Abstract
Measurements of body condition are typically used to assess an individual’s quality, health, or energetic state. Most indices of body condition are based on linear relationships between body length and mass. Although these indices are simple to obtain, nonlethal, and useful indications of energetic state, their accuracy at predicting constituents of body condition (e.g., fat and lean mass) are often unknown. The objectives of this research were to (1) validate the accuracy of another simple and noninvasive method, quantitative magnetic resonance (QMR), at estimating body composition in a small-bodied lizard, Anolis sagrei, and (2) evaluate the accuracy of two indices of body condition (based on length–mass relationships) at predicting body fat, lean, and water mass. Comparisons of results from QMR scans to those from chemical carcass analysis reveal that QMR measures body fat, lean, and water mass with excellent accuracy in male and female lizards. With minor calibration from regression equations, QMR will be a reliable method of estimating body composition of A. sagrei. Body condition indices were positively related to absolute estimates of each constituent of body composition, but these relationships showed considerable variation around regression lines. In addition, condition indices did not predict fat, lean, or water mass when adjusted for body mass. Thus, our results emphasize the need for caution when interpreting body condition based upon linear measurements of animals. Overall, QMR provides an alternative noninvasive method for accurately measuring fat, lean, and water mass in these small-bodied animals.
Body condition is often used as an indicator of an individual’s energetic state, health, or quality (Schulte-Hostedde et al., 2001; Peig and Green, 2009), and is generally assumed to be positively related to survival or reproductive success (Millar and Hickling, ‘90; Linden et al., ‘92; Hayes and Shonkwiler, 2001; Le Galliard et al., 2004; Barnett et al., 2015). For example, body condition is associated with foraging success (Gaëtan et al., 2014) and immune function (Navarro et al., 2003), and can influence how well individuals withstand harsh environments (Evans, ‘69; Millar and Hickling, ‘90). Thus, accurate measurements of body condition are critical to evolutionary ecologists, but yet body condition is often poorly defined and is estimated in many different ways (Labocha et al., 2014). Although body condition is traditionally viewed as an animal’s body reserves (accumulated via feeding) after accounting for maintenance costs (Rowe and Houle, ‘96; Peig and Green, 2009; Barnett et al., 2015), most estimates of condition do not directly measure energy reserves, but instead use an index based on the linear relationship between body length and mass (e.g., length–mass residuals, Hayes and Shonkwiler, 2001; Schulte-Hostedde et al., 2005). Such estimates have been shown to correlate well with energy reserves or other constituents of body composition (Schulte-Hostedde et al., 2001, 2005), but this is not the case for some other organisms (e.g., Schulte-Hostedde et al., 2001; Kelly et al., 2014). This problem arises due to the fact that measures of body mass do not necessarily reflect fat mass, but instead could be reflective of other constituents of body composition at the time of measurement. Moreover, some key assumptions in regression-based indices of condition are often violated (Green, 2001; but see Schulte-Hostedde et al., 2005; Peig and Green, 2009).
Despite some drawbacks of using length–mass residuals for estimates of body condition, a major advantage is that this method is relatively simple and noninvasive. This is important in population-wide studies that require quick and feasible measures of individual quality. Low stress, noninvasive approaches like this are also necessary in studies that focus on species of conservation concern (Stevenson and Woods, 2006) or longitudinal studies designed to assess relationships between body condition and fitness (Civantos and Forsman, 2000; Merilä et al., 2001; Cox and Calsbeek, 2015). Despite the abundant use of regression-based estimates of body condition, however, the accuracy of these condition indices is rarely validated. Moreover, alternative noninvasive and simple methods of estimating body condition are rare, because direct measures of body composition (e.g., fat, lean, water mass) require dissections or euthanasia. The most accurate, but terminal, method for measuring body composition is chemical carcass analysis (CCA), which is the “gold standard” to which other methods are compared (Reynolds and Kunz, 2001).
Nevertheless, several alternative, nonlethal methods to measure body composition exist. For example, absorption of cyclopropane gas by animals can provide accurate predictions of lipid content of live animals (Henen, ‘91); however, this method is labor intensive and is not necessarily practical for ecological studies. Total body electrical conductivity (TOBEC) and total body water (TBW) have been used to predict lean mass and nonpolar lipid mass in many different animals (Castro et al., ‘90; Boyd et al., ‘93; Fischer et al., ‘96; Angilletta, ‘99). However, validation of these methods has yielded mixed results and their accuracy of predicting body composition of small animals (<25 g) is poor and understudied (Fischer et al., ‘96; Scott et al., 2001). Dual-energy X-ray absorptiometry has been validated in snakes (Secor and Nagy, 2003) and fish (Johnson et al., 2016) and is more easily and accurately used for measuring body composition than TOBEC and cyclopropane gas. Bioimpedance spectroscopy has also shown excellent accuracy in predicting TBW, fat-free mass, and fat mass in rats (Smith et al., 2009). Importantly, one drawback of some of these methods (e.g., dual-energy X-ray absorptiometry and bioimpedance spectroscopy) is that anesthesia or sedation may be required to immobilize animals, which could have unwanted side effects.
