concept in the pharmacology of neuroprotection. J Mol Neurosci 14: 61–68, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Niikura T, Hashimoto Y, Tajima H, Nishimoto I. Death and survival of neuronal cells exposed to Alzheimer’s insults. J Neurosci Res 70: 380–391, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Vicario-Abejon C, Owens D, McKay R, Segal M. Role of neurotrophins in central synapse formation and stabilization. Nat Rev Neurosci 3: 965–974, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science 298: 789–791, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24: 1217–1281, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Chao MV. Neurotrophins and their receptors: a convergence point for many signaling pathways. Nature Rev 4: 299–309, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Mufson EJ, Kordower JH. Cortical neurons express nerve growth factor receptors in advanced age and Alzheimer’s disease. Proc Natl Acad Sci 89: 569–573, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drake CT, Milner TA, Patterson SL. Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity. J Neurosci 19: 8009–8026, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savaskan E, Muller-Spahn F, Olivieri G, Bruttel S, Otten U, Rosenberg C, Hulette C, Hock C. Alterations in trkA, trkB and trk C receptor immunoreactivities in parietal cortex and cerebellum in Alzheimer’s disease. Eur Neurol 44: 172–180, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Pitts AF, Miller MW. Expression of nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 in the somatosensory cortex of the mature rat: coexpression with high-affinity neurotrophin receptors. J Comp Neurol 418: 241–254, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Hu X-Y, Zhang H-Y, Qin S, Xu H, Swaab DF, Zhou J-N. Increased p75NTR expression in hippocampus neurons containing hyperphosphorylated τ in Alzheimer patients. Exp Neurol 178: 104–111, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Park H-S, Kim M-S, Huh S-H, Park J, Chung J, Kang SS, Choi E-J. Akt (protein kinase B) negatively regulates SEK1 by means of protein phosphorylation. J Biol Chem 277: 2573–2578, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Dugan LL, Creedon DJ, Johnson EM, Holtzman DM. Rapid suppression of free radical formation by nerve growth factor involves the mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA 94: 4086–4091, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol 67: 203–233, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Rabizadeh S, Bredesen DE. Ten years on: mediation of cell death by the common neurotrophin receptor p75NTR. Cytokine Growth Factor Rev 14: 224–239, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Dobrowsky RT, Carter BD. Coupling of the p75 neurotrophin receptor to sphingolipid signaling. Ann NY Acad Sci 845: 32–45, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol Cell Neurosci 18: 210–220, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Hempstead BL. The many faces of p75NTR. Curr Opin Neurobiol 12: 260–267, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Longo, FM, Manthorpe M, Xie Y, Varon S. Synthetic NGF peptide derivatives prevent neuronal death via a p75 receptor-dependent mechanism. J Neurosci Res 48: 1–17, 1997. [DOI] [PubMed] [Google Scholar]
- 65.Tuszynski MH. Growth-factor gene therapy for neurodegenerative disorders. Lancet Neurol 1: 51–57, 2002. [DOI] [PubMed] [Google Scholar]
- 66.Robner S, Ueberham U, Schliebs R, Perez-Polo JR, Bigl V. The regulation of amyloid precursor protein metabolism by cholinergic mechanisms and neurotrophin receptor signaling. Prog Neurobiol 56: 541–569, 1998. [DOI] [PubMed] [Google Scholar]
- 67.Mufson EJ, Kroin JS, Sendera TJ, Sobreviela T. Distribution and retrograde transport of trophic factors in the central nervous system: functional implications for the treatment of neurodegenerative diseases. Prog Neurobiol 57: 451–484, 1999. [DOI] [PubMed] [Google Scholar]
- 68.Cooper JD, Salehi A, Delcroix JD, Howe CL, Belichenko PV, Chua-Couzens J et al. Failed retrograde transport of NGF in a mouse model of Down syndrome: reversal of cholinergic neurodegenerative phenotype following NGF infusion. Proc Natl Acad Sci USA 98: 10439–10444, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salehi A, Delcroix JD, Mobley WC. Traffic at the intersection of neurotrophic factor signaling and neurodegeneration. Trends Neurosci 26: 73–80, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Capsoni S, Giannotta S, Cattaneo A. β-amyloid plaques in a model for sporadic Alzheimer’s disease based on transgenic anti-nerve growth factor antibodies. Mol Cell Neurosci 21: 15–28, 2002. [DOI] [PubMed] [Google Scholar]