In this study, we assess the accuracy of an alternative and nondestructive method for estimating body composition in small animals. Quantitative magnetic resonance (QMR) was developed for quantifying body fat and lean mass in small mammals used primarily in medical research, and in studies of nutrition and obesity (Taicher et al., 2003; Johnson et al., 2009; Schwartz et al., 2015). Validation studies of this method in rodents, bats, birds, and fish demonstrate that it is accurate and precise (Johnson et al., 2009; McGuire and Guglielmo, 2010; Guglielmo et al., 2011; Fowler et al., 2016). QMR is based on the principles of nuclear magnetic resonance, which uses radio waves to manipulate how nuclei of atoms spin in order to identify the molecules being tested (Taicher et al., 2003). Any atom with an odd number mass can be used, such as hydrogen, carbon-13, oxygen-17, and phosphorus-31 as they produce a magnetic moment when their atoms spin. The QMR instrument evaluated in this study uses hydrogen (proton)-NMR principles. Briefly, the animal is placed within a magnetic field that aligns the protons in the body. Radio waves then excite the protons and the energy released when they return to baseline, and the time it takes is measured. These characteristics of the protons are different depending whether the protons are associated with fat or lean tissue.
Our primary objective is to assess the use of QMR as a practical method for determining body composition in a small lizard (Anolis sagrei; body mass range: ~2–8 g) that serves as an important model for studies in animal behavior, ecology, and evolution (Losos, ‘94, 2009). Our specific goals are (1) to validate the accuracy of QMR in predicting body composition of small lizards, (2) to determine if the accuracy differs between males and females (which differ substantially in body size), and (3) to quantify the relationship between two estimates of body condition (based on mass–length relationships) with body composition from CCA. This final goal will provide important insights into the biological significance of traditional body condition indices that are commonly used in ecological and evolutionary studies.
METHODS
Forty-three A. sagrei were used to test the accuracy of QMR at determining water, fat, and lean tissue mass. Tests were performed on two cohorts of lizards. The first cohort consisted of 22 individuals (11 male and 11 female) collected in Palm Coast, Florida in October 2012. These lizards were housed in captivity at the University of Alabama at Birmingham for 1 year prior to QMR validation. The body mass of these individuals ranged from 1.97 to 8.60 g at the time of testing. For the second cohort, 21 individuals (10 males and 11 females) were captured in Ormond Beach, FL (about 30 km south of the Palm Coast site) about 1 year later (16 October 2013) and were used for QMR validation 15 days after capture. The body mass of individuals in the second cohort ranged from 1.54 to 5.86 g at the time of testing. The details of housing conditions are described in Warner et al. (2015). All lizards were measured and weighed, and their body composition using QMR and CCA was determined on a single day.
Quantitative Magnetic Resonance
In vivo body composition (fat, lean, and total water) was determined using the EchoMRI™ 3-in-1 quantitative magnetic resonance machine (Echo Medical Systems, Houston, TX) with software version 2013. This system has three probes (mouse, tissue, and biopsy) and the mouse probe and holder (53-mm diameter) were used in this study. Quality control was performed using a known fat standard prior to any animals being scanned. Lizards were weighed and then placed into a clear holding tube (designed for mice) capped with a stopper that restricted vertical movement, but allowed constant air flow. The tube was inserted into the machine and the lizards scanned using the mouse setting with the primary accumulation = 3 setting. The scan time was 2.5 min and the average room temperature was 21.5°C.
Chemical Carcass Analysis
Immediately after the QMR scans, lizards were euthanized by decapitation, females were opened to determine the presence of oviductal eggs (only two individuals from the first cohort had eggs and were removed from statistical analyses), and CCA was performed for each individual. The lizards were dried in an oven at 60°C until constant weight (approximately 5 days). Water content was determined as the weight lost during drying. The dried carcasses were then ground using a mortar and pestle and placed in a weighed cellulose thimble and reweighed. The thimbles were placed in a Soxhlet apparatus for fat extraction using petroleum ether as the solvent (Dobush and Ankey ‘85). The thimbles were then dried in the oven overnight and reweighed. The weight lost during the extraction was the fat content. The remaining fat-free dry mass was placed in weighed porcelain crucibles, reweighed, and ashed in a muffle furnace at 600°C overnight. The remaining ash was the mineral content. Lean mass was calculated as the sum of water mass and fat-free dry mass minus ash mass.
Statistical Analyses
Statistical analyses were performed with SAS software (version 9.3). Linear regressions were used to determine the accuracy of QMR at predicting fat, lean tissue, and water mass; independent variables were the tissue estimates (g) from the QMR scans and dependent variables were the measurements from the CCA. Differences in predictability of QMR scans between sexes and cohorts were tested with slopes tests from analysis of covariance (interaction between sex or cohort with the covariate; tissue measurement from the QMR scan). In all cases, interactions between sex and cohort with the covariate were not significant and therefore this interaction term was removed from final models. Biases in QMR estimates of body composition were visualized by subtracting the CCA values from QMR values and regressing the difference against CCA values for each constituent of body composition.
A cross-validation procedure was performed to assess the ability of the regression equations at estimating each body component. To do this, regression equations for males and females based on relationships between QMR and CCA values using only data from the first cohort was used to predict lean, fat, and water mass of the second cohort. The predicted values for each body component were then regressed against the observed value from the CCA. Paired t-tests were also used to compare observed and expected values for each body component.
Body condition was calculated using two commonly used indices based on relationships between body mass and length. First, the residual index of condition (Ri) was calculated from ordinary least squares (OLS) regressions of log-transformed body mass on log-transformed snout–vent length (SVL); regressions were performed separately for each sex. Second, we used the scaled mass index (Mi) of condition using the following equation:
where M and SVL are the body mass and the snout–vent length of the individual, respectively. SVL0 is the mean SVL of the population, and bSMA is the standardized major axis slope from the OLS regression of log-transformed body mass on log-transformed SVL divided by Pearson’s correlation coefficient (LaBarbera, ‘89; Peig and Green, 2009).
Regression analyses were used to quantify the relationship between each index of body condition and CCA estimates of body composition (i.e., lean, fat, and water mass). However, because of the tight positive correlations of body mass with lean mass (r2 = 0.999, P < 0.001), fat mass (r2 = 0.736, P < 0.001), and water mass (r2 = 0.998, P < 0.001), these measures of body composition greatly reflected body size, which differs substantially between male and female A. sagrei. Therefore, to account for body size variation additional analyses were used to assess each body component expressed as the residual score from the regression of each body component versus body mass. Residual scores (i.e., lean, fat, and water relative to body mass) were used as dependent variables to assess their relationships with each body condition index for males and females.
RESULTS
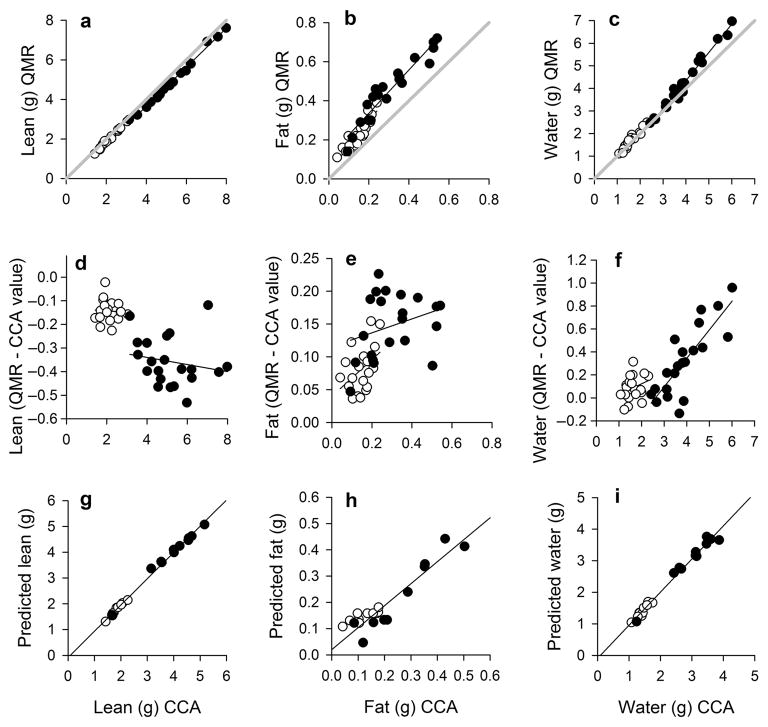
On average, lizard body mass was composed of 71% water (SD = 2.1), 5.9% fat (SD = 1.9), and 23.0% fat-free dry mass (SD = 1.4). Of the fat-free dry mass, 4.6% (SD = 1.0) was ash (mostly bone tissue). Males had greater absolute lean, fat, and water mass (Fig. 1; Table 1) due to their overall larger size than females. However, the sexes did not differ in lean mass (F1,37 = 0.0, P = 0.986), fat mass (F1,38 = 0.2, P = 0.683), and water mass (F1,38 = 0.1, P = 0.749) when adjusted by their total body mass. QMR scans provided accurate measurements of lean, fat, and water mass in A. sagrei (Fig. 1; Table 2). This method nearly perfectly estimated lean mass for males and females (r2-values > 0. 99, slopes ranging from 0.995 to 1.054), but estimates were slightly underestimated (Fig. 1d). The QMR method also provided accurate measures of fat mass (slopes ranging from 0.65 to 0.83), but slightly overestimated the actual value obtained from the chemical carcass analysis (Fig. 1e). Similarly, QMR scans also overestimated water mass, particularly for larger individuals that contained relatively high absolute levels of water (Fig. 1f). The slopes of regression equations for each body component did not differ significantly between sexes or cohorts (All P-values > 0.05).
Figure 1.
Accuracy of quantitative magnetic resonance (QMR) at estimating body composition of brown anoles (Anolis sagrei). Relationship between QMR values and values from a chemical carcass analysis (CCA) for (a) lean mass, (b) fat mass, and (c) water mass. The gray line represents slope = 1. Regressions for the difference in QMR and CCA values for (d) lean mass, (e) fat mass, and (f) water mass. Results from the cross-validation procedure using predicted estimates of (g) lean mass, (h) fat mass, and (i) water mass from regression equations in Table 1 (see text for details). Females are represented by open circles, and males are represented by closed circles. Statistics are reported in Table 1. Although CCA values are on the x-axis (which is the convention in the literature), this variable was the dependent variable in the statistical analyses.
Table 1.
Effect of sex, cohort, and their interaction on body composition of Anolis sagrei
| Dependent variable | Sex | Cohort | Sex × cohort | Covariate |
|---|---|---|---|---|
| Lean mass | F1,35 = 5.4, P = 0.026 | F1,35 = 1.3, P = 0.264 | F1,35 < 0.0, P = 0.991 | F1,35 = 2310.2, P < 0.001 |
| Fat mass | F1,36 = 4.2, P = 0.047 | F1,36 = 3.9, P = 0.055 | F1,36 = 1.8, P = 0.182 | F1,36 = 330.3, P < 0.001 |
| Water mass | F1,36 = 15.8, P < 0.001 | F1,36 = 0.8, P = 0.373 | F1,36 < 0.1, P = 0.900 | F1,36 = 627.1, P < 0.001 |
Cohort represents individuals that were raised in the laboratory for 1 year prior to testing versus individuals that were recently captured in the field. Dependent variables are estimates of body composition from the chemical carcass analysis. Covariates are the measurements of lean, fat, and water mass from the QMR scans. Interactions between main effects and covariates were never significant.
Table 2.