- 71.Pizzo DP, Winkler J, Sidiqi I, Waite JJ, Thal LJ. Modulation of sensory inputs and ectopic presence of Schwann cells depend upon the route and duration of nerve growth factor administration. Exp Neurol 178: 91–103, 2002. [DOI] [PubMed] [Google Scholar]
- 72.Eriksdotter JM, Nordberg A, Amberla K, Backman L, Ebendal T, Meyerson B, Olson L et al. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 9: 246–257, 1998. [DOI] [PubMed] [Google Scholar]
- 73.Xie Y, Longo FM. Neurotrophin small-molecule mimetics. Prog Brain Res 128: 333–347, 2000. [DOI] [PubMed] [Google Scholar]
- 74.Saragovi HU, Gehring K. Development of pharmacological agents for targeting neurotrophins and their receptors. Trends Pharmacol Sci 21: 93–98, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Massa SM, Xie YM, Longo FM. Alzheimer’s therapeutics: neurotrophin small molecule mimetics. J Mol Neurosci 19: 107–111, 2002. [DOI] [PubMed] [Google Scholar]
- 76.Xie Y, Tisi MA, Yeo TT, Longo FM. Nerve growth factor (NGF) loop 4 dimeric mimetics activate ERK and AKT and promote NGF-like neurotrophic effects. J Biol Chem 275: 29868–29874, 2000. [DOI] [PubMed] [Google Scholar]
- 77.Auld DA, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer’s disease and the basal forebrain cholinergic system: relations to β-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol 68: 209–245, 2002. [DOI] [PubMed] [Google Scholar]
- 78.Farlow M, Anand R, Messina J Jr, Hartman R, Veach J. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol 44: 236–241, 2000. [DOI] [PubMed] [Google Scholar]
- 79.Coyle J, Kershaw P. Galantamine, a cholinesterase inhibitor that allosterically modulates nicotinic receptors: effects on the course of Alzheimer’s disease. Biol Psychiatry 49: 289–299, 2001. [DOI] [PubMed] [Google Scholar]
- 80.Doraiswamy PM, Krishnan KR, Anand R, Sohn H, Danyluk J, Hartman RD, Veach J. Long-term effects of rivastigmine in moderately severe Alzheimer’s disease: does early initiation of therapy offer sustained benefits? Prog Neuropsychopharmacol Biol Psychiatry 26: 705–712, 2002. [DOI] [PubMed] [Google Scholar]
- 81.Farlow M, Potkin S, Koumaras B, Veach J, Mirski D. Analysis of outcome in retrieved dropout patients in a rivastigmine vs placebo, 26-week, Alzheimer disease trial. Arch Neurol 60: 843–848, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Fisher A, Brandeis R, Haring R, Kliger-Spatz M, Natan N, Sonego H et al. AF150(S) and AF267B: M1 muscarinic agonists as innovative therapies for Alzheimer’s disease. J Mol Neurosci 19: 145–153, 2002. [DOI] [PubMed] [Google Scholar]
- 83.Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science 258: 304–307, 1992. [DOI] [PubMed] [Google Scholar]
- 84.Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE, Greengard P. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta-A4 amyloid protein precursor. Proc Natl Acad Sci USA 89: 10075–10078, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hung AY, Haass C, Nitsch RM, Qui WQ, Citron M, Wurtman RJ, Growdon JH, Selkoe DJ. Activation of protein kinase C inhibits cellular production of the amyloid beta-protein. J Biol Chem 268: 22959–22962, 1993. [PubMed] [Google Scholar]
- 86.Lin L, Georgievska B, Mattson A, Isacson O. Cognitive changes and modified processing of amyloid precursor protein in the cortical and hippocampal system after cholinergic synapse loss and muscarinic receptor activation. Proc Natl Acad Sci USA 96: 12108–12113, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hellstrom-Lindahl E. Modulation of β-amyloid precursor protein processing and tau phosphorylation by acetylcholine receptors. Eur J Pharmacol 393: 255–263, 2000. [DOI] [PubMed] [Google Scholar]
- 88.Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Shibasaki H, Kume T, Akaike A. α7 Nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block Aβ-amyloid-induced neurotoxicity. J Biol Chem 276: 13541–13546, 2001. [DOI] [PubMed] [Google Scholar]
- 89.Knipper M, de Penha Berzaghi M, Blochl A, Breer H, Thoenen H, Lindholm D. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived growth neurotrophic factor in the rat hippocampus. Eur J Neurosci 6: 668–671, 1994. [DOI] [PubMed] [Google Scholar]
- 90.Isacson O, Seo H, Lin L, Albeck D, Granholm AC. Alzheimer’s disease and Down’s syndrome: roles of APP, trophic factors and Ach. Trends Neurosci 25: 79–84, 2002. [DOI] [PubMed] [Google Scholar]
- 91.Nitsch RM, Deng M, Tennis M, Schoenfeld D, Growdon JH. The selective muscarinic M1 agonist AF102B decreases levels of total Aβ in cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol 48: 913–918, 2000. [PubMed] [Google Scholar]