Regression analyses of body composition using values from the chemical carcass analysis (dependent variable, y) and values from quantitative magnetic resonance (independent variable, x)
| N | r2 | P-value | Regression equation | |
|---|---|---|---|---|
| Lean mass (both sexes) | 40 | 0.997 | <0.0001 | y = 1.054x + 0.069 |
| Female | 19 | 0.989 | <0.0001 | y = 0.995x + 0.154 |
| Male | 21 | 0.994 | <0.0001 | y = 1.009x + 0.314 |
| Fat mass (both sexes) | 41 | 0.931 | <0.0001 | y = 0.752x − 0.031 |
| Female | 20 | 0.828 | <0.0001 | y = 0.647x − 0.002 |
| Male | 21 | 0.915 | <0.0001 | y = 0.827x − 0.070 |
| Water mass (both sexes) | 41 | 0.989 | <0.0001 | y = 0.867x + 0.190 |
| Female | 20 | 0.928 | <0.0001 | y = 0.849x + 0.168 |
| Male | 21 | 0.980 | <0.0001 | y = 0.785x + 0.586 |
Note that the variables on the x and y axes in Fig. 1a–c are switched to remain consistent with the presentation of body composition data in the literature. Two females that had oviductal eggs were removed from the analyses.
Cross-validation procedure indicated that QMR precisely estimates body composition (Fig. 1g–i). Lean, fat, and water mass of A. sagrei can be predicted with QMR with average errors of 1.4%, 4.5%, and 2.5%, respectively. Predicted values did not differ significantly from observed values for lean mass (t = −1.25, P = 0.225), fat mass (t = −0.97, P = 0.341), or water mass (t = 0.67, P = 0.510).
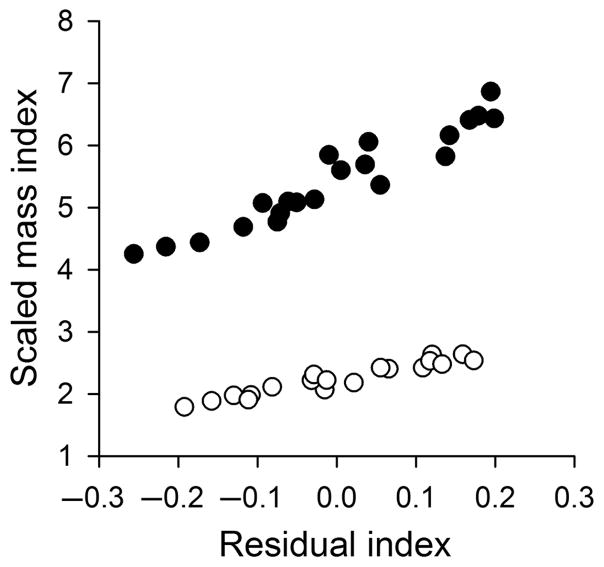
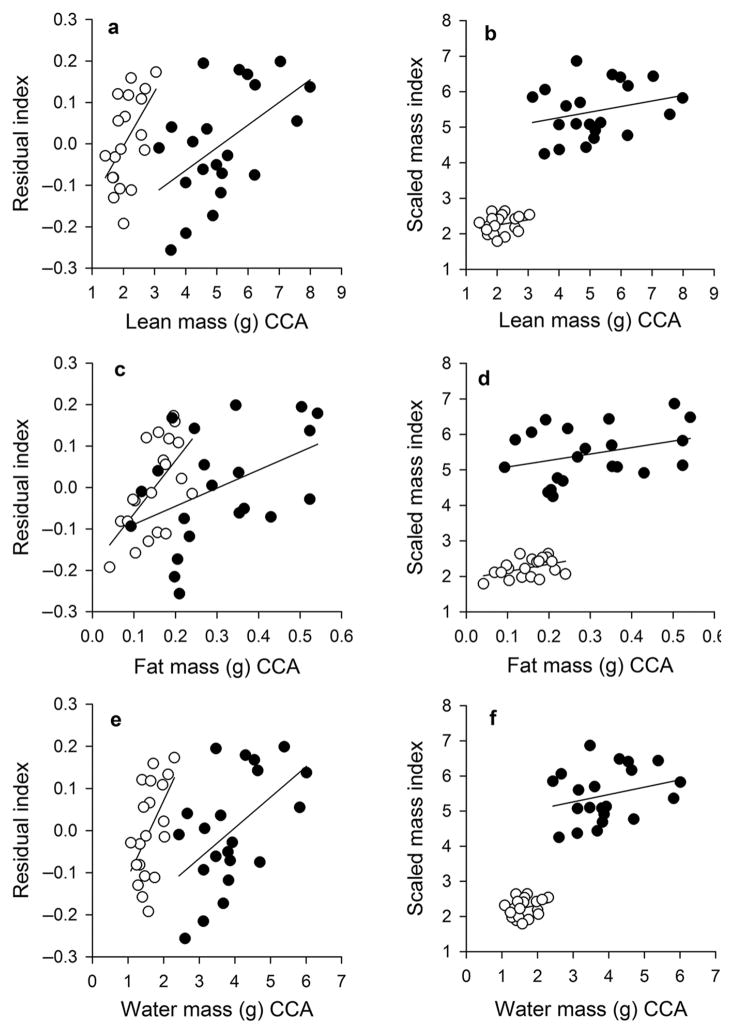
The residual and scaled mass indices of body condition were positively correlated for males (r = 0.955, P < 0.001) and females (r = 0.958, P < 0.001; Fig. 2). Absolute measures of lean, fat, and water mass increased with both body condition indices (Table 3; Fig. 3), but these relationships were not significant within males and females for the scaled mass index (Table 3). Lean, fat, and water relative to body mass were not related to the residual index or scaled mass index of body condition for either sex (Table 4).
Figure 2.
Correlations between the residual and the scaled mass indices of body condition in male (solid circles) and female (open circles) brown anoles (Anolis sagrei).
Table 3.
Regression analyses of the residual index and scaled mass index of body condition versus body components measured from chemical carcass analysis
| Dependent variable | N | Residual index of body condition (Ri)
|
Scaled mass index of body condition (Mi)
|
||||
|---|---|---|---|---|---|---|---|
| r2 | P-value | Regression equation | r2 | P-value | Regression equation | ||
| Lean mass (both sexes) | 40 | 0.058 | 0.135 | y = 3.620x + 3.698 | 0.710 | <0.001 | Y = 0.903x + 0.157 |
| Female | 19 | 0.283 | 0.019 | y = 2.149x + 2.088 | 0.067 | 0.284 | Y = 0.451x + 1.085 |
| Male | 21 | 0.284 | 0.013 | y = 5.180x + 5.166 | 0.072 | 0.239 | Y = 0.463x + 2.645 |
| Fat mass (both sexes) | 41 | 0.135 | 0.018 | y = 0.388x + 0.228 | 0.430 | <0.001 | Y = 0.049x + 0.036 |
| Female | 20 | 0.371 | 0.004 | y = 0.288x + 0.149 | 0.178 | 0.064 | Y = 0.086x − 0.044 |
| Male | 21 | 0.198 | 0.043 | y = 0.453x + 0.303 | 0.108 | 0.146 | Y = 0.059x − 0.019 |
| Water mass (both sexes) | 41 | 0.071 | 0.092 | y = 2.995x + 2.789 | 0.715 | <0.001 | Y = 0.677x + 0.156 |
| Female | 20 | 0.282 | 0.016 | y = 1.557x + 1.609 | 0.076 | 0.241 | Y = 0.348x + 0.829 |
| Male | 21 | 0.286 | 0.013 | y = 3.938x + 3.912 | 0.075 | 0.231 | Y = 0.356x + 1.972 |
Regression equations use body condition indices as the independent variable (x), and each body component as a dependent variable (y).
Figure 3.
Linear relationships between two body condition indices and lean mass (a and b), fat mass (c and d), and water mass (e and f) in the brown anole (Anolis sagrei). Body composition values are based on results from the chemical carcass analyses (CCA). Graphs in the left column show relationship with the residual index of body condition, and graphs in the right column show relationships with the scaled mass index of body condition. Males are represented by solid circles and females by open circles. Statistics are reported in Table 3.
Table 4.
Regression analyses of two body condition indices versus body components (expressed as the residual scores from the regression of body component vs. body mass) measured from chemical carcass analysis
| Dependent variable | N | Residual index
|
Scaled mass index
|
||
|---|---|---|---|---|---|
| r2 | P-value | r2 | P-value | ||
| Percent Lean mass (both sexes) | 40 | 0.035 | 0.247 | 0.004 | 0.684 |
| Female | 19 | 0.150 | 0.101 | 0.086 | 0.222 |
| Male | 21 | 0.026 | 0.484 | 0.035 | 0.415 |
| Percent Fat mass (both sexes) | 41 | 0.060 | 0.124 | 0.002 | 0.787 |
| Female | 20 | 0.258 | 0.022 | 0.161 | 0.080 |
| Male | 21 | 0.040 | 0.384 | 0.047 | 0.347 |
| Percent water mass (both sexes) | 41 | 0.010 | 0.544 | 0.004 | 0.715 |
| Female | 20 | 0.081 | 0.225 | 0.072 | 0.253 |
| Male | 21 | 0.003 | 0.804 | 0.006 | 0.731 |
DISCUSSION
Body composition has strong ecological implications. For example, the level of stored fat can influence reproductive output (Bonnet et al., 2001; Milenkaya et al., 2015), and affect an individual’s likelihood of survival under certain environments (e.g., food shortages or nutrient imbalances; Dussutour et al., 2016) or during critical events (e.g., migration, Sandberg and Moore, ‘96). Body water mass could also be important for survival in arid environments or during periods of drought (Hillman and Gorman, ‘77). Variation in lean mass (as well as fat mass) can affect long distance migrations (Karasov and Pinshow, ‘98) or influence performance during physically demanding activities (Perez-Gomez et al., 2008). Despite potential fitness consequences of body composition, however, many ecological studies do not assess composition directly, but instead rely upon condition indices based on mass–length measurements of individuals. Condition indices are rarely assessed for their accuracy compared to measures of body composition.
The objectives of this research were to validate the accuracy of QMR at estimating body composition in a small-bodied lizard species (A. sagrei) and evaluate the utility of two commonly used indices of body condition. Our results demonstrate that with proper calibration using regression equations, QMR measures lean, fat, and water mass with excellent accuracy for male and female lizards, even in individuals that weigh as little as 1.54 g. Although one body condition index significantly predicted lean, fat, and water mass, there was still considerable variation around regression lines (Fig. 3) and these indices did not significantly predict these aspects of body composition when adjusted to body mass. Thus, our results further emphasize the need for caution when interpreting body condition based upon linear measurements of animals (Kelly et al., 2014; Labocha et al., 2014).