- 92.Hock C, Maddalena A, Raschig A, Muller-Spahn F, Eschweiler G, Hag K et al. Treatment with the selective muscarinic m1 agonist talsaclidin decreases cerebrospinal fluid levels of Aβ42 in patients with Alzheimer’s disease. Amyloid 10: 1–6, 2003. [DOI] [PubMed] [Google Scholar]
- 93.Butterfield DA. Amyloid β-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic Res 36: 1307–1313, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging 23: 795–807, 2002. [DOI] [PubMed] [Google Scholar]
- 95.Pratico D, Clark CM, Liun F, Lee VY-M, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment. Arch Neurol 59: 972–976, 2002. [DOI] [PubMed] [Google Scholar]
- 96.Kourie JI. Mechanisms of amyloid β protein-induced modification of ion transport systems: implications of neurodegenerative diseases. Cell Mol Neurobiol 21: 173–213, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beal MF. Mitochondria, free radicals, and neurodegeneration. Curr Opin Neurobiol 6: 661–666, 1996. [DOI] [PubMed] [Google Scholar]
- 98.Bush AI. The metallobiology of Alzheimer’s disease. Trends Neurosci 26: 207–214, 2003. [DOI] [PubMed] [Google Scholar]
- 99.Pratico D. Alzheimer’s disease and oxygen radicals: new insights. Biochem Pharmacol 63: 563–567, 2002. [DOI] [PubMed] [Google Scholar]
- 100.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci 21: 8370–8377, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cole GM. Ironic fate: can a banned drug control metal heavies in neurodegenerative disease? Neuron 37: 889–893, 2003. [DOI] [PubMed] [Google Scholar]
- 102.Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 30: 665–676, 2001. [DOI] [PubMed] [Google Scholar]
- 103.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Wilson RS, Scherr PA. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer’s disease in a biracial community study. JAMA 287: 3230–3237, 2002. [DOI] [PubMed] [Google Scholar]
- 104.Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer’s disease. JAMA 287: 3223–3229, 2002. [DOI] [PubMed] [Google Scholar]
- 105.Foley DJ, White LR. Dietary intake of antioxidants and risk of Alzheimer’s disease: food for thought. JAMA 287: 3261–3263, 2002. [DOI] [PubMed] [Google Scholar]
- 106.Luchsinger JA, Tang M-X, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer’s disease. Arch Neurol 60: 203–208, 2003. [DOI] [PubMed] [Google Scholar]
- 107.Sano M, Ernesto MS, Thomas RG, Klauber MR, Schafer K, Grundman M et al. A controlled trial of selegiline, alpha-tocopherol or both as a treatment for Alzheimer’s disease. N Engl J Med 336: 1216–1222, 1997. [DOI] [PubMed] [Google Scholar]
- 108.Grundman M, Delaney P. Antioxidant strategies for Alzheimer’s disease. Proc Nutr Soc 61: 191–202, 2002. [DOI] [PubMed] [Google Scholar]
- 109.Gutzmann H, Hadler D, Erzigkeit H. Long-term treatment of Alzheimer’s disease with idebenone. In: Alzheimer’s disease: biology, diagnosis and therapeutics (Iqbal K, Winbald B, Nishimura T, Takeda M, Wisniewski HM, eds), pp 687–705. UK: Wiley, 2002 1997.
- 110.Choi DW. Calcium and excitotoxic neuronal injury. Ann NY Acad Sci 747: 162–171, 1994. [DOI] [PubMed] [Google Scholar]
- 111.Butterfield DA, Pocernich C. The glutamatergic system in Alzheimer’s disease: therapeutic implications. CNS Drugs 17: 641–652, 2003. [DOI] [PubMed] [Google Scholar]
- 112.Olney JW, Wozniak DF, Farber NB. Glutamate receptor dysfunction and Alzheimer’s disease. Restor Neurol Neurosci 13: 75–83, 1998. [PubMed] [Google Scholar]
- 113.Harris ME, Carney JM, Cole PS, Hensley K, Howard K, Howard BJ, Martin L et al. Beta-amyloid peptide-derived, oxygen-dependent free radicals inhibit glutamate uptake in cultured astrocytes: implications for Alzheimer’s disease. Neuroreport 6: 1875–1879, 1995. [DOI] [PubMed] [Google Scholar]
- 114.Erdo SL, Schafer M. Memantine is highly potent in protecting cortical cultures against excitotoxic cell death evoked by glutamate and N-methyl-D-aspartate. Enr J Pharmacol 198: 215–217, 1991. [DOI] [PubMed] [Google Scholar]
- 115.Parsons CG, Gruner J, Rozental J, Millar J, Lodge D. Patch clamp studies on the kinetics and selectivity of N-methyl-D-aspartate receptor antagonism by memantine (1-amino-3, 5-dimethyladamantan). Neuropharmacology 32: 1337–1350, 1993. [DOI] [PubMed] [Google Scholar]
- 116.Miguel-Hidalgo JJ, Alvarez XA, Cacabelos R, Quack G. Neuroprotection by memantine against neurodegeneration induced by β-amyloid(1–40). Brain Res 958: 210–221, 2002. [DOI] [PubMed] [Google Scholar]
- 117.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 348: 1333–1341, 2003. [DOI] [PubMed] [Google Scholar]