QMR overcomes many of the problems associated with other techniques used for measuring body composition. For example, many techniques are necessarily lethal to the animal, time consuming, generate chemical waste (e.g., CCA), or may require anesthesia (e.g., dual-energy X-ray absorptiometry, isotope dilution, and TOBEC). QMR overcomes all these issues, as live, conscious individuals are simply placed in the QMR machine for a very brief scan (~2.5 min). Moreover, QMR is highly accurate and precise in quantifying lean, fat, and water mass in small mammals (Johnson et al., 2009; Jones et al., 2009; McGuire and Guglielmo, 2010), birds (Guglielmo et al., 2011), and small fish (Fowler et al., 2016), and we see similar accuracy reported here in a small lizard. Indeed, slopes of the relationship between values from CCA with values from QMR scans were nearly 1 for each body component. With slight calibration using regression equations reported in Table 2, estimates of lean, fat, and water mass can be highly accurate. Although we did not assess the precision of QMR estimates in this study by performing multiple scans on the same individual, the high r2 values (>0.9) suggest that QMR values are precise. Moreover, studies that validate QMR in small mammals and birds show very low coefficients of variation for fat mass (~2%), lean mass (~0.4%), and water mass (~2.2%) based on multiple scans of individuals (Johnson et al., 2009; Guglielmo et al., 2011). In addition, our cross-validation analyses suggest that QMR makes excellent predictions of different constituents of body composition. Importantly, the only other study that has used QMR to estimate body composition in reptiles shows excellent accuracy for body water and lean mass in snakes, but the QMR prediction for fat mass has a high relative error (Riley et al., 2016). The difference in QMR estimates of fat mass between the current study and that of Riley et al. (2016) may be attributed to the small amounts of fat within their snakes (~3%) compared to that measured in A. sagrei (~6%) in the current study.
Simple, noninvasive and accurate methods for measuring body composition are critical in longitudinal studies that require repeated measures through time, or in studies that assess impacts of different body components on energetically costly activities, such as growth, dispersal and reproduction. Although QMR provides many advantages over other methods for measuring body composition, the expense ($125,000) and size (59 × 59 × 130 cm3) of a QMR machine may limit its use in some field research programs. Nevertheless, field-portable QMR machines have been used (e.g., in a temperature-controlled trailer; Guglielmo et al., 2011), and depending on the proximity of field sites to QMR-equipped laboratories or field stations, animals could be removed from the wild, scanned, and then released within short timeframes.
The utility of body condition indices has received considerable criticism as estimates of energy reserves and individual health (Green, 2001; Peig and Green, 2009; Labocha and Hayes, 2012). By examining relationships between two commonly used body condition indices with body composition data from chemical analysis (i.e., lean, fat, and water mass), we further assessed the utility of body condition. In line with other studies (e.g., Labocha et al., 2014), our results show that the residual index (but not the scaled mass index) of condition was better at predicting absolute constituents of body composition (e.g., fat, lean, and water mass) than predicting composition adjusted for body mass. Indeed, neither index of body condition explained variation in size-adjusted body fat, lean, or water mass of A. sagrei. Any combination of these body components may influence the mass of an individual, or variation in mass (relative to length) may be explained by body components that were not measured here (e.g., bone density). At best, condition indices can provide an estimation of body mass relative to length, and may explain variation in all aspects of body composition (Schulte-Hostedde et al., 2001). Such an estimate might provide an impression of relative “robustness” of an individual, but it is difficult to draw conclusions about which body component contributes most to variation in relative body mass based on linear measurements.
QMR has been extremely useful in ecological studies on birds (Guglielmo et al., 2011) and bats (McGuire and Guglielmo, 2010) to better understand energetics during different activities (e.g., reproduction and migration; Seewagon and Guglielmo, 2010), and how body composition changes in response to experimental manipulations (Schmidt et al., 2012). Based on our results, QMR technology can be applied in the same way to better understand lizard energetics in the wild, as well as address a wealth of other topics (e.g., the impact of diet on body composition, energy available to fuel reproduction or long distance movements). In particular, this application of QMR has interesting implications for better understanding Anolis reproductive ecology. For example, because A. sagrei lay a single-egg clutch every 7–10 days across an extensive reproductive season (~April–October; Lee et al., ‘89), QMR could be used to assess temporal changes in body fat and its contribution to female fecundity. Moreover, given that prey availability affects reproductive allocation (Warner and Lovern, 2014; Warner et al., 2015), this application could provide new insights into Anolis nutritional ecology and reproductive investment. This technology could also be incorporated into field research (Guiglielmo et al., 2011) and useful in assessing fitness consequences of different body components; such studies rely upon longitudinal measurements via mark-recapture studies where simple and noninvasive methods are required (e.g., QMR). Although body condition indices are simple to calculate and have provided insight into individual body composition, QMR provides an alternative and more accurate measurement of fat reserves and lean mass. Overall, this study provides additional evidence that QMR is an effective method for estimating body composition and provides specific validation in small-bodied Anolis lizards.
Research Highlights.
Quantitative Magnetic Resonance (QMR) is a quick, noninvasive method for providing accurate measurements of fat, lean, and water in small lizards.
Body condition indices based on mass–length relationships poorly predict body composition in small lizards.
Acknowledgments
Grant sponsor: NIH; Grant numbers: P30DK056336, P30DK0709626, and P30AG050886.
We thank A. Buckelew, C. Cates, D. Delaney, A. Dhawan, A. Durso, T. Mitchell, P. Pearson, and A. Reedy for their help collecting lizards and/or captive lizard care. This research was approved by the University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committee (project # 120909764). Body composition studies were carried out by the UAB Small Animal Phenotyping Core (NIH: P30DK056336, P30DK0709626, and P30AG050886).
LITERATURE CITED
- Angilletta MJ., Jr Estimating body composition of lizards from total body electrical conductivity and total body water. Copeia. 1999;1999:587–595. [Google Scholar]
- Barnett CA, Suzuki TN, Sakaluk SK, Thompson CF. Mass-based condition measures and their relationship with fitness: in what condition is condition? J Zool. 2015;196:1–5. doi: 10.1111/jzo.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet X, Naulleau G, Shine R, Lourdais O. Short-term versus long-term effects of food intake on reproductive output in a viviparous snake, Vipera aspis. Oikos. 2001;92:297–308. [Google Scholar]
- Boyd T, Arnbom T, Fedak M. Water flux, body composition, and metabolic rate during molt in female southern elephant seals (Mirounga leonina) Physiol Zool. 1993;66:43–60. [Google Scholar]
- Castro G, Wunder BA, Knopf FL. Total body electrical conductivity (TOBEC) to estimate total body fat of free-living birds. Condor. 1990;92:496–499. [Google Scholar]
- Civantos E, Forsman A. Determinant of survival in juvenile Psammodromus algirus lizards. Oecologia. 2000;124:64–72. doi: 10.1007/s004420050025. [DOI] [PubMed] [Google Scholar]
- Cox RM, Calsbeek R. Survival of the fattest? Indices of body condition do not predict viability in the brown anole (Anolis sagrei) Funct Ecol. 2015;29:404–413. [Google Scholar]
- Dobush GR, Ankey CD, Krementz DG. The effect of apparatus, extraction time, and solvent type on lipid extractions of snow geese. Can J Zool. 1985;63:1917–1920. [Google Scholar]
- Dussutour A, Poissonnier L-A, Buhl J, Simpson SJ. Resistance to nutritional stress in ants: when being fat is advantageous. J Exp Biol. 2016;219:824–833. doi: 10.1242/jeb.136234. [DOI] [PubMed] [Google Scholar]
- Evans PR. Winter fat deposition and overnight survival of yellow buntings (Emberiza citronella L.) J Anim Ecol. 1969;38:415–423. [Google Scholar]
- Fischer RU, Congdon JD, Brock M. Total body electrical conductivity (TOBEC): a tool to estimate lean mass and nonpolar lipids of an aquatic organism? Copeia. 1996;1996:459–462. [Google Scholar]
- Fowler LA, Dennis LN, Barry RJ, et al. In vivo determination of body composition in zebrafish (Danio rerio) by quantitative magnetic resonance. Zebrafish. 2016;13:170–176. doi: 10.1089/zeb.2015.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaëtan R, Vacquié-Garcia J, Jouma’a J, et al. Variation in body condition during post-moult foraging trip of southern elephant seals and its consequences on diving behaviour. J Exp Biol. 2014;217:2609–2619. doi: 10.1242/jeb.088542. [DOI] [PubMed] [Google Scholar]
- Green AJ. Mass/length residuals: measures of body conditions or generators of spurious results? Ecology. 2001;82:1473–1483. [Google Scholar]
- Guglielmo CG, McGuire LP, Gerson AR, Seewagon CL. Simple, rapid, and non-invasive measurement of fat, lean and total water masses of live birds using quantitative magnetic resonance. J Ornithol. 2011;152:S75–S85. [Google Scholar]
- Hayes JP, Shonkwiler JS. Morphometric indicators of body condition: worthwhile or wishful thinking? In: Speakman JR, editor. Body composition analysis of animals: a handbook of non-destructive methods. Cambridge: Cambridge University Press; 2001. pp. 8–38. [Google Scholar]
- Henen BT. Measuring the lipid content of live animals using cyclopropane gas. Am J Physiol. 1991;261:R752–R759. doi: 10.1152/ajpregu.1991.261.3.R752. [DOI] [PubMed] [Google Scholar]
- Hillman SS, Gorman GC. Water loss, desiccation tolerance, and survival under desiccating conditions in 11 species of Caribbean Anolis: evolutionary and ecological implications. Oecologia. 1977;29:105–116. doi: 10.1007/BF00345791. [DOI] [PubMed] [Google Scholar]
- Fowler LA, Dennis LN, Barry RJ, et al. In vivo determination of body composition in zebrafish (Danio rerio) by quantitative magnetic resonance. Zebrafish. 2016;13:170–176. doi: 10.1089/zeb.2015.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MS, Smith DL, Jr, Nagy TR. Validation of quantitative magnetic resonance (QMR) for determination of body composition in rats. Int J Body Comp Res. 2009;7:99–107. [PMC free article] [PubMed] [Google Scholar]
- Johnson MS, Watts RJ, Hammer HS, Nagy TR, Watts SA. Validation of duel-energy X-ray absorptiometry (DXA) to predict body composition of channel catfish, Ictalurus punctatus. J World Aquaculture Soc. 2016 doi: 10.1111/jwas.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AS, Johnson MS, Nagy TR. Validation of quantitative magnetic resonance for the determination of body composition of mice. Int J Body Comp Res. 2009;7:67–72. [PMC free article] [PubMed] [Google Scholar]
- Karasov WH, Pinshow B. Changes in lean mass and in organs of nutrient assimilation in a long-distance passerine migrant at a springtime stopover site. Physiol Biochem Zool. 1998;71:435–448. doi: 10.1086/515428. [DOI] [PubMed] [Google Scholar]
- Kelly CD, Tawes BR, Worthington AM. Evaluating indices of body condition in two cricket species. Ecol Evol. 2014;4:4476–4487. doi: 10.1002/ece3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBarbera M. Analyzing body size as a factor in ecology and evolution. Ann Rev Ecol Evol Syst. 1989;20:97–117. [Google Scholar]
- Labocha MK, Hayes JP. Morphometric indices of body condition in birds: a review. J Ornithol. 2012;153:1–22. [Google Scholar]
- Labocha MK, Schutz H, Hayes JP. Which body condition index is best? Oikos. 2014;123:111–119. [Google Scholar]
- Lee JC, Clayton D, Eisenstein S, Perez I. The reproductive cycle of Anolis sagrei in southern Florida. Copeia. 1989;1989:930–937. [Google Scholar]
- LeGalliard J-F, Clobert J, Ferriere R. Physical performance and Darwinian fitness in lizards. Nature. 2004;432:502–505. doi: 10.1038/nature03057. [DOI] [PubMed] [Google Scholar]
- Lindén M, Gustafsson L, Pärt T. Selection on fledging mass in the collard flycatcher and the great tit. Ecology. 1992;73:336–343. [Google Scholar]
- Losos JB. Integrative approaches to evolutionary ecology: Anolis lizards as model systems. Ann Rev Ecol Syst. 1994;25:467–493. [Google Scholar]
- Losos JB. Lizards in and evolutionary tree: ecology and adaptive radiation of anoles. Berkeley: University of California Press; 2009. [Google Scholar]
- McGuire LP, Guglielmo GC. Quantitative magnetic resonance: a rapid, noninvasive body composition analysis technique for live and salvaged bats. J Mammol. 2010;91:1375–1380. [Google Scholar]
- Merilä J, Kruuk LEB, Sheldon BC. Natural selection on the genetical component of variance in body condition in a wild bird population. J Evol Biol. 2001;14:918–929. [Google Scholar]
- Milenkaya O, Catlin DH, Legge S, Walters JR. Body condition indices predict reproductive success but not survival in a sedentary, tropical bird. PLoS One. 2015 doi: 10.1371/journal.pone.0136582. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JS, Hickling GJ. Fasting endurance and the evolution of mammalian body size. Funct Ecol. 1990;4:5–12. [Google Scholar]
- Navarro C, Marzal A, de Lope F, Moller AP. Dynamics of an immune response in house sparrows Passer domesticus in relation to time of day, body condition, and blood parasite infection. Oikos. 2003;101:291–298. [Google Scholar]
- Peig J, Green AJ. New perspectives for estimating body condition form mass/length data: the scaled mass index as an alternative method. Oikos. 2009;118:1883–1891. [Google Scholar]
- Perez-Gomez J, Rodriguez GV, Ara I, et al. Role of muscle mass on sprint performance: gender differences? Eur J Appl Physiol. 2008;102:685–694. doi: 10.1007/s00421-007-0648-8. [DOI] [PubMed] [Google Scholar]
- Reynolds DS, Kunz TH. Standard methods for destructive body composition analysis. In: Speakman JR, editor. Body composition analysis of animals. Cambridge: Cambridge University Press; 2001. pp. 39–55. [Google Scholar]
- Riley JL, Baxter-Gilbert JH, Guglielmo CG, Litzgus JD. Scanning snakes to measure condition: a validation of quantitative magnetic resonance. J Herpetol. 2016 doi: 10.1670/15-113. [DOI] [Google Scholar]
- Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc R Soc Lond B. 1996;263:1415–1421. [Google Scholar]
- Sandberg R, Moore FR. Fat stores and arrival on the breeding grounds: reproductive consequences for passerine migrants. Oikos. 1996;77:577–581. [Google Scholar]
- Schmidt KL, MacDougall-Shackleton EA, Macdougall-Shackleton SA. Developmental stress has sex-specific effects on nestling growth and adult metabolic rates but no effect on adult body size or body composition in song sparrows. J Exp Biol. 2012;215:3207–3217. doi: 10.1242/jeb.068965. [DOI] [PubMed] [Google Scholar]
- Schwartz TS, Gainer R, Dohm ED, et al. Second-hand eating? Maternal perception of the food environment affects reproductive investment in mice. Obesity. 2015;23:927–930. doi: 10.1002/oby.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Hostedde AI, Millar JS, Hickling GJ. Evaluating body condition in small mammals. Can J Zool. 2001;79:102–1029. [Google Scholar]
- Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ. Restitution of mass-size residuals: validating body condition indices. Ecology. 2005;86:155–163. [Google Scholar]
- Scott I, Selman C, Mitchell PI, Evans PR. The use of total body electrical conductivity (TOBEC) to determine body composition in vertebrates. In: Speakman JR, editor. Body composition analysis of animals. Cambridge: Cambridge University Press; 2001. pp. 127–160. [Google Scholar]
- Secor SM, Nagy TR. Non-invasive measure of body composition of snakes using dual-energy X-ray absorptiometry. Comp Biochem Physiol A. 2003;136:379–389. doi: 10.1016/s1095-6433(03)00176-4. [DOI] [PubMed] [Google Scholar]
- Seewagon CL, Guglielmo GC. Effects of fat and lean body mass on migratory landbird stopover duration. Wilson J Ornithol. 2010;122:82–87. [Google Scholar]
- Smith DL, Jr, Johnson MS, Nagy TR. Precision and accuracy of bioimpedance spectroscopy for determination of in vivo body composition of rats. Int J Body Comp Res. 2009;7:21–26. [PMC free article] [PubMed] [Google Scholar]
- Stevenson RD, Woods WA., Jr Condition indices for conservation: new uses for evolving tools. Integ Comp Biol. 2006;46:1169–1190. doi: 10.1093/icb/icl052. [DOI] [PubMed] [Google Scholar]
- Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem. 2003;377:990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- Warner DA, Lovern MB. The maternal environment affects offspring viability via an indirect effect of yolk investment on offspring size. Physiol Biochem Zool. 2014;87:276–287. doi: 10.1086/674454. [DOI] [PubMed] [Google Scholar]
- Warner DA, Buckelew AM, Pearson PR, Dhawan A. The effect of prey availability on offspring survival depends on maternal food resources. Biol J Linn Soc. 2015;115:437–447. [Google Scholar